Studies on Stem Cells Research and Therapy
Potential therapeutic applications of mesenchymal stem cells for erectile dysfunction in diabetes mellitus: From preclinical/clinical perspectives
Serap Gur1,2*
2Departments of Urology, Tulane University Health Sciences Center, New Orleans, LA, USA
Cite this as
Gur S (2021) Potential therapeutic applications of mesenchymal stem cells for erectile dysfunction in diabetes mellitus: From preclinical/clinical perspectives. Stud Stem Cells Res Ther 7(1): 001-011. DOI: 10.17352/sscrt.000017Diabetes-related complications affect all body organ systems, including the penis. Diabetes-induced erectile dysfunction (ED) is caused by neuropathy of the penile nerves and vasculopathy of the smooth muscle and endothelium corpus cavernosum. To present an overview of Mesenchymal Stem Cell (MSC) research in diabetic animal models of ED, focusing on the function, signaling, and niches that have a prominent role in the regeneration of cavernosal cells and restoration of penile tissues. We highlight common erectile pathologies caused by diabetes and review relevant preclinical trials. We also discuss paracrine mechanisms of various MSC therapies involved in the repair of endothelial cells and cavernous nerves in these diabetic models. A PubMed search was performed, with dates ranging from inception until November 20, 2019. This review provides a comprehensive evaluation of the various strategies that have been investigated for improving MSC delivery methods, through preclinical literature and published clinical trials regarding ED in men with diabetes. MSC-type applications have been beneficial to erectile function in diabetic models of ED. This review examines the progress and remaining challenges in diabetes-related SC research regarding ED. Moving forward, it is only with a combined effort of basic biology and translational work that the potential of MSC-based therapies in diabetes can be realized.
Introduction
Diabetes mellitus affects millions of people globally, and its complications can be harmful to all body systems[1,2]. Diabetic men exhibit a higher prevalence of erectile dysfunction (ED) than non-diabetic men[3-5], with epidemiological studies documenting that up to 75% of diabetic men suffer from ED [6,7].
Diabetic ED involves nerve damage, endothelial injury, and cavernosal muscle fibrotic alterations (Figure 1) [3,8-10]. Complications due to diabetes can limit blood flow into the penis if atherosclerotic damage corresponds to major blood vessels in the vascular system [11]. Advanced glycation endproducts (AGE) cause microvascular complications as pathogenesis of ED in streptozotocin-induced diabetic rats[12]. AGE and its receptor (RAGE) interaction develop a metabolic memory [13-15]. Insulin therapy for glycemic control can reduce AGE and RAGE and control the corresponding inflammatory response in the penis[14,16].
Diabetic ED is currently managed by Phosphodiesterase (PDE)-5 inhibitors, vacuum erection devices, and prosthetic surgery. Though they are considered first-line ED treatments, the efficacy of oral PDE-5 inhibitor treatments, such as tadalafil, vardenafil, sildenafil, and avanafil, is lower in diabetic men compared with nondiabetic men, as these treatments do not alter the existing pathological changes caused by diabetes[17]. The effectiveness of PDE-5 inhibitors is further reduced due to inadequate nitric oxide (NO) bioavailability from both neuropathic and endothelial dysfunction in diabetic men [18]. However, the inhibition of PDE-5 improves the therapeutic efficacy of adipose-derived stem cells for ED in diabetic rats[19].
The past decade has witnessed no significant developments in treatment options for men with diabetes-related ED; however, there has been considerable attention given to preclinical studies centered on stem cell therapies for ED in diabetic men [20]. Recent efforts to treat ED at a preclinical level have consisted of isolating SCs from various organs, such as bone marrow, adipose tissue, and human umbilical cord blood [20,21].
This review focuses on the advances of various mesenchymal stem cell (MSC) delivery methods, through preclinical literature and published clinical trials regarding ED in men with diabetes.
Penile erection and diabetic ED
Erectile function is a multifaceted neurovascular phenomenon that necessitates the healthy coordination of endothelial, nerve, and smooth muscle cells [22,23]. NO is formed by an enzymatic pathway involving both endothelial NO synthase [24] and neuronal (n) NOS and plays a crucial role in a normal erection [23,25]. For instance, the main cavernous nerve of the rat branches into the dorsal and intracavernous nerves. There is a loss of nNOS nitrergic fibers due to damage to both locations of the cavernous nerve [26,27]. Cavernous nerve integrity is critically important for normal erectile physiology.
Burke, et al. [28] documented that nearly 50% of diabetic men aged 40–79 years suffer from ED. Additionally, many men seeking help for their ED are unaware they have diabetes [8]; however, clinical studies demonstrate that early metabolic control may delay the onset of diabetic complications. The analysis of both semen testosterone levels and hemoglobin A1c is a vital part of the clinical evaluation and treatment of diabetes, as diabetic men with sexual disorders have elevated glycated-hemoglobin levels (>6.5%) (Figure 1) [29]. Angulo, et al. [30] described that the NO-cyclic guanosine monophosphate (cGMP) pathway is severely affected by diabetic ED men.
Physiological loss of the properties of the endothelium leads to vascular endothelial dysfunction and a shift to a prothrombotic, vasoconstrictor, and proinflammatory state. This status is considered to be the initial insult in the progression of diabetic ED [31]. The smooth muscle relaxation by NO-mediated neurotransmission is reduced, and vascular hemodynamics in penile tissue is altered by chronic hyperglycemia [23,32]. Severe diabetes decreases NO bioavailability as a result of damage to the nitrergic nerves that serve the penile Corpus Cavernosum (CC). Furthermore, the effect of vasoconstrictor mediators, including endothelin-1 and angiotensin II, is increased in diabetes [23,33,34].
The tight control of glycemia is an essential first step in the management of diabetes-related ED. In a recent study of ED in a rat model of type 1 diabetes, islet transplantation with improved hyperglycemic status allowed for smooth muscle cell regeneration, and reduced CC fibrosis to its normal state in rats with advanced-stage diabetes [35]. Hyperglycemia-induced tissue damage occurs via increased levels of oxygen-free radicals [36,37]. The underlying mechanism in the restoration of CC fibrosis by islet transplantation may be due to inhibition of transforming growth factor (TGF)-β1/Samd2/ connective tissue growth factor in a rat model of type 1 diabetes [35]. Diabetic microvascular complications are associated with AGE in the ED pathogenesis in streptozotocin-induced diabetic rats [12].
Type-2 diabetic patients have a higher incidence of hypogonadism and ED[38]. Erectile function and sexual desire in type-2 diabetic men can be improved by testosterone replacement therapy (TRT). However, available data are inadequate, and the long-standing benefits of TRT are not well described in this diabetic population. TRT may be offered to type-2 diabetic men after all potential risks and benefits of treatment have been discussed [39].
In earlier studies using animal models of ED, SC treatment prevented neuronal and endothelial dysfunction in the penises of diabetic rats[40]. Erectile function was restored by bone marrow-derived SCs (BMSCs) and adipose-derived SCs (ADSCs), with increased generation of penile endothelial, smooth muscle, and neuronal cells [41-43]. nNOS and eNOS expressions in the cavernous tissues were increased by SCs in a diabetic rat model. Angulo, et al. [30] described that the NO-cyclic guanosine monophosphate (cGMP) pathway is severely affected by diabetic ED men. Also, various types of cytokines, such as brain-derived neurotrophic factor (BDNF) and Vascular Endothelial Growth Factor (VEGF) are increased, and cavernous tissues are protected from apoptosis with the promotion of cell survival[44]. Intracavernous BMSCs differentiate into cells expressing both endothelial and smooth muscle markers. In the case of diabetes, numerous studies have reported that intracavernous-transplanted mesenchymal stem cells (MSCs) regenerate endothelial and smooth muscle cells in the CC of diabetic rats[45,46].
