Scientific J Genet Gene Ther
Lenalidomide as potential treatment in small cell neuroendocrine lung cancer with del 5q
Paul Hartel*
Cite this as
Hartel P (2018) Lenalidomide as potential treatment in small cell neuroendocrine lung cancer with del 5q. Scientific J Genet Gene Ther 4(1): 002-003. DOI: 10.17352/sjggt.000014Introduction
Lung cancer is a leading cause of cancer deaths world-wide, with the carcinogens in tobacco smoke playing a major etiologic role. Genetic changes responsible for carcinogenesis include activation of proto-oncogenes and inactivation of tumor suppressor genes. Tumor suppressor gene inactivation is contributed to, in part, by loss of chromosomal DNA. While cytogenetic findings in small cell neuroendocrine lung carcinoma are complex, 5q deletion is among the most frequently identified [1,2].
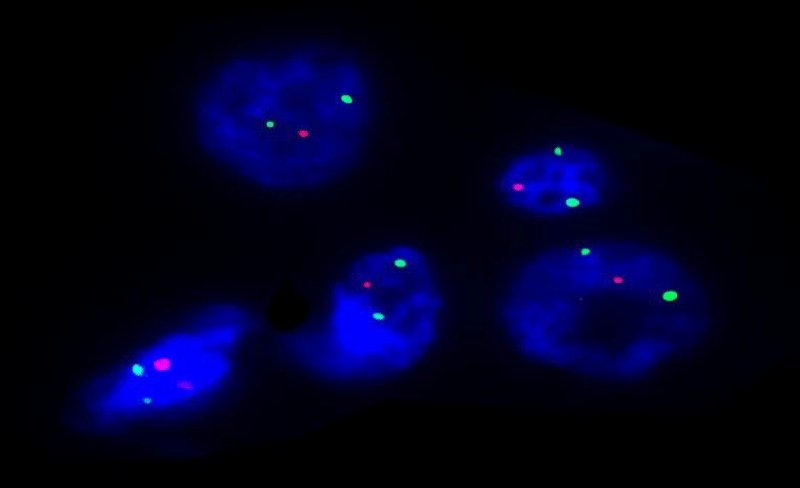
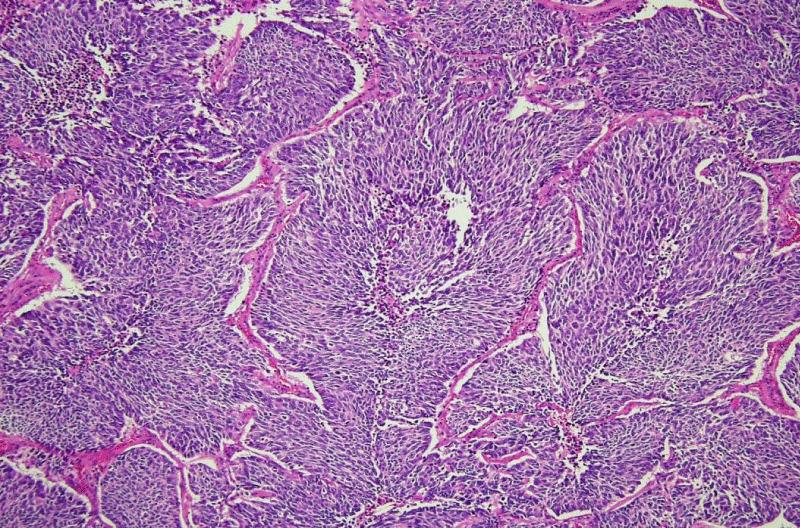
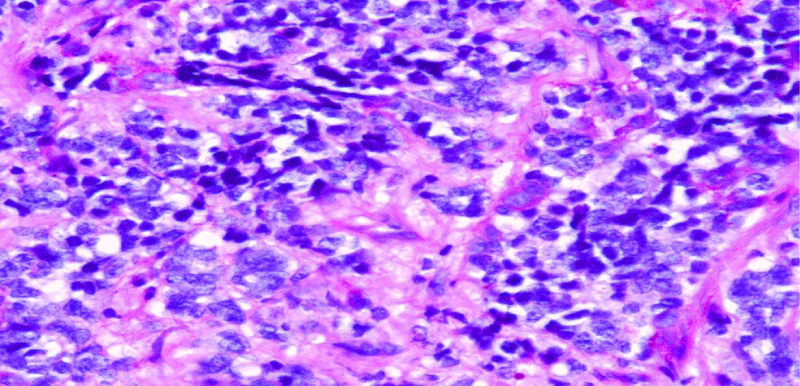
Very few studies have investigated the relationship between such cytogenetic alterations and clinical or pathologic factors in small cell neuroendocrine lung carcinoma [2-4]. Our recent investigation found that patients with small cell neuroendocrine carcinoma with del (5q) (Figure 1), had a greater quantity of spindle cells (Figure 2), and more tumour-associated mucin [5] (Figure 3), compared to patients with tumours without del (5q). Interestingly, when symptomatic, patients in this group more often reported cough as the most common presenting symptom. Patients with tumors having del (5q) were also older than patients without del (5q), and had greater pack-year smoking histories. While this may be explained by their older age, it potentially is the result of longer exposure to mutagenic influences, i.e. smoking, and that del (5q) in the genetic evolution of lung carcinogenesis occurs as a later “hit” in the tumorigenic pathway of small cell neuroendocrine lung carcinoma. Chromosomal losses have been shown to have meaningful clinical correlates, whether prognostically favorable or unfavorable, in oligodendroglioma (loss of 1p and 19q) [6], myelodysplastic syndromes (loss of 5q, 7q, and 20q) [7], acute myeloid leukemia (loss of 5q and 9q) [8], and chronic lymphocytic leukemia (loss of 13q) [9].
