Scientific Journal of Genetics and Gene Therapy
Heredo-Familial and Pediatric GISTs: Spot the Differences
Alessandro Perez and Daniele Fanale*
Cite this as
Perez A, Fanale D (2016) Heredo-Familial and Pediatric GISTs: Spot the Differences. Scientific J Genetics Gen Ther 2(1): 001-009. DOI: 10.17352/sjggt.000007Gastrointestinal stromal tumors (GISTs) are rare sporadic tumors that typically occur late in life, although they are the most common mesenchymal neoplasms of the gastrointestinal tract. GISTs are believed to originate from the Interstitial Cells of Cajal (ICC), a group of cells identified in the wall of the organs of the gastrointestinal tract, which act as a pace-maker for peristalsis and gut movements. However, familial and pediatric cases have also been reported. These rare subsets of GISTs have clinicopathological and molecular features different from their sporadic counterparts. Pediatric GISTs account for only 1% of all the identified cases of GISTs. Most of them lack the gain-of-function mutation in c-KIT or PDGFRA commonly found in adult cases, and may harbor mutations in other genes such as SDH. Gene expression profiling studies of pediatric GISTs show distinct molecular signatures, suggesting a unique origin as compared with adult GISTs. The review discusses also familial GISTs with germline c-KIT or PDGFRA gene mutations and variants harbouring no mutations in these genes such as Carney triad, Carney-Stratakis syndrome, and neurofibromatosis type 1. However, the initial phases of familial GISTs appear biologically similar to those of sporadic GISTs, with similar cytogenetic progression mechanisms and genic expression profiles.
Introduction
Gastrointestinal stromal tumors (GISTs) are rare tumors whose incidence has been recently clarified after the redefinition of the diagnostic criteria following the recognition of the peculiar immunohistochemical profile of these tumors [1]. These tumors were initially considered exceptional with an estimated incidence in the past of about 1.5 cases per 100.000 per year, but now this assumption is no longer accepted given a new estimated incidence more than ten times higher than that previously assumed; approximately 1,000 cases per year in Italy and almost 4500-5000 cases in USA [2,3]. GISTs represent less than 1% of all malignancies, but are the most common mesenchymal tumors of the gastrointestinal tract (0.1-3%) [4]. Their identification is relatively recent, since as a result of their myogen or neurogen pattern they were previously classified as leiomyomas, leiomyosarcomas, neurofibromas, and schwannomas [5]. GISTs are believed to originate from the Interstitial Cells of Cajal, a group of cells identified in the wall of the organs of the gastrointestinal tract, which act as a pace-maker for peristalsis and gut movements [6]. Data proving this relationship are based on similar histological profiles and above all on the common expression of certain antigens such as CD117, the product of the oncogene c-KIT, and myoid antigens. Although GISTs are well characterized histochemically, the data reported in literature until now are not much consistent at molecular level [7]. They may originate from any part of the gastrointestinal tract, but are more frequently found in the stomach (40-70%) followed by the small intestine (20-40%) [8]. Other sites are considered rare. Moreover, GISTs usually present as friable, non-capsulated masses ranging from 1-2 to more than 20cm wide and may present areas of necrosis and hemorrhage [9]. Hystological findings usually showed a spindle cell pattern, resembling smooth muscle cells, an epithelyoid pattern and occasionally a mixture of these. However, this distinction has no prognostic relevance [10].
In over 99% of cases they occur in the sporadic form, but there is a small percentage of heredo-familial GISTs characterized by germline mutations in different susceptibility genes. Specifically, these GISTs can be distinguished in two different forms: the familial form occurring when within the same family most subjects are affected by the same type of cancer and the hereditary form whenever it’s demonstrated that susceptibility to the development of cancer is genetically transmitted from parents to children [11]. Familial tumors are due to a combination of risk factors such as inherited susceptibility genes and environmental factors, whereas hereditary tumors, which represent a subgroup of the familial forms, are caused exclusively by inherited genetic changes.