A challenge in the management of diabetic ED
The first-line treatment for ED is oral PDE-5 inhibitors, which are well recognized as less clinically useful in diabetic ED. While approximately 35% of patients with ED will fail to respond to PDE5 inhibitors, in diabetic men the figure is 70%. The inadequate response to these agents stems from cGMP levels not reaching the threshold needed to induce a penile erection. This is due to insufficient NO caused by severe endothelial dysfunction and neuropathy. These agents generally inhibit GMP breakdown and augment the NO-cGMP pathway [30,47]. Combination therapy for diabetic ED involves PDE-5 inhibitors and TRT. Several antioxidants (e.g., ascorbic acid, melatonin, vitamin E, and sodium selenate) have been documented to partially restore ED in experimental diabetic animal models[36].
MSCs
MSCs are easily isolated from bone marrow and adipose tissue (BMSCs and ADSCs). The hypoimmunogenicity and immunomodulatory effects of MSCs have been well characterized and they have been widely used in clinical practice [48]. MSCs show both transdifferentiation capability and self-renewal potential and can be expanded in vitro, and then directed to various cell lineages to differentiate into cell types such as endothelial, smooth muscle, neuronal, and Schwann cells [49,50]. MSCs also secrete cytokines and paracrine factors that may enhance cell survival and angiogenesis and induce anti-apoptotic, pro-neurogenic, anti-inflammatory, and anti-fibrotic effects[51,52].
The pore-forming “large potassium conductance calcium-activated channel” proteins of cell membranes are encoded by the gene KCNMA1 (potassium large-conductance calcium-activated channel) [23,53]. In an earlier study, KCNMA1-transfected BMSCs improved erectile function in diabetic rats[54]. Hyperpolarization of the membrane decreased the excitability of cells, and functional ion channel-mediated intracellular K+ outflow was increased by its expression [55].
Bahk et al. reported that penile blood flow and erectile function were improved by chronic placental matrix-derived MSC injections for ED in type-2 diabetic patients [23,56]. Although not all of the patients had satisfactory erections without oral medications or injectables, none of them requested a penile prosthesis [23].
Diabetes-associated ED has been reported to benefit from low-energy shockwave therapy (LESWT), with the claims that nNOS positive nerves, endothelial, and smooth muscle cells in the penile tissue were regenerated by this treatment. SC transplantation combined with LESWT may be one suitable treatment for diabetic ED[57].
The improved erectile function observed by the combination of LESWT and BMSC in diabetic rats was more efficient than BMSC transplantation alone[23,58]. Zhu, et al. [59] suggested that the combination approach stimulated autophagy and decreased apoptosis in the diabetic rat CC. After intracavernous transplantation of BMSCs, smooth muscle and endothelial content increased, inducing normal erectile function in diabetic rats. Labeled BMSCs were observed in the penis post-injection after four weeks, caused by the secretion of neurotrophic factors [20,60,61]. Erectile function was restored with the transplantation of Flk-1(+)Sca-1(-) mesenchymal SCs(Flk-1(+)Sca-1(-) MSCs) in STZ diabetic rats [62]. Flk-1(+)Sca-1(-) MSCs were engrafted and led to homing of damaged muscle, myofibers restoration, partial reconstitution of the sarcolemmal expression of myocardin, and restored specific pathological marker levels [62].
Intracavernosal injection of clonal BMSCs in streptozotocin-induced diabetic mice significantly recovered erectile function [63]. Increased cavernosal smooth muscle and endothelial cells, penile eNOS phosphorylation (Ser1177), and nNOS and neurofilament were restored to 80–90% of the control values[23].
Recently, Bcl-2-modified BMSCs were transplanted to treat diabetic ED in a rat model of type 2 diabetes [64]. Bcl-2 contributed to the function of BMSCs and improved intracavernosal pressure (ICP)/ mean arterial pressure (MAP) and erections in diabetic ED rats [64].
Recently, BM-MSCs with a significantly lowered maternally expressed gene (MEG)-3 were implanted intracavernous and improved ED of diabetic rats [65]. FOXM1 protein can be degraded by MEG3, and the differentiation of the endothelium is ultimately regulated by BM-MSCs [65].
A significant aspect of diabetic complications is an injury caused by oxidative stress due to hyperglycemia; therefore, future studies of the antioxidant capacity of MSCs are warranted. Endothelial-progenitor cells (EPCs) from the bone marrow can be mobilized to counter diabetes-induced oxidative stress. In 2012, Qui et al. documented that treatment with melatonin promoted EPC mobilization and thereby preserved erectile function in type-1 diabetic rats [66]. ED was improved by transplantation of VEGF165-transfected EPCs into the CC of diabetic rats [67]. In a previous study, tissue repair and angiogenesis were synergistically promoted by the combined transplantation of MSCs and EPCs [68].
There are many unanswered questions, e.g., the question of single versus multiple injections. A single injection of MSCs may be insufficient for the maintenance of a long-term therapeutic effect. Similarly, the management of potency by multiple MSC injections within a short interval and the dosage of MSC infusions need to be further explored.
Because of the complicated pathogenesis of diabetes mellitus, intrinsic dysfunction of the bone marrow SC niche ultimately results in MSC failure [23]. Some strategies for reducing the functional inability of BM-MSCs need to be recognized. HbA1c reduction and insulin requirements require close clinical follow-up to improve the efficacy of MSC treatment in type II diabetes[23]. Similarly, ED studies using a diabetic animal model need to observe changing insulin resistance when using MSC therapies. While available data from animal and human studies are encouraging, MSC therapy may signify a new paradigm for glycemic control in type-2 diabetes.
The main biocomponent of the secretome is the exosome, which is a naturally occurring membrane nanoparticle of 30-120 nm in diameter that mediates intercellular communication by delivering biomolecules into recipient cells[69,70]. Exosomes carry many molecules, including miRNAs, proteins, and lipids as a composite cargo, as well as the exosome cargo, which is transferable to different cell types. These recipient cells undergo expressional and functional changes with exosome uptake [71,72]. The role of exosomes in diabetic ED needs further study.
The nanosized exosomes derived from MSCs may become a valuable therapeutic strategy in regenerative therapies compared with transplanted exogenous MSCs. There are many advantages of nanosized exosomes compared with exogenous MSCs. Exosomes are more natural to preserve and transfer, have lower immunogenicity, and are safer for therapeutic administration [73]. Exosomes derived from BMSCs may become a treatment for diabetes-induced ED. MSCs with hypoxic preconditioning may provide additional benefit in diabetes-induced ED, due to increased angiogenesis and neuroprotection [74].
Erythropoietin [75] is a potent cytokine capable of reducing apoptosis of Schwann cells. However, the expression of EPO in MSC is limited, though overexpression of EPO in MSC significantly improves neuroprotective actions. EPO-MSCs have the potential to reduce apoptosis of diabetes-triggered Schwann cells. Thus, suppression is likely due to the reduction of oxidative stress and apoptosis-related protein factors. Studies have revealed that the placenta (P)-derived MSCs have potent paracrine and differentiation potential effects in diabetic nude rats[76]. P-MSCs that survived three weeks accelerated the recovery of ischemic damage by increased generation of arterioles, the formation of capillaries, and the secretion of various proangiogenic factors [76].
