Studies on Stem Cells Research and Therapy
The potential effects of pomegranate peel extract and bee venom in improving the diabetes induced damaging of spiral artery
El-Sayyad HIH1*, El-Ghawet HA1 and El-Sayed AMA2
2Department of Zoology, Kafr-El-Sheikh University, Egypt
Cite this as
El-Sayyad HIH, El-Ghawet HA, El-Sayed AMA (2019) The potential effects of pomegranate peel extract and bee venom in improving the diabetes induced damaging of spiral artery. Studies on Stem Cells Research and Therapy 5(1): 007-011. DOI: 10.17352/sscrt.000013Back ground: Diabetes Mellitus is a public health problem associated with increased maternal blood sugar level and impair insulin secretion leading to complication of pregnancy and retarded placental development. Maternal diabetes developed premature term and mortalities of their child. The alterations of placental spiral artery during the progress of diabetes paid attention and little of work is concerned with this public health problem. The traditional increase of phytotherapy especially pomegranate peel extract and bee venom therapy encourage author to carry out this study in spiral artery of placenta at 14days of pregnancy in order to clarify the best choice of improvement the histological picture of the spiral artery.
Methods: Thirty six pregnant rats were used in the present study and arranged into 6 groups (n=6); control (saline-treated), pomegranate peel-extract (), bee venom-treatment (i.p. 0.12mg/kg body weight, single dose), diabetes (Single i.p. inection of streptozotocin (60mg/kg in citrate buffer pH 4.5 plus 100mg/kg nicotinamide). diabetic and pomegranate and diabetic and bee venom-treatment. Pregnant were anaesthetized followed by cervical dislocation and dissection at 14days of gestation. Placenta were removed and immediately fixed in 10% phosphate buffered formalin pH 7.4 and processed for histological examination and decidual spiral artery was investigated.. Immunohistochemistry with caspase 3 to observe the degree of cell death.
Results: The present results showed that diabetic placenta of mother rats possessed abnormal shrinked spiral artery with damaged endothelial lining and collagen genesis in the outer covering sheath. There was a detected increase of immunohistochemical reaction of caspase 3. Mother received pomegranate peel extract exhibited a considerable improved with slightly immunohistochemical reaction, compared to slightly altered spiral artery of placenta of diabetic mother received bee venom-treatment. Moderate immunohistochemical reaction of caspase 3 was observed.
Conclusion: The author finally concluded that administration of pomegranate juice as a natural product may give sufficient antioxidant materials needed for improved diabetic complication of the placenta.
At the same time, bee venom therapy with complete supervision of physicians had to be done carefully.
Introduction
Diabetes Mellitus is a public health condition associated with increased blood sugar level and impair insulin secretion affected nearly 184 million peoples with diabetes in 2013, reaching 288 million by 2030 [1]. It is known that early gestation is critical especially during organogenesis due to a higher susceptibility of embryonic tissues to alterations in the intrauterine environment such as diabetes and obesity [2] . The endometrium gives the demand for menstruation, implantation and placentation [3]. Uterine spiral arteries facilitated the nutrient supply for the placenta and fetus. During gestation, they undergoe remodeling directed by maternal natural killer cells and embryo-derived invasive trophoblast lineages [4].During early organogenesis, the embryo depend mainly on maternal nutrition through the trophoblast cells, the elementary components of the placenta, at the maternal-fetal interface. Maternal blood bathes trophoblast directly and induces the reconstruction of the uterine spiral artery that facilitates the flow of nutrients from the distal maternal portion of the uterine spiral artery to the placenta [5]. The distal uterine spiral artery facilitated remodeling trophoblast invasion into the vascular wall and widening of the vascular lumen [6,7]. The vasculature and development of the spiral artery explains the successful fetal growth [8].