Biomolecular features of GISTs: c-KIT and PDGFRA
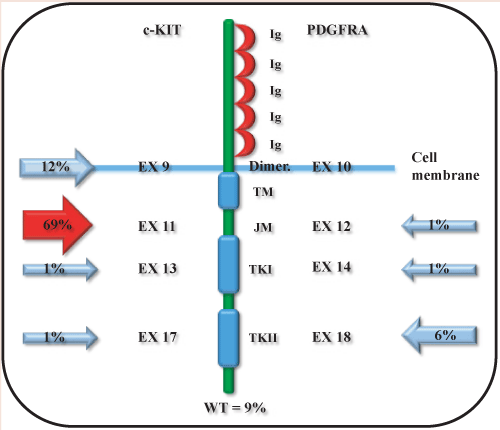
Molecular genetics has not only revealed the mechanism involved in the genesis of GIST but, also, gave us an immunophenotypic marker, the c-KIT protein [12]. The majority of GISTs (90-95%) show activating mutations of c-KIT with KIT/CD117 immunopositivity of approximately 95%. This marker allows then to deliver a very reliable diagnosis in 90-95% of cases [13,14]. c-KIT is an oncogene mapping on chromosome 4q12 and is composed of 21 coding exons [15]. The codifying sequence presents 4 mutational “hot spot” falling, in frequency order, in exons 11, 9, 13 and 17 [16]. KIT is a 145KD transmembrane glycoprotein with tyrosine kinase activity, a pivotal enzyme involved in development (differentiation) and cell growth (proliferation) [17]. In non-pathological conditions c-KIT is a receptor with TK activity that acts s a “cellular switch”. Indeed, if activated from the interaction of its ligand (stem cell factor, SCF) with the extracellular portion of the receptor via dimerization, triggers the activation of an intracellular pathway that initiates a cascade of signals whose final aim is to increase proliferation, differentiation and inhibition of apoptosis [18]. More in detail, the binding of a SCF molecule with each of the c-KIT monomers gives rise to the formation of a c-KIT dimer. The interaction of two adjacent receptors (homodimer) is specifically mediated by two molecules of SCF [19]. The homodimerization involves structural changes in the receptor with the activation of the intracellular tyrosine kinase that, firstly, catalyzes the auto-phosphorylation of specific tyrosine residues, which antagonizes the self-inhibition and is necessary for the transduction of the downstream signal triggered by the initial ligand-receptor interaction. The main activated pathways are PI3-kinase/AKT, RAS/ RAF/MAPK and JAK/STAT [20-22]. In pathological conditions most of the mutations on this gene are responsible of spontaneous homodimerization that, ultimately, causes an uncontrolled activation with advantages in terms of proliferative and anti-apoptotic effects. To date, it has been well recognised that mutations in c-KIT are mainly responsible of the pathogenetic event (90% of cases), but they do not are the only cause [23,24]. Indeed, a percentage of GIST cases that show no mutations in c-KIT exhibits alterations in PDGFRA gene (platelet-derived growth factor receptor), with a mutually exclusive relationship. The codifying sequence contains 23 exons and preferred mutational “hot spot” falling in exons 12, 18 and 14 [25]. The gene sequence encodes for a molecule with tyrosine kinase activity with similar function to c-KIT [26,27]. Structurally, the receptor consists of three main portions:
1. An extracellular portion with the ligand binding sites, consisting of 5 Ig-like domains. In this region it is possible to recognize:
• The ligand binding domain (consisting of 4 Ig-like domains) through which the ligand or growth factor specifically binds the receptor;
• The dimerization domain, which is fundamental for the receptor activation.
2. A transmembrane portion.
3. A portion containing the intracellular tyrosine kinase activity, divided in two domains:
• The juxtamembrane domain or autoinhibitory loop (encoded by the exon 11 and able to autoinhibit the kinase activity in the absence of the specific ligand) below the cell membrane;
• The kinase domain (catalytic), which represents the active part of the receptor and has the biochemical properties required for transfering a phosphate group from ATP to a substrate. In particular, the TK1 domain contains the ATP binding site, while the TK2 domain contains the phosphotransferase activity [24,28,29].