MSCs combined with pioglitazone, or exendin-4 demonstrated substantial benefit compared with MSCs alone in regards to cardioprotective effects [77]. Recently, Jeon et al. [78] showed that stromal cell-derived factor-(SDF)-1-expressing engineered MSCs improved erectile function in STZ-induced diabetic ED rats [23]. A recent phase-I clinical study proved the safety, tolerability, and efficiency of intracavernous autologous BM-MSC injections to treat ED in diabetic patients [79].
ADSCs
Adipose tissue is also a possible source for SCs, as ADSCs have self-renewal and multipotency characteristics similar to BMSCs [20,21]. The main advantages of ADSCs are that they are accessible to culture and easily collected from patients by a minimally invasive procedure, such as liposuction. The successful transplantation of allogeneic and xenogeneic ADSC illustrates their low immunogenicity [80,81].
Growing evidence suggests the success of ADSC in several ED models[82]. Intracavernosal unmodified ADSCs have been shown to restore erectile function in numerous rat ED models[42,83].
The preservation of neuronal and endothelial cells of CC in rat ED models has been observed after the intracavernous administration of cultured ADSCs [42,46]. Rats with diabetic ED treated with autologous ADSCs displayed improvement of erectile function, as well as reduced apoptosis of cavernosal tissues, but few labeled ADSCs were identified [46]. The therapeutic benefit of ADSCs appears to be an indirect mechanism, whereby ADSCs improve the extracellular environment and local tissue function via the direct transformation of ADSCs into local cell types[46]. Intracavernosal injection of ADSC to a VEGF-treated group of ED in a rodent diabetic model demonstrated improved erectile function linked to an amplified expression of smooth muscle, endothelial, and pericyte markers[84]. The potential of ADSCs to regenerate and repair various tissues deserves more focus [85].
An ideal source for SC and stromal cells are the ADSC stromal vascular fraction (SVF). Human SVF was isolated from five patients undergoing reduction mammoplasty and administered to C57BL/6J mice after induction of diabetes. At eight weeks, erectile function was restored by increased endothelial and smooth muscle cells, nNOS-positive nerve fibers, and eNOS phosphorylation in diabetic mice [86].
This benefit witnessed in animal models [45,87] suggests that SC therapy may recover erectile function in humans [23]. Furthermore, the overexpression of adrenomedullin by ADSC enhanced erectile function in diabetic rats, likely by amplified VE-cadherin and eNOS expressions in diabetic rats [88].
Various forms of fibrosis involve the TGFβ1-Smad signaling pathway. Hepatocyte growth factor (HGF) is known to inhibit the TGFβ1-Smad signaling pathway and attenuate renal fibrosis in diabetic rats[89,90]. Similarly, penile fibrosis occurs as a pathological response to diabetes. Erectile function was improved by ADSC monotherapy in streptozotocin-induced diabetic rats, and the benefit was augmented when combined with HGF, resulting in a higher number of endothelial and smooth muscle cells and a lower cell apoptotic index in the CC [91].
Tissue inhibitors of matrix metalloproteinase -1 (MMP-1), lipopolysaccharide-inducible CXC chemokine (LIX), and VEGF are expressed after ADSC delivery [12]. Cavernous endothelium, smooth muscle, and nNOS-positive nerves were partially preserved, and apoptosis was reduced in ADSC-treated diabetic rats[12]. Theoretically, the addition of insulin may further control the inflammatory response and decrease AGE-product accumulation in the penis[12,23,63].
In other experiments, a streptozotocin-diabetic rat, transplantation with pigment epithelium-derived factor (PEDF)-transfected ADSCs successfully improved ICP/MAP ratios as compared with untreated ADSC [92]. Overexpression of PEDF resulted in higher survival rates and decreased apoptosis of ADSC[92]. ADSC transplantation restored erectile function in a diabetic rat model by attenuating the harmful effects of hyperglycemia. Thus, the therapeutic potential of ADSC for treating ED, as well as the additional benefits of PEDF overexpression, is an exciting development [92]. At the early stages of elevated glucose levels in type-2 diabetic rats, nNOS and eNOS were unregulated and corporal veno-occlusive dysfunction (CVOD), caused by contracted smooth muscle, increased collagen, and fat infiltration was improved by SC delivery [23,93]. However, SCs administered at a later stage in this high-glucose in a streptozotocin-diabetic rat model were unsuccessful in restoring functional corporal tissue [23,92].
In a recent study, the in vivo homing efficiency provided by superparamagnetic iron oxide nanoparticles (SPION)-labeled ADSCs to the CC region was improved by the use of an external magnetic field in a rat model of diabetic ED [23,94]. Smooth muscle and endothelial density increased after magnetic field-guided SPION-labeled ADSCs in the CC, and erectile function was improved when compared to ADSC treatment alone[94]. The combination of intracavernous injection of ADSCs and icariin via daily gastric gavage augmented ICP and ICP/MAP values[23,95]. Thus, icariin has a positive effect on ADSC treatment of diabetic ED [95]. ADSC-derived exosomes also induced a beneficial effect on erectile function in a type-2 diabetic rat model [96]. Exosomes were isolated from the supernatants of cultured ADSC by ultracentrifugation. ADSC-derived exosomes, similar to ADSC, were capable of rescuing CC endothelial and smooth muscle cells by inhibiting apoptosis and thus promoting the recovery of erectile function in a type-2 diabetic rat model (using a high-fat diet and low-dose streptozotocin administered by intraperitoneal injection) [64]. ADSCs-based Microtissues (MT) in STZ-induced diabetic rats with ED induced expression of Nerve Growth Factor (NGF), VEGF, and tumor necrosis factor-stimulated gene-6 [97]. Also, MT treatment improved ICP, nNOS levels, and endothelial and smooth muscle contents and reduced local inflammation in the CC of diabetic rats. MTs combined with intracavernosal ADSC enhanced erectile function and histopathological changes in streptozotocin-induced diabetic rats [97]. Very recently, the injection of ADSCs into the tunica albuginea during the active phase of Peyronie’s disease prevents the development of fibrosis[98].
ADSCs and platelet-rich plasma co-transplantation is an attractive option in therapies using autologous cells. However, transplantation of ADSCs is often exposed to hostile environments in which local oxidative stress, hypoxia, and inflammation induce early cell loss. Reduced survival of transplanted ADSCs will dramatically reduce their therapeutic effects. Of note, a current study in a rat model of type 2 diabetes showed that hypoxia-preconditioning promoted ADSC-based repair of diabetes-induced ED by augmenting angiogenesis and neuroprotection [99].
The efficacy of ADSC in improving ED in diabetic rats is mainly derived through a paracrine effect. ADSC-derived exosomes, similar to ADSC, are capable of restoring CC endothelial and smooth muscle cells by inhibiting apoptosis and promoting recovery of erectile function [64]. ADSC-derived exosomes display in vitro proangiogenic properties, and restored erectile function in vivo, by the proliferation of endothelial cells and decreasing fibrosis of CC.
Molecular mechanisms underlying MSC dysfunction
The accumulation of AGEs is one of the recognized mechanisms of MSC dysfunction in diabetes. In short, the formation of AGEs due to the overproduction of Reactive Oxygen Species (ROS)[29] mediates the intracellular glycation of mitochondrial respiratory chain proteins and triggers a cascade of events through activation of the receptor for RAGE [24,100,101]. Hyperglycemia exerts metabolic stress, leading to the production of AGE and the generation of ROS. In turn, mitochondrial DNA polymerase-γ mutations are stimulated, and ROS production is exacerbated. The apoptosis and senescence of stem/progenitor cells contribute to these pathological effects of diabetes. Chronic RAGE stimulation causes defects in cellular membrane repair [102]. This triggered damage via oxidative and endoplasmic reticulum stress by ROS production and increases inflammation through TNF-α signaling[100-102]. The effect of AGEs on BM-MSC function results from amplified oxidative stress and inflammatory response[23,103,104]. It is unclear whether the beneficial effects of in vitro preconditioning can be sustained when MSCs are transplanted into a hostile environment. Together, these studies suggest that the use of anti-inflammatory and antioxidant agents should be concurrently employed in patients with diabetes undergoing MSC therapy.