Failure differentiation of the spiral arteries is associated with uteroplacental ischemia and development of acute atherosis in the spiral arteries of the decidua parietalis or basalis leading to preeclampsia [9]. Diabetes was found to reduce the volumes of both the intervillous space and the villous membrane specific diffusing capacity [10]. The vascular wall distensibility was increased associated with reduction of the wall stiffness in diabetes [11,12]. Two of the 18 diabetic patients developed intramural fibrosis in the decidual portion of the spiral artery. Hyperglycaemia and abnormal level of C-peptide values in amniotic fluid and cord blood had been influenced in the induction of spiral artery lesions [13]
The Pomegranate fruit’s peel, seeds and juice are rich in antioxidants Pomegranate (Punica granatum L.), Punica granatum Linn. (Family , Punicaceae ), is commonly known as pomegranate. It is the old native tree of the Mediterranean regions especially Egypt [14]. In vivo studies revealed that consumption of pomegranate juice improved the diabetic associated oxidative stress in placenta. Moreover, pomegranate juice reduced in vitro oxidative stress, apoptosis, and global cell death in trophoblast cultures subjected to hypoxic stress [15]. Wild type (WT, C57Bl/6J) and eNOS−/− mice supplemented orally pomegranate juice during utero life reduced relaxation of uterine arteries in response to oxidative stress [16] .
Phenolic compounds of pomegranate, have been found to show anti-oxidant activities and activated the reactivity of isolated blood vessel in rats as well as reduce the systolic blood pressure in both a hypertensive rat model [17].
Bee venom consists of several forms of peptides (mellitine and apamine), enzymes (phospholipase A2, hyaluronidase, acid phosphomonoesterase, lysophospholipase), active amines (histamine, dopamine, norepinephrine, serotonine) and many other substances [18]. In the meantime, mellitin and phospholipase A2 had anti-inflammatory effects [19]. In addition, mellitin had a strong activator for A2 phospholipase [20].
Diabetic rats received interperitoneal injection of 5mg BV/kg every day for four consecutive weeks improved blood glucose and serum triglyceride and total cholesterol level [21].
In addition, BV treatment demonstrated a high therapeutic potential for improved improved wound healing in diabetic mice through increased collagen and BD-2 expression and restored Ang-1 and Nrf2 levels. This was affected by the activities of antioxidant enzymes in the damaged tissue and the elimination of chemokines [22]).
Oxidative stress represents a key pathophysiological elements in vascular diseases influenced in congenital malformations. Little information about the role of diabetes in damaging the spiral artery and consequently the placental complications.
The present study explored the antidiabetic capacity of either bee venom –treatment or pomegranate supplementation on the spiral artery of 14days old placenta of mother rats.
Materials and methods
Pomegranate peel extract and applied dose-treatment
Pomegranate fruits were purchased from the market. Their peel were separated, washed with distilled water, cleaned and dried. A known weight of the pomegranate peel was kept with 100mL methyl alcohol for seven day to complete extraction of polar and non-polar compounds and filtered. The filtrate was allowed to evaporate under vaccum and replaced the residual methyl alcohol was replaced by 70% ethyl alcohol and complete evaporation near almost drying , then mix with 10ml saline solution to obtain dose of 30mg/kg.body weight. Each pregnant rat was oral administered every other day from 6th day of gestation till 14days old 7.
Bee venom-treatment
Bee venom was supplied from Bee Lab, Faculty of Agriculture, Assiut University. A known weight of the bee venom was dissolved in saline solution and interperitoneally administered at single dose of 0.12mg/kg body weight at the 6th day of gestation.
Induction of diabetes
Experimental type 2 diabetes mellitus was induced by a single intraperitoneal injection of streptozotocin (60mg/kg, diluted with buffer, pH 4.5) and 100mg nicotinamide /kg body weight. Control animals were received physiological saline. Hyperglycemia was assessed by ranging the blood sugar level within the limits of 240-280mg/dL by glucometer.