Lenalidomide, used conventionally to treat multiple myeloma and more recently myelodysplasia with del (5q), is undergoing clinical trial as a treatment for solid tumors, including carcinoma of the pancreas and non-small cell lung cancer [10-12]. Lenalidomide has multiple mechanisms of action, and they can be simplified by organizing them as mechanisms of action in vitro and in vivo [13]. In vitro, lenalidomide has direct anti-tumor effect, inhibition of angiogenesis, and immunomodulation. In vivo, lenalidomide induces tumor cell apoptosis directly and indirectly by inhibition of bone marrow stromal cell support, by anti-angiogenic and anti-osteoclastogenic effects, and by immunomodulatory activity. Lenalidomide has a broad range of activities that can be potentially exploited to treat many hematologic and solid cancers. Side effects, however, can be severe and include thrombosis, pulmonary embolus, and hepatotoxicity, as well as bone marrow toxicity resulting in neutropenia and thrombocytopenia. Yelo suppression is the major dose limiting toxicity [13].
Future studies are warranted to confirm the cytogenetic associations with clinical and histological variables in small cell neuroendocrine lung carcinoma utilizing larger sample sizes, larger tumor samples (i.e., wedge resections), and more extensive cytogenetic analyses. Lenalidomide, specifically targeting the del (5q) clone, may offer potential as an adjunct treatment in small cell lung cancer. We are eager to see more studies in this regard, as FISH offers an easy, economic means toward this end.
- Miura I, Graziano SL, Cheng JQ, Doyle LA, Testa JR (1992) Chromosome alterations in human small cell lung cancer: frequent involvement of 5q. Cancer Res 52: 1322-1328. Link: https://tinyurl.com/yaec9kb7
- Hosoe S, Ueno K, Shigedo Y, Tachibana I, Osaki T, et al. (1994) a frequent deletion of chromosome 5q21 in advanced small cell and non-small cell carcinoma of the lung. Cancer Res 54: 1787-1790. Link: https://tinyurl.com/yaxrv3ko
- Schwendel A, Langreck H, Reichel M, Schröck E, Ried T, et al. (1997) Primary small-cell lung carcinomas and their metastases are characterized by a recurrent pattern of genetic alterations. Int J Cancer 74: 86-93. Link: https://tinyurl.com/yc52d3b5
- Onuki N1, Wistuba II, Travis WD, Virmani AK, Yashima K, et al. (1999) Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 85: 600-607. Link: https://tinyurl.com/y89ezssa
- Hartel PH, Shackelford AL, Hartel JV, Wenger SL (2008) Del(5q) is associated with clinical and histological parameters in small cell neuroendocrine lung carcinoma. Int J Surg Pathol 16: 419-423. Link: https://tinyurl.com/yc5d7hk9
- Smith JS, Perry A, Borell TJ, Lee HK, OFallon J, (2000) Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol 18: 636-645. Link: https://tinyurl.com/yal4usnv
- Morel P, Hebbar M, Lai JL, Duhamel A, Preudhomme C, et al. (1993) Cytogenetic analysis has strong independent prognostic value in de novo myelodysplastic syndromes and can be incorporated in a new scoring system: a report on 408 cases. Leukemia 7: 1315-1323. Link: https://tinyurl.com/y7uajd4w
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, et al. (1998) The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 92: 2322-3233. Link: https://tinyurl.com/y9n97vv5
- Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, et al. (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med 343: 1910-1916. Link: https://tinyurl.com/y9lc8xp8
- Khade P, Devarakonda S (2017) Coexisting multiple myeloma, lymphoma, and non-small cell lung cancer: a case report and review of the literature. Int Med Case Rep J 10: 373-376. Link: https://tinyurl.com/yajw4mcg
- Kim K, An S, Cha HJ, Choi YM, Choi SJ, et al. (2013) Lenalidomide induces apoptosis and alters gene expression in non-small cell lung cancer cells. Oncol Lett 5: 588-592. Link: https://tinyurl.com/ycow8h5h
- (2009) ClinicalTrials.gov. US National Institutes of Health .Link: https://tinyurl.com/yb44uzrl
- Vallet S, Palumbo A, Raje N, Boccadoro M, Anderson KC (2008) "Thalidomide and lenalidomide: Mechanism-based potential drug combinations". Leukemia & Lymphoma 49: 1238–1245. Link: https://tinyurl.com/y93offgy
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