Future perspectives
The effects and safety issues for diabetes-associated ED treatment need further delineation to improve the quality of life for afflicted men.
Metabolic disorders are commonly observed after the pathological effects of diabetes have occurred [105]. The loss of penile smooth muscle and impaired vasculogenesis in patients with diabetic ED can hopefully be reversed by MSC regenerative therapy. However, issues to be considered include cellular senescence due to hyperglycemia-induced metabolic changes caused by epigenetic changes. In this condition, post-translational histone modification, DNA methylation, and regulation by noncoding RNA-like miRNA and lncRNA have reduced the regenerative abilities of MSCs. Similarly, exosomes regulate the efficacy of endogenous/transplanted cells and carry the molecular cargo that influences the angiogenesis of the diabetic penis. The regenerative ability of diabetic stem/progenitor cells is affected by reduced secretion of therapeutic exosomes or the aberrant release of diseased exosomes. Progress to enable cell differentiation to include increasing the yield of cells, enabling grafting via direct cell or tissue transplantation and overcoming legal issues regarding national regulations [106].
In regenerative medicine, MSCs that can differentiate and migrate are a necessity. Paracrine action is vital to realize the curative effect of MSCs to improve erectile function. LESWT, in theory, recruits endogenous SC to the cavernous bodies to improve the diabetic microenvironment in the CC, and possibly provide some benefit to transplanted SC survival. Combined SC and gene therapy would similarly promote SC survival and increase cell differentiation for tissue repair[107]. However, single-cell-based injections have a low cellular survival, which counters any long-lasting success. Adult SCs may be altered from their regular adherent culture[82].
A wide range of growth factors is secreted by ADSCs, and these, in turn, stimulate tissue regeneration. In clinical applications, the use of ADSCs is a reasonable concept for the repair of damaged tissues and the stimulation of angiogenic activity. Hypoxia preconditioning might also improve the therapeutic efficacy of ADSC in diabetes-induced ED. Additional issues to consider are unwanted side effects, the survival rate of ADSC in cavernous tissues, and changes that occur to ADSC in the cavernosal microenvironment[23].
Further discovery of the pathophysiological mechanisms will come. SPIONs effectively incorporated into ADSCs had no adverse effects on SC properties. ADSCs and platelet-rich plasma co-transplantation is another novel approach to cell therapy in regenerative medicine.
Platelet-rich plasma can enhance the properties of ADSCs and also needs further investigation [108]. Conversely, the processes related to culturing and isolating ADSCs have boundaries; these comprise the high cost of amenities and staff, the underlying threat of contamination with undefined proteins and foreign serum, and changes in functional characteristics due to repeated culturing procedures[109,110].
Soluble factors released from MSCs may benefit MSC effects [111]. High levels of cellular senescence, apoptosis, and altered differentiation capacity in ADSC isolated from type-2 diabetics, have been observed [112]. Thus, the addition of adjuncts that increase differentiation and proliferation is needed to fortify ADSCs.
Modification of MSCs with growth factor genes may enhance the efficiency of MSC therapy. Groups treated with modified MSCs demonstrated better erectile function than that is unmodified. Among these strategies, MSC-based treatment is the most promising due to its ability to recover function in cells and tissues.
The investigational procedures of ADSC and BM-MSC are similar when comparing studies with only minor alterations regarding the cells examined and monitored. ICP-measured, post-SC injection into the CC is significantly higher than the control populations. The addition of specific growth factors to SCs by gene transfection may recover the efficacy of damaged cells. Until now, no reproducible tracking markers of these cells have been developed. The encouraging effect by injection of SCs on the ICP is attributed to cellular trans-differentiation, and various paracrine effects [113]. The positive impact of the injection of SCs on the ICP belongs to the cellular transdifferentiation effect and particularly to the paracrine effects, which have not yet been understood[114].
The pathogenesis of diabetes mellitus may induce intrinsic MSC dysfunction that eventually will be unsuccessful. This highlights the need for a strategy to counteract the functional decline of MSCs. Compromised BM-MSCs may be ineffective, as impairment of BM-MSCs may lead to disease progression and the development of comorbidities. The emergence of autologous MSC therapy in diabetes necessitates a deeper understanding of the SC alterations that occur when these cells are chronically exposed to a pathological environment. Future studies need to mimic the changes in MSC in diabetes and either rectify them before transplantation or prevent them from occurring. There is still a need for preclinical studies investigating the efficacy of antioxidants and anti-inflammatory agents in reversing the functional decline of MSCs[23]. Chronic RAGE exposure induces changes in cellular membrane repair, triggers intracellular damage by causing oxidative and endoplasmic reticulum stress through elevations in cytosolic ROS production, and amplifies inflammation through NFκB-mediated TNF-α signaling. The RAGE signaling pathway may be a pivotal point to preserve SC function in diabetic ED.
As type-2 diabetes is a multifactorial disease that is associated with insulin resistance-induced hyperglycemia[23], the majority of preclinical MSC studies in diabetic ED have focused on type-1 diabetes, which is an autoimmune condition characterized by a complete loss of insulin secretion, leading to hyperglycemia. Therefore, the development of autologous MSC therapies depends on a better understanding of the extrinsic host milieu on MSC function.
Advancements in technology and experimental techniques have provided an insight into how aging affects the properties of MSCs. Given that human life expectancy is expected to increase, the topic of cell aging and therapeutic applications continues to be an area of interest.
The more recent evidence suggests a developmental affiliation between pericytes and MSCs based on cell markers and differentiation potential[115, 116]. As a novel stem cell source, pericytes are generally considered to be the origin of MSCs. Pericytes have crucial roles in blood vessel function/stability, angiogenesis, endothelial cell proliferation/differentiation, wound healing, blood-brain barrier function, and hematopoietic stem cell maintenance [117]. All of these properties make pericytes preferred cells in the field of tissue engineering. Similar to other types of stem cells, pericytes act as a repair system in response to injury by maintaining the structural integrity of blood vessels[118]. Pericytes have recently been recognized for their central role in blood vessel formation. Pericytes are multipotent cells that are heterogeneous in their origin, function, morphology, and surface markers. In situ, pericytes are recognized by their localization to the abluminal side of the blood vessel wall and closely associated with endothelial cells, in combination with the expression of markers such as CD146, neural glial 2, platelet derived growth factor receptor β, α-smooth muscle actin, nestin and/or leptin receptor[116]. Similar to other types of stem cells, pericytes act as a repair system in response to injury by maintaining the structural integrity of blood vessels The role of pericytes is not restricted to the formation and development of the vasculature: they have been shown to possess stem cell-like characteristics and may differentiate into cell types from different lineages. While this assumption relies mainly on indirect evidence, the data supports the possibility that a precursor of the MSC is natively associated with the blood vessel wall and belongs to a subset of perivascular cells. Addressing this aspect may help improve the novelty of the subject in diabetes.