Experimental work
Forty-eight fertile male and virgin female mice weighing approximately 125-150g (1male: 3 female) body weight obtained from Hellwan Breeding Farm, Ministry of Health, Egypt and used for experimentation. Free access of standard diet and water were allowed ad-libitum. They were kept with 12hours light and dark cycle in good aerated room. Pregnancy occurred by keeping virgin females with fertile male for overnight and investigating in the next morning for observing sperm in the vaginal smears and recording the onset of gestation. The pregnant rats were arranged into 6 groups (n=6) as follows: control (saline-treated), pomegranate peel-extract, bee venom-treatment, diabetic alone or with either pomegranate or bee venom-treatment. Pregnant were anaesthetized by combined subcutaneous injection with medetomidine (0.5mg/kg) and ketamine (75mg/kg), followed by cervical dislocation and dissection. Placenta were removed and immediately fixed in 10% phosphate buffered formalin pH 7.4. The specimens were dehydrated in ascending grades of ethyl alcohol, cleared in xylol and mounted in molten paraplast 58-62ºC. Five µm histological sections were cut and stained with Harris hematoxylin & eosin. For immunohistochemically, the formalin fixed tissue sections were dewaxed, hydrated and incubated with antibodies against caspase 3 (Thermo Fisher Scientific, Fremont, CA, USA; Cat. No. A1-70007) and counterstained with hematoxylin for background of examined tissues. The specimens were investigated with a light microscope and photographed.
Results
Histopathological observations
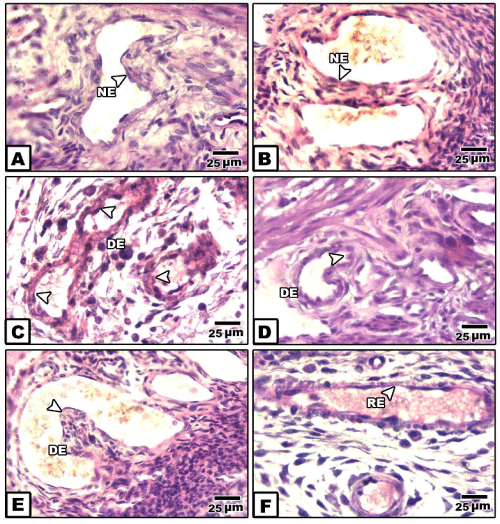
In placenta of control and pomegranate extract treatment, the spiral artery is characterized by dilated lumen. The wall is composed of tunica intima line with endothelial cells and thin layer of elastic fiber.
It is surrounded by intima tunica formed by a thin layer of smooth muscle fibers and accompanied by a thin adventitia tunica (Figure 1A).
In bee venom-treated mother’s placenta, the spiral artery’s tunica media and adventitia showed dense cellularity. The endothelium remain intact (Figure 1B).
In placenta of diabetic mother, the spiral artery attained a considerable congestion, associated with degeneration of the endothelium lining layer. The outer surrounding covering sheath possessed dense collection of macrophage cells and thin collagenous material. Red blood cells appeared grouping within their lumina of the blood vessel (Figure 1C,D).
In placenta of diabetic mother and received bee venom-treatment, there was a dense collection of inflammatory cells in both the tunica media and adventitia. The lumen of the spiral artery showed congestion associated with protrusion of the tunica media inside the luminal cavity (Figure 1E ).
However, there was apparent improvement of the spiral artery of diabetic mother received pomegranate-extract treatment. The blood vessel dilated and the endothelium become regenerated (Figure 1F ).