Conclusion
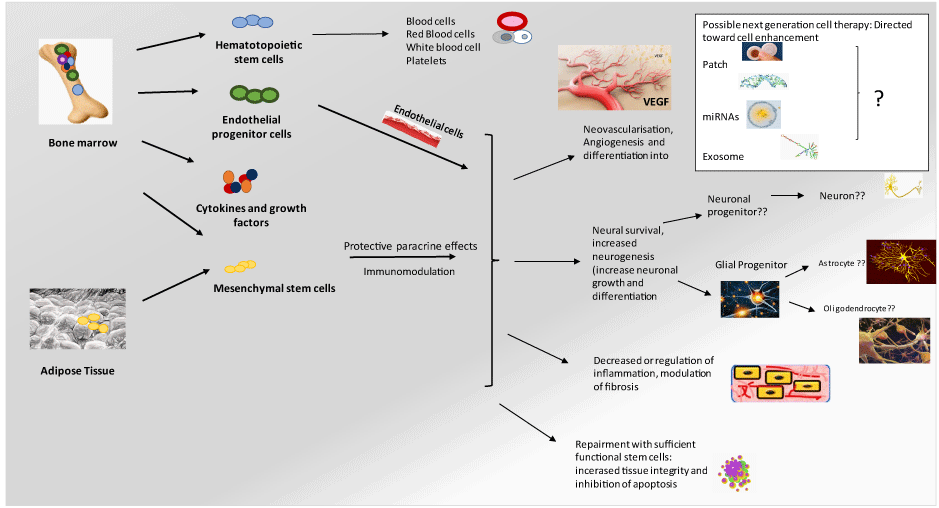
Though few stem cell-based studies have been directed toward type-2 diabetes, MSC-based therapies may provide better multifaceted metabolic corrections and concurrently offer long-term benefits to diabetic patients (Figure 1). MSC therapy in diabetic men with ED appears very close to addressing the effectiveness and safety of regenerative technology (Figure 1) [119]. MSC seems to be safe and effective in the shorter term and may provide genomic or epigenetic changes in the longer term.
It is useful for future MSC clinical trials to include histology confirmation and more extensive multicenter trials with various study protocols to compare treatment templates, including dose, duration, and a number of MSC injections[120]. Adult MSC has the advantage of avoiding the ethical issues of ESCs, and besides, published literature shows a very low probability of malignant transformation and tumor formation [121]. Diabetic patients need to be counseled and treated for many problems [122] hopefully, and regenerative effects will soon be brought into clinical practice.
- Cheng SK, Park EY, Pehar A, Rooney AC, Gallicano GI, et al. (2016) Current progress of human trials using stem cell therapy as a treatment for diabetes mellitus. Am J Stem Cells 5: 74-86. Link: https://bit.ly/3xMwGSE
- Habtemariam S, Lentini G (2015) The therapeutic potential of rutin for diabetes: an update. Mini Rev Med Chem 15: 524-528. Link: Link: https://bit.ly/3uc6grn
- Giuliano FA, Leriche A, Jaudinot EO, de Gendre AS (2004) Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology 64: 1196-201. Link: https://bit.ly/3aVhgSr
- De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, et al. (2002) Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care 25: 284-291. Link: https://bit.ly/3uhgPJV
- Cho NH, Ahn CW, Park JY, Ahn TY, Lee HW, et al. (2006) Prevalence of erectile dysfunction in Korean men with Type 2 diabetes mellitus. Diabet Med 23: 198-203. Link: https://bit.ly/3el6xmx
- Teles AG, Carreira M, Alarcão V, Sociol D, Aragüés JM, et al. (2008) Prevalence, severity, and risk factors for erectile dysfunction in a representative sample of 3,548 portuguese men aged 40 to 69 years attending primary healthcare centers: results of the Portuguese erectile dysfunction study. J Sex Med 5: 1317-1324. Link: https://bit.ly/3nKs1Mm
- Yang G, Pan C, Lu J ()2010 Prevalence of erectile dysfunction among Chinese men with type 2 diabetes mellitus. Int J Impot Res 22: 310-317. Link: Link: https://bit.ly/2PRUt2E
- Malavige LS, Levy JC (2009) Erectile dysfunction in diabetes mellitus. J Sex Med 6: 1232-1247. Link: https://bit.ly/3eP22iZ
- Moore CR, Wang R (2006) Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl 8: 675-684. Link: https://bit.ly/2RoJIoU
- Hatzimouratidis K, Hatzichristou D (2014) How to treat erectile dysfunction in men with diabetes: from pathophysiology to treatment. Curr Diab Rep 14: 545. Link: https://bit.ly/3vzPzWY
- Maiorino MI, Bellastella G, Esposito K (2014) Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes 7 95-105. Link: https://bit.ly/3eS4zZJ
- Zhou F, Hui Y, Xu Y, Lei H, Yang B, et al. (2016) Effects of adipose-derived stem cells plus insulin on erectile function in streptozotocin-induced diabetic rats. Int Urol Nephrol 48: 657-669. Link: https://bit.ly/33f5LAR
- Wang L, Xu Y, Li H, Lei H, Guan R, et al. (2015) Antioxidant icariside II combined with insulin restores erectile function in streptozotocin-induced type 1 diabetic rats. J Cell Mol Med 19: 960-969. Link: https://bit.ly/3thpkTZ
- Wang L, Tian W, Uwais Z, Li G, Li H, et al. (2014) AGE-breaker ALT-711 plus insulin could restore erectile function in streptozocin-induced type 1 diabetic rats. J Sex Med 11: 1452-1462. Link: https://bit.ly/3vIsD7Z
- Usta MF, Kendirci M, Gur S, Foxwell NA, Bivalacqua TJ, et al. (2006) The breakdown of preformed advanced glycation end products reverses erectile dysfunction in streptozotocin-induced diabetic rats: preventive versus curative treatment. J Sex Med 3: 242-250. Link: https://bit.ly/3ee3tZ1
- Usta MF, Bivalacqua TJ, Koksal IT, Toptas B, Surmen S, et al. (2004) The protective effect of aminoguanidine on erectile function in diabetic rats is not related to the timing of treatment. BJU Int 94: 429-432. Link: https://bit.ly/3uhLhDA
- Redrow GP, Thompson CM, Wang R (2014) Treatment strategies for diabetic patients suffering from erectile dysfunction: an update. Expert Opin Pharmacother 15: 1827-1836. Link: https://bit.ly/3h044iX
- Dorsey P, Keel C, Klavens M, Hellstrom WJ (2010) Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin Pharmacother 11: 1109-1122. Link: https://bit.ly/3th5qbA
- Yang J (2014) inhibition of phosphodiesterase-5 improves therapeutic efficacy of adipose-derived stem cells for erectile dysfunction in diabetic rats. Journal of Urology 191: E481-E481.