Immunohistochemistry of caspase 3
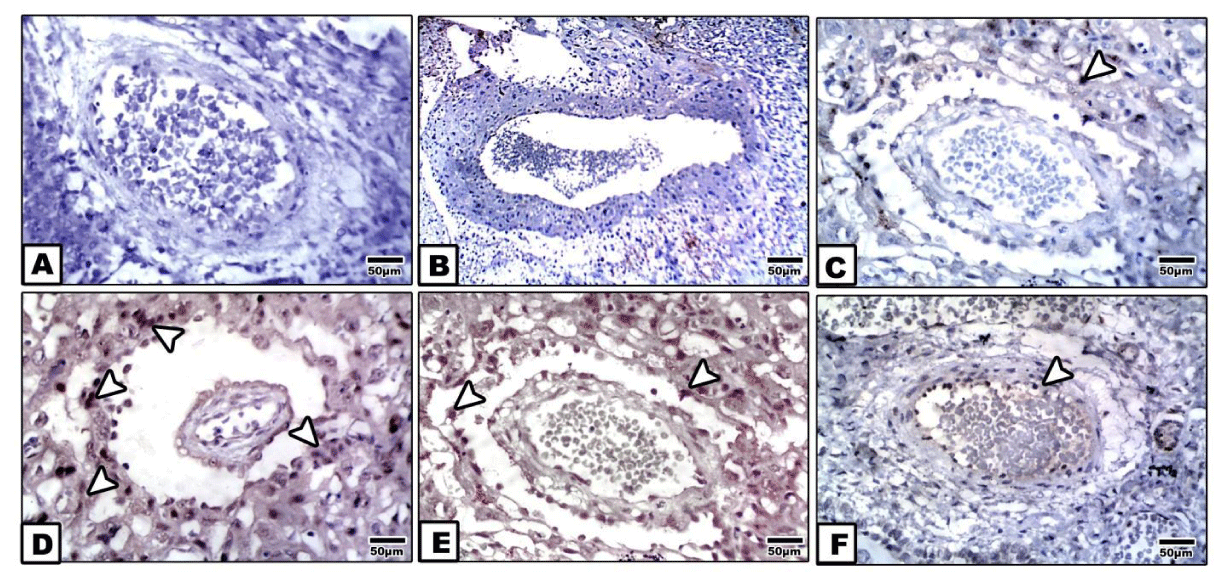
Following examining placenta of control, pomegranate-extract treatment and bee venom-treatment mother, the spiral arteries showed missing immunohistochemical reaction (Figure 2A,B). However, bee venom-treated placenta possessed mild dark-brown immunohistochemical reaction in the tunica media and adventitia (Figure 2C).
In diabetic placenta, there was a detected dense dark-brown immunohistochemical reaction in the tunica intema and endothelial layer (Figure 2D).
Bee venom-treatment plus diabetes showed a moderate immunohistochemical reaction in both the tunica intema and endothelium compared with marked improvement and almost missing of the caspase 3 immunohistochemical reaction in spiral artery of diabetic mother supplemented pomegranate (Figures 2E,F ).
Image analysis showed marked increased intensity of caspase 3 in spiral artery of diabetic mother compared to less degree in those of diabetic mother and bee venom-treatment. However, there was a detected negative immunostaining activity in the other studied groups (Figure 3 ).
Discussion
The placenta is a main structural organ involved for fetal growth. It supplied the nutrients, oxygen and steroids required for differention of fetal organs [23]. Uterine spiral arteries allowed placenta and fetus nutrient supply.
They undergo remodeling during gestation, directed by natural maternal killer cells and invasive trophoblast lineages derived from embryos [14].
From the present work, there was a detected congestion of the spiral artery associated with damaging of the inner lining endothelial cells. The congested blood vessel become surrounded with collagenous materials.
The present findings were consistent with Bjork, et al., [13], whom reported two of the 18 diabetic patients showed intramural fibrosis in the decidual portion of the spiral artery associated with diabetic angiopathy (White’s class D and F).
Abnormal spiral artery was also detected in 69/203 of obese cases (33.9%) [24].
High fat-fed dams showed delayed invasion of trophoblast due to over expression of smooth actin around the placental spiral arteries. This led to impairment of remodeling of the spiral artery and consequently deformed the placent and increased fetal mortality [25] .
It is known that diabetes was associated with altering maternal environment and increased oxidative stress [26], resulting in retarding their remodeling and impaired uteroplacental function leading to preeclampsia [21] .
The diabetic associated deformation of the spiral artery associated damage of endothelium and vascular dysfunction predicted the early sign of preeclampsia . These may result from reduction of nutrients like calcium, protein, vitamins and essential fatty acids and consequently impair the trophoblast invasion [27].