- Lin CS, Xin Z, Wang Z, Deng C, Huang YC, et al. (2012) Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev 21: 343-351. Link: https://bit.ly/3vHDteO
- Condorelli RA, Calogero AE, Vicari E, Favilla V, Morgia G, et al. (2013) Vascular regenerative therapies for the treatment of erectile dysfunction: current approaches. Andrology 1: 533-540. Link: https://bit.ly/33fzxWa
- Yohannes E, Chang J, Tar MT , Davies KP, Chance MR (2010) Molecular targets for diabetes mellitus-associated erectile dysfunction. Mol Cell Proteomics 9: 565-578. Link: https://bit.ly/3nLMv7N
- Gur S, Abdel-Mageed AB, Sikka SC, Hellstrom WJG (2018) Advances in stem cell therapy for erectile dysfunction. Expert Opin Biol Ther 18: 1137-1150. Link: https://bit.ly/3vCmTwI
- Piperi C, Goumenos A, Adamopoulos C, Papavassiliou AG (2015) AGE/RAGE signalling regulation by miRNAs: associations with diabetic complications and therapeutic potential. Int J Biochem Cell Biol 60: 197-201. Link: https://bit.ly/3h0XzfB
- Alwaal A, Zaid UB, Lin CS, Lue TF (2015) Stem cell treatment of erectile dysfunction. Adv Drug Deliv Rev 82-83: 137-144. https://bit.ly/3eg8nEX
- Albersen M, Shinde A, Mwamukonda K, Lue T (2010) The future is today: emerging drugs for the treatment of erectile dysfunction. Expert Opin Emerg Drugs 15: 467-480. Link: https://bit.ly/2SevDKX
- Albersen M, Lin G, Fandel TM, Zhang H, Qiu X, et al. (2011) Functional, metabolic, and morphologic characteristics of a novel rat model of type 2 diabetes-associated erectile dysfunction. Urology 78: 476 e1-8. Link: https://bit.ly/2SgEgVm
- Burke JP, Jacobson DJ, McGree ME, Nehra A, Roberts RO, et al. (2017) Diabetes and sexual dysfunction: results from the Olmsted County study of urinary symptoms and health status among men. J Urol 177: 1438-1442. Link: https://bit.ly/3eiEHr0
- Bak E, Marcisz C, Krzeminska S, Dobrzyn-Matusiak D, Foltyn A (2018) Does Type 1 Diabetes Modify Sexuality and Mood of Women and Men? Int J Environ Res Public Health 15. Link: https://bit.ly/2QR1bGw
- Angulo J, González-Corrochano R, Cuevas P, Fernández A, La Fuente JM, et al. (2010) Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med 7: 758-768. Link: https://bit.ly/3nKxFy2
- Saenz de Tejada I, Angulo J, Cellek S, González-Cadavid N (2005) Pathophysiology of erectile dysfunction. J Sex Med 2: 26-39. Link: https://bit.ly/3aZ3qyv
- Tarhan F, Demirel GY, Kuyumcuoğlu U, Faydaci G, Eryildirim B (2009) Apoptosis of corpus cavernosum in patients with organic erectile dysfunction. World J Urol 27: 235-240. Link: https://bit.ly/3eV4pka
- Tousoulis D, Tsarpalis K, Cokkinos D, Stefanadis C (2008) Effects of insulin resistance on endothelial function: possible mechanisms and clinical implications. Diabetes Obes Metab 10: 834-842. Link: https://bit.ly/2SgsXwm
- Jansson PA (2007) Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med 262: 173-183. Link: https://bit.ly/3xJQg1Q
- Wu Z, Wang H, Ni F, Jiang X, Xu Z, et al. (2018) Islet transplantation improved penile tissue fibrosis in a rat model of type 1 diabetes. BMC Endocr Disord 18: 49. Link: https://bit.ly/2QQHYEV
- Gur S, Kadowitz PJ, Hellstrom WJ (2009) A critical appraisal of erectile function in animal models of diabetes mellitus. Int J Androl 32: 93-114. Link: https://bit.ly/3tfLkhX
- Boyer F, Vidot JB, Dubourg AG, Rondeau P, Essop MF, et al. (2015) Oxidative stress and adipocyte biology: focus on the role of AGEs. Oxid Med Cell Longev 534873. Link: https://bit.ly/2QT7GbJ
- Corona G, Mannucci E, Petrone L, Ricca V, Balercia G, et al. (2006) Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res 18: 190-197. Link: https://bit.ly/3xKBckQ
- Algeffari M, Jayasena CN, MacKeith P, Thapar A, Dhillo WS, et al. (2018) Testosterone therapy for sexual dysfunction in men with Type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med 35: 195-202.Link: https://bit.ly/2Rhz6bf
- Li M, Li H, Ruan Y, Wang T, Liu J (2016) Stem Cell Therapy for Diabetic Erectile Dysfunction in Rats: A Meta-Analysis. PLoS One 11: e0154341. Link: https://bit.ly/3nLvri6
- Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, et al. (2007) Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol 292: H1278-H1290. Link: https://bit.ly/3ee60lZ
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, et al. (2010) Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 7: 3331-3340. Link: https://bit.ly/2QQIIdb
- Gokce A, Abd Elmageed ZY, Lasker GF, Bouljihad M, Braun SE, et al. (2015) Intratunical Injection of Genetically Modified Adipose Tissue-Derived Stem Cells with Human Interferon alpha-2b for Treatment of Erectile Dysfunction in a Rat Model of Tunica Albugineal Fibrosis. J Sex Med 12: 1533-1544. Link: https://bit.ly/3b2SLTs
- Wu JH, Xia SJ (2011) Stem cell-based therapy for erectile dysfunction. Chin Med J (Engl) 124: 3810-3815. Link: https://bit.ly/3b0C9eW
- Qiu X, Sun C, Yu W, Lin H, Sun Z, et al. (2012) Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl 33: 37-44. Link: https://bit.ly/3xJl0QK
- Garcia MM, Fandel TM, Lin G, Shindel AW , Banie L, et al. (2010) Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med 7: 89-98. Link: https://bit.ly/3umHx3G
- Musicki B, Burnett AL (2007) Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res 19: 129-138. Link: https://bit.ly/3nMrwl9
- Wu H, Mahato RI (2014) Mesenchymal stem cell-based therapy for type 1 diabetes. Discov Med 17: 139-143. Link: https://bit.ly/3nPjHen
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, et al. (2008) Recent advances in andrology-related stem cell research. Asian J Androl 10: 171-175. Link: https://bit.ly/33eXYmA
- Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, et al. (2012) Endothelial differentiation of mesenchymal stromal cells. PLoS One 7: e46842. Link: https://bit.ly/2QJwRhb
- Lee RH, Oh JY, Choi H, Bazhanov N (2011)Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem 112: 3073-3078. Link: https://bit.ly/3h0evD2
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, et al. (2012) Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 7: e47559. Link: https://bit.ly/2RrG1Pm
- Long X, Tharp DL, Georger MA, Slivano OJ , Lee MY, et al. (2009) The smooth muscle cell-restricted KCNMB1 ion channel subunit is a direct transcriptional target of serum response factor and myocardin. J Biol Chem 284: 33671-33682. Link: https://bit.ly/339Rndc
- He Y, He W, Qin G, Luo J, Xiao M (2014) Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia 46: 479-486. Link: https://bit.ly/3b2fH55
- Salkoff L, Butler A, Ferreira G , Santi C , Wei A, et al. (2006) High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921-931. Link: https://bit.ly/3ui8oxS
- Bahk JY, Jung JH, Han H, Min SK, Lee YS (2010) Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant 8: 150-160. Link: https://bit.ly/3tlGy2d
- Lei H, Liu J, Li H, Wang L, Xu Y, et al. (2013) Low-intensity shock wave therapy and its application to erectile dysfunction. World J Mens Health 31: 208-214. Link: https://bit.ly/3nJhhy1
- Shan HT, Zhang HB, Chen WT, Chen FZ, Wang T, et al. (2017) Combination of low-energy shock-wave therapy and bone marrow mesenchymal stem cell transplantation to improve the erectile function of diabetic rats. Asian J Androl 19: 26-33. Link: https://bit.ly/3ujsFD5
- Zhu GQ, Jeon SH, Bae WJ, Choi SW, Jeong HC, et al. (2018) Efficient Promotion of Autophagy and Angiogenesis Using Mesenchymal Stem Cell Therapy Enhanced by the Low-Energy Shock Waves in the Treatment of Erectile Dysfunction. Stem Cells Int 2018: 1302672. Link: https://bit.ly/3vCkH8o
- Qiu X, Lin H, Wang Y, Yu W, Chen Y, et al. (2011) Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med 8: 427-436. Link: https://bit.ly/3vHI7cK
- Sun C, Lin H, Yu W, Li X, Chen Y, et al. (2012) Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int J Androl 35: 601-607. Link: https://bit.ly/3tf90mv
- Li Y, Pan E, Wang Y, Zhu X, Wei A, et al. (2015) Flk-1(+)Sca-1(-) mesenchymal stem cells: functional characteristics in vitro and regenerative capacity in vivo. Int J Clin Exp Pathol 8: 9875-9888. Link: https://bit.ly/3h12PzR
- Ryu JK, Kim DH, Song KM, Ryu DS, Kim SN, et al. (2016) Intracavernous delivery of clonal mesenchymal stem cells rescues erectile function in the streptozotocin-induced diabetic mouse. Andrology 4: 172-184. Link: https://bit.ly/3b0FzOO
- Chen F, Zhang H, Wang Z, Ding W, Zeng Q, et al. (2017) Adipose-Derived Stem Cell-Derived Exosomes Ameliorate Erectile Dysfunction in a Rat Model of Type 2 Diabetes. J Sex Med 14: 1084-1094. Link: https://bit.ly/3nNkE7e
- Sun X, Luo LH, Feng L, Li DS (2018) Down-regulation of lncRNA MEG3 promotes endothelial differentiation of bone marrow derived mesenchymal stem cells in repairing erectile dysfunction. Life Sci 208: 246-252. Link: https://bit.ly/3xNX0vF
- Qiu XF, Li XX, Chen Y, Lin HC, Yu W, et al. (2012) Mobilisation of endothelial progenitor cells: one of the possible mechanisms involved in the chronic administration of melatonin preventing erectile dysfunction in diabetic rats. Asian J Androl 14: 481-486. Link: https://bit.ly/3ujo8AK
- Gou X, He YW, Xiao MZ, Qiu MZ, Wang M, et al. (2011) Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl 13: 332-338. Link: https://bit.ly/3tgSVg4
- Sun K, Zhou Z, Ju X, Zhou Y, Lan J, et al. (2016) Combined transplantation of mesenchymal stem cells and endothelial progenitor cells for tissue engineering: a systematic review and meta-analysis. Stem Cell Res Ther 7: 151. Link: https://bit.ly/3ui3zV3
- Salem ESB, Fan GC (2017) Pathological Effects of Exosomes in Mediating Diabetic Cardiomyopathy. Adv Exp Med Biol 998: 113-138. Link: https://bit.ly/3eXYnQ6
- Hassanpour M, Cheraghi O, Brazvan B, Hiradfar A, Aghamohammadzadeh N, et al. (2018) Chronic Exposure of Human Endothelial Progenitor Cells to Diabetic Condition Abolished the Regulated Kinetics Activity of Exosomes. Iran J Pharm Res 17: 1068-1080. Link: https://bit.ly/3vI7QBQ
- Kim SM, Kim HS (2017) Engineering of extracellular vesicles as drug delivery vehicles. Stem Cell Investig 4: 74. Link: https://bit.ly/2PLMZOr
- Conigliaro A, Fontana S, Raimondo S, Alessandro R (2017) Exosomes: Nanocarriers of Biological Messages. Adv Exp Med Biol 998: 23-43. Link: https://bit.ly/3edHDVM
- Altanerova U, Jakubechova J, Repiska V, Altaner C (2017) Exosomes of human mesenchymal stem/stromal/medicinal signaling cells. Neoplasma 64: 809-815. Link: https://bit.ly/3eVxjRp
- Wang X, Liu C, Li S, Xu Y, Chen P, et al. (2015) Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One 10: e0118951. Link: https://bit.ly/2PQYbJQ
- Gharaee H, Kargozar A, Daneshvar-Kakhki R, Sharepour M, Hassanzadeh S (2011) Correlation between Corneal Endothelial Cell Loss and Location of Phacoemulsification Incision. J Ophthalmic Vis Res 6: 13-17. Link: https://bit.ly/2RoRGy4
- Liang L, Li Z, Ma T, Han Z, Du W, et al. (2017) Transplantation of Human Placenta-Derived Mesenchymal Stem Cells Alleviates Critical Limb Ischemia in Diabetic Nude Rats. Cell Transplant 26: 45-61. Link: https://bit.ly/2RnWXpI
- Wassef MAE, Tork OM, Rashed LA, Ibrahim W, Morsi H, et al. (2018) Mitochondrial Dysfunction in Diabetic Cardiomyopathy: Effect of Mesenchymal Stem Cell with PPAR-gamma Agonist or Exendin-4. Exp Clin Endocrinol Diabetes 126: 27-38. Link: https://bit.ly/3uimdwe
- Jeon SH, Zhu GQ, Bae WJ, Choi SW, Jeong HC, et al. (2018) Engineered Mesenchymal Stem Cells Expressing Stromal Cell-derived Factor-1 Improve Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats. Int J Mol Sci 19: 3730. Link: https://bit.ly/2QSBwNF
- Al Demour S, Jafar H, Adwan S, AlSharif A, Alhawari H, et al. (2018) Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase I Clinical Trial. Urol Int 101: 358-365. Link: https://bit.ly/3vCKrBy
- Ren ML, Peng W, Yang ZL, Sun XJ, Zhang SC, et al. (2012) Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant 21: 2711-2721. Link: https://bit.ly/3tnR2ys
- Chang SH, Park CG (2019) Allogeneic ADSCs induce CD8 T cell-mediated cytotoxicity and faster cell death after exposure to xenogeneic serum or proinflammatory cytokines. Exp Mol Med 51: 1-10. Link: https://go.nature.com/3eRpxbc
- Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL, et al. (2007) Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res 22: 1943-1956. https://bit.ly/33aBALn
- Qiu X, Fandel TM, Ferretti L Albersen M, Zhang H, et al. (2012) Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol 62: 720-727. Link: https://bit.ly/33fw9uo
- Liu G, Sun X, Bian J, Wu R, Guan X, et al. (2013) Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One 8: e72790. Link: https://bit.ly/3nKPD3y
- Paek HJ, Kim C, Williams SK (2014) Adipose stem cell-based regenerative medicine for reversal of diabetic hyperglycemia. World J Diabetes 5: 235-243. Link: https://bit.ly/3uizrZX
- Das ND, Song KM, Yin GN, Batbold D, Kwon MH, et al. (2014) Xenogenic transplantation of human breast adipose-derived stromal vascular fraction enhances recovery of erectile function in diabetic mice. Biol Reprod 90: 66. Link: https://bit.ly/3nIqbvG
- Skakun NP, Shman'ko VV (1986) Antioxidant effectiveness in isoniazid-induced lesions of the liver. Farmakol Toksikol 49: 86-89. Link: https://bit.ly/2PL5IcW
- Nishimatsu H, Suzuki E, Kumano S, Nomiya A, Liu M, et al. (2012) Adrenomedullin mediates adipose tissue-derived stem cell-induced restoration of erectile function in diabetic rats. J Sex Med 9: 482-493. Link: https://bit.ly/3egHe51