On the other hand, two trials of treatments were carried out by either oral administration of pomegranate peel extract and or intraperitoneal injection of therapeutic dose of bee venom. The observed improvement of pomegranate peel extract may result from pomegranate ellagic polyphenols which manage the blood glucose within almost the normal level. Also, they impair the diabetes associated inflammation through decrease of tumor necrosis factor-alpha , interleukin-6 and C-reactive protein [28]. Diabetic patients received daily administration of three capsules containing 1g pomegranate seed oil showed improved fasting blood sugar level, Insulin concentration, HbA1C, and increased the GLUT-4 gene expression [29]. In vivo studies revealed that consumption of pomegranate juice improved the diabetic associated oxidative stress in placenta. Moreover, pomegranate juice reduced in vitro oxidative stress, apoptosis, and global cell death in trophoblast cultures subjected to hypoxic stress [15]. Wild type (WT, C57Bl/6J) and eNOS−/− mice supplemented orally pomegranate juice during utero life reduced relaxation of uterine arteries in response to oxidative stress [16].
The capacity of improvement of pomegranate extract exceed bee venom treatment. The tunica media and adventitia of the spiral artery of bee venom-treated placenta possessed densely grouping inflammatory cells. Although bee venom-treatment was found to improve diabetes via managing blood sugar, triglyceride and total cholesterol level [30], it possessed sign of cytotoxicity manifested by a detected sign of inflammation.
The author finally concluded that administration of pomegranate juice as a natural product may give sufficient antioxidant materials needed for improved diabetic complication of the placenta.
At the same time, bee venom therapy with complete supervision of physicians had to be done carefully.
- International Diabetes Federation (IDF) (2015) Diabetes Atlas. IDF: Brussels, Belgium, 7th ed. 1-144. Link: http://bit.ly/2RU2lxi
- Metzger BE (1991) Biphasic effects of maternal metabolism on fetal growth; quintessential expression of fuel-mediated teratogenesis. Diabetes 40: 99-105. Link: http://bit.ly/2uKI31i
- Nagy S, Bush M, Stone J, Lapinski RH, Gardo S (2003) Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol 102: 94-100. Link: http://bit.ly/2vwWZ3J
- Soares MJ, Chakraborty D, Kubota K, Renaud SJ, Rumi MA (2014) Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int J Dev Biol 58: 247-259. Link: http://bit.ly/36ycnth
- Osol G, Moore LG (2014) Maternal uterine vascular remodeling during pregnancy. Microcirculation 21: 38-47. Link: http://bit.ly/37EbUar
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473-482. Link: http://bit.ly/2REqF7L
- Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R (2005) Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta 26: 574–584. Link: http://bit.ly/2vw498q
- Osol G, Mandala M (2009) Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58-71. Link: http://bit.ly/38MSrED
- Kim JY, Kim YM (2015) Acute Atherosis of the uterine spiral arteries: Clinicopathologic Implications. J Pathol Transl Med 49: 462-471. Link: http://bit.ly/2RyFybA
- Jauniaux E, Burton GJ (2006) Villous histomorphometry and placental bed biopsy investigation in Type I diabetic pregnancies. Placenta 27: 468-474. Link: http://bit.ly/2S1OR2V
- Crijns FR, Wolffenbuttel BH, De Mey JG, Struijker Boudier HA (1999) Mechanical properties of mesenteric arteries in diabetic rats: consequences of outward remodeling. Am J Physiol 276: H1672–H1677. Link: http://bit.ly/2GypPCY
- Phillips JK, Vance AM, Raj RS, Mandalà M, Linder EA, et al. (2012) Impact of experimental diabetes on the maternal uterine vascular remodeling during rat pregnancy. Reprod Sci 19: 322-331. Link: http://bit.ly/36E0C4C