- Shen YH, Jiang HX, Qin SY, Wei LP, Meng YC, et al. (2014) Activation of hepatocyte growth factor promotes apoptosis of hepatic stellate cells via the Rho pathway. Zhonghua Gan Zang Bing Za Zhi 22: 136-141. Link: https://bit.ly/3eR3wJv
- Yi SL, Liu JX, Zhong ZQ, Zhang Y (2014) Role of caveolin-1 in atrial fibrillation as an anti-fibrotic signaling molecule in human atrial fibroblasts. PLoS One 9: e85144. Link: https://bit.ly/3vGCYkZ
- Liu T, Peng Y, Jia C, Fang X, Li J, et al. (2015) Hepatocyte growth factor-modified adipose tissue-derived stem cells improve erectile function in streptozotocin-induced diabetic rats. Growth Factors 33: 282-289. Link: https://bit.ly/33dGerU
- Lu J, Xin Z, Zhang Q, Cui D, Xiao Y, et al. (2016) Beneficial effect of PEDF-transfected ADSCs on erectile dysfunction in a streptozotocin-diabetic rat model. Cell Tissue Res 366: 623-637. Link: https://bit.ly/3xO14Mr
- Kovanecz I, Vernet D, Masouminia M, Gelfand R, Loni L, et al. (2016) Implanted Muscle-Derived Stem Cells Ameliorate Erectile Dysfunction in a Rat Model of Type 2 Diabetes, but Their Repair Capacity Is Impaired by Their Prior Exposure to the Diabetic Milieu. J Sex Med 13: 786-797. Link: https://bit.ly/3eQD9n8
- Zhu LL, Zhang Z, Jiang HS, Chen H, Chen Y, et al. (2017) Superparamagnetic iron oxide nanoparticle targeting of adipose tissue-derived stem cells in diabetes-associated erectile dysfunction. Asian J Androl 19: 425-432. Link: https://bit.ly/3thKZLE
- Seftel AD (2017) Re: Combination of Mesenchymal Stem Cell Injection with Icariin for the Treatment of Diabetes-Associated Erectile Dysfunction. J Urol 198: 238. Link: https://bit.ly/2RlYTzd
- Zhu LL, Huang X, Yu W, Chen H, Chen Y, et al. (2018) Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50. Link: https://bit.ly/3vCNKbW
- Zhou F, Hui Y, Xin H, Xu YD, Lei HE, et al. (2017) Therapeutic effects of adipose-derived stem cells-based microtissues on erectile dysfunction in streptozotocin-induced diabetic rats. Asian J Androl 19: 91-97. Link: https://bit.ly/2QK1SBD
- Castiglione F, Hedlund P, Weyne E, Hakim L, Montorsi F, et al. (2019) Intratunical Injection of Human Adipose Tissue-Derived Stem Cells Restores Collagen III/IRatio in a Rat Model of Chronic Peyronie's Disease. Sex Med 7: 94-103. Link: https://bit.ly/3b0QTKI
- Chen F, Zhang H, Wang Z, Ding W, Zeng Q, et al. (2017) Adipose-Derived Stem Cell-Derived Exosomes Ameliorate Erectile Dysfunction in a Rat Model of Type 2 Diabetes. J Sex Med 14: 1084-1094. Link: https://bit.ly/3nNkE7e
- Litwinoff E, Hurtado Del Pozo C, Ramasamy R, Schmidt AM (2015) Emerging Targets for Therapeutic Development in Diabetes and Its Complications: The RAGE Signaling Pathway. Clin Pharmacol Ther 98: 135-144. Link: https://bit.ly/2RnuW1y
- Ramasamy R, Shekhtman A, Schmidt AM (2016) The multiple faces of RAGE--opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Ther Targets 20: 431-446. Link: https://bit.ly/3uxrGj3 .
- Howard AC, McNeil AK, Xiong F, Xiong WC, McNeil PL (2011) A novel cellular defect in diabetes: membrane repair failure. Diabetes 60: 3034-3043. Link: https://bit.ly/3eS8ndn .
- Liu S, Chen F, Wang L, Sun W, Liu Q, et al. (2016) 2,5-hexanedione induced apoptosis of rat bone marrow mesenchymal stem cells by reactive oxygen species. J Occup Health 58: 170-178. Link: https://bit.ly/3uxdVk0
- Lu YQ, Lu Y, Li HJ, Cheng XB (2012) Effect of advanced glycosylation end products (AGEs) on proliferation of human bone marrow mesenchymal stem cells (MSCs) in vitro. In Vitro Cell Dev Biol Anim 48: 599-602. Link: https://bit.ly/3vHYohH
- Wespes E, Amar E, Hatzichristou D, Montorsi F, Pryor J, et al. (2002) Guidelines on erectile dysfunction. Eur Urol 41: 1-5. Link: https://bit.ly/3aZ9Vkz
- Stoltz JF, Bensoussan D, De Isla N, Zhang L, Han Z, et al. (2016) Stem cells and vascular regenerative medicine: A mini review. Clin Hemorheol Microcirc 64: 613-633. Link: https://bit.ly/3b20dhz
- Liu S, Zhang H, Zhang X, Lu W, Huang X, et al. (2011) Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair. Tissue Eng Part A 17: 725-739. https://bit.ly/3h1RX55
- Tobita M, Tajima S, Mizuno H (2015) Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness. Stem Cell Res Ther 6: 215. Link: https://bit.ly/3nZo9aN
- Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, et al. (2007) Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev 16: 91-104. Link: https://bit.ly/3efUc2O
- Rombouts WJ, Ploemacher RE (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17: 160-170. Link: https://bit.ly/3xM4C1H
- Baer PC (2014) Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells 6: 256-265. Link: https://bit.ly/3ukGcKx
- Cramer C, Freisinger E, Jones RK, Slakey DP, et al. (2010) Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 19: 1875-1884. Link: https://bit.ly/3nP1FsX
- El Osta R, Decot V, Bensoussan D, Stoltz JF, Eschwege P, et al. (2018) Treatment by stem cell therapy of erectile dysfunction of diabetic origin: State of the art. Prog Urol 28: 74-84. Link: https://bit.ly/3nPVRPW
- El Osta R, Decot V, Bensoussan D, Stoltz JF, Eschwege P, et al. (2018) Treatment by stem cell therapy of erectile dysfunction of diabetic origin: State of the art. Prog Urol 28: 74-84. Link: https://bit.ly/3nPVRPW
- Zhang J, Du C, Guo W, Li P , Liu S, et al. (2017) Adipose Tissue-Derived Pericytes for Cartilage Tissue Engineering. Curr Stem Cell Res Ther 12: 513-521. Link: https://bit.ly/2POp9Sb
- Sa da Bandeira D, Casamitjana J, Crisan M (2017) Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther 171: 104-113. Link: https://bit.ly/3umtDOW
- Celebi-Saltik B (2018) Pericytes in Tissue Engineering. Adv Exp Med Biol 1109: 125-137. Link: https://bit.ly/3upNQ6A
- Ahmed TA, El-Badri N (1079) Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv Exp Med Biol 1079: 69-86. Link: https://bit.ly/33dqzsr
- Chung E (2019) Stem cell therapy in diabetic men with erectile dysfunction: a step closer to safe and effective regenerative technology. Annals of Translational Medicine 7. Link: https://bit.ly/3um14RU
- Chung E (2019) Stem cell therapy in diabetic men with erectile dysfunction: a step closer to safe and effective regenerative technology. Annals of Translational Medicine 7. Link: https://bit.ly/3um14RU
- Chung E (2015) Stem-cell-based therapy in the field of urology: a review of stem cell basic science, clinical applications and future directions in the treatment of various sexual and urinary conditions. Expert Opin Biol Ther 15: 1623-1632.
- Mushtaq S, Khan K, Abid S, Umer A, Raza T, et al. (2018) Frequency of Hypogonadism and Erectile Dysfunction in Type-II Diabetic Patients. Cureus 10: e2654. Link: https://bit.ly/3tl6oU4
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