- Björk O, Persson B, Stangenberg M, Václavínková V (1984) Spiral artery lesions in relation to metabolic control in diabetes mellitus. Acta Obstet Gynecol Scand 63: 123-127. Link: http://bit.ly/36xxYSJ
- Johanningsmeier SD, Harris GK (2011) Pomegranate as a functional food and nutraceutical source. Annu Rev Food Sci Technol 2: 181–201. Link: http://bit.ly/2RVKF4w
- Chen B, Tuuli MG, Longtine MS, Shin JS, Lawrence R, et al. (2012) Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab 302: E1142-E1152. Link: http://bit.ly/2O8zzs1
- Finn-Sell SL, Cottrell EC, Greenwood SL, Dilworth MR, Cowley EJ, et al. (2018) Pomegranate Juice Supplementation Alters Utero-Placental Vascular Function and Fetal Growth in the eNOS-/- Mouse Model of Fetal Growth Restriction. Front Physiol 9: 1145. Link: http://bit.ly/2O7Tp6p
- Delgado NTB, Rouver WDN, Freitas-Lima LC, de Paula TDC, Duarte A, et al. (2017). Pomegranate extract enhances endothelium-dependent coronary relaxation in isolated perfused hearts from spontaneously hypertensive ovariectomized rats. Front. Pharmacol 7: 522. Link: http://bit.ly/2O943tH
- Son DJ, Lee JW, Lee YH, Song HS, Lee CK, et al. (2007) Therapeutic application of anti-arthrities, pain-releasing, and anti-cancer effect of Bee Venom and its compounds. Pharmacol Ther 115: 246–270. Link: http://bit.ly/2RD48YR
- Mazzanti C, Spanevello R, Ahmed M, Schmatz R, Mazzanti A, et al. (2007) Cyclosporine inhibits acetyl cholinesterase activity in rats experimentally demyelinated with ethidium bromide. Int J Devl Neuroscience 25: 259-264. Link: http://bit.ly/319gJ9h
- Zalat S, Nabil Z, Hussein A, Rakha M (1999) Biochemical and haematological studies of some solitary and social bee venoms. Egypt J Biology 1: 57-71. Link: http://bit.ly/36JJoTK
- Mousavi SM, Imani S, Haghighi S, Mousavi SE, Karimi A (2012) Effect of Iranian Honey bee (Apis mellifera) Venom on Blood Glucose and Insulin in Diabetic Rats. J Arthropod Borne Dis 6: 136-143. Link: http://bit.ly/2U4yQMb
- Hozzein WN, Badr G, Badr BM, Allam A, Ghamdi AA, et al. (2018) Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol Immunol103: 322-335. Link: http://bit.ly/37EeTQb
- Honda M, Toyoda C, Nakabayashi M, Omori Y (1992) Quantitative investigations of placental terminal villi in maternal diabetes mellitus by scanning and transmission electron microscopy. Tohoku J Exp Med 167: 247-257. Link: http://bit.ly/38P11mw
- Avagliano L, Marconi AM, Romagnoli S, Bulfamante GP (2012) Abnormal spiral arteries modification in stillbirths: the role of maternal prepregnancy body mass index. J Matern Fetal Neonatal Med 25: 2789-2792. Link: http://bit.ly/2RAPVLZ
- Hayes EK, Tessier DR, Percival ME, Holloway AC, Petrik JJ, et al. (2014) Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod Sci 21: 648-657. Link: http://bit.ly/2uE7otY
- Hoch D, Gauster M, Hauguel-de Mouzon S, Desoye G (2019) Diabesity-associated oxidative and inflammatory stress signalling in the early human placenta. Mol Aspects Med 66: 21-30. Link: http://bit.ly/36AyBef
- Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, et al. (2014) Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med 211: 165-179. Link: http://bit.ly/38OUlEW
- Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C (2018) Obesity and Preeclampsia: Common Pathophysiological Mechanisms. Front Physiol 9: 1838. Link: http://bit.ly/38Lf78j
- Sun YL, Zhou FM, Wang HR (2019) Mechanism of pomegranate ellagic polyphenols reducing insulin resistance on gestational diabetes mellitus rats. Am J Transl Res 11: 5487-5500. Link: http://bit.ly/2GvKXts
- Khajebishak Y, Payahoo L, Alivand M, Hamishehkar H, Mobasseri M, et al. (2019) Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J Cell Physiol 234: 19s621-19628. Link: http://bit.ly/38S7ywJ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