A schematic representation of c-KIT and PDGFRA protein domains and the respective codying exons is showed in Figure 1.
Mutational “hot spots” in c-KIT and PDGFRA
More than 90% of GISTs carry activating mutations in the c-KIT gene (approximately 90-95% of cases) or less commonly in the PDGFRA gene (about 5-10% of cases) [30,31]. In c-KIT four mutational “hot spots” in exons 9, 11, 13 and 17 were identified. It exists a hierarchy for the mutations involved in GIST [32]. The most interested exon is the exon 11, in approximately 70-80% of new cases, which encodes the “autoinhibitory loop”. This domain plays its autoinhibitory effect against the tyrosine kinase receptor [33,34]. Moreover, exon 9 is mutated in 10-20% of GIST. The exon 9 encodes for the fifth immunoglobulin-like domain of the receptor extracellular region. Unlike the aforementioned exons, point mutations in the exons 13 and 17 codifying for the tyrosine kinase domains of the receptor intracytoplasmic portion are detected with significant lower frequency (about 1.5%) [35,36]. The PDGFRA gene encodes for the platelet-derived growth factor receptor and shares with c-KIT about 35% of the amino acid sequence. This is a molecule with also a tyrosine kinase activity. The pathway that follows the activation of the receptor is basically similar to that of c-KIT [23,25]. The most frequent genetic alterations in c-KIT and PDGFRA including missense mutations as well as insertions and deletions are summarized in Tables 1, 2, respectively. Moreover, since PDGFRA and c-KIT genes share a high amino acid sequence homology, the functional consequences of their alterations are similar. Indeed, in both cases the mutations can generate autophosphorylation, which triggers the activation of two main signaling pathways, the RAS-RAF-MEK-ERK and the PI3KAKT-mTOR cascades, which leads to uncontrolled cell growth [25].
Tumor progression model in GIST
Activating mutations in c-KIT or PDGFRA are observed almost in the whole GIST cell population, while chromosome alterations are observed only in a percentage of neoplastic cells [37]. This suggests that mutations of c-KIT represent an early event, presumably, having a role in the initiation of the neoplastic transformation process, while subsequent cytogenetic abnormalities are involved in the neoplastic progression process [38]. Low grade GISTs have often a normal karyotype or only loss of chromosome 1p or 14 or 22 (50% of cases). High-grade GISTs are often accompanied by loss of at least 3 chromosomal portions (1p, 9p, 11p or 22p). Metastatic GISTs are characterized by loss in chromosome 9p often accompained by gain in 5q, 8q, 17q and 20q. Loss of heterozygosity (LOH) of c-KIT and TP53 and MSI downregulation are also frequent [39,23]. However, it must be emphasized that this sequence of events represents a simplification and that not all GISTs acquire genetic alterations in the aforementioned order [33].
About 10-15% of adult and pediatric gastrointestinal stromal tumors (GIST) do not show any activating mutation in c-KIT or PDGFRA. Indeed, it has been demonstrated that about 4% of wild-type GISTs show BRAF exon 15 mutation (V600E) [40]. It is widely reported that GISTs harboring activating c-KIT or PDGFRA mutations well respond to imatinib administration. This selective tyrosine kinase inhibitor is able to interfere with receptor activity by binding its ATP-binding pocket. However 80-85% of the cases seem to overcome drug efficacy becoming resistant to the treatment mainly due to the onset of secondary resistance. Indeed, an in vitro approach recently showed that imatinib-sensitive c-KIT mutated cells no longer respond to imatinib administration if KRAS or BRAF mutants are transfected [41].
Pediatric GISTs
Any GIST case diagnosed before the age of eighteen is defined as pediatric. The pediatric GISTs described until now represent between 1 and 2% of all cases of GISTs, and then represent rarely diagnosed tumors in youth. Basic research on GIST biology has been hampered by the rarity of this disease and by the lack of reliable experimental models [42]. Despite these limitations, the knowledge acquired through the study conducted in adults and other tumor types associated with them (eg. paragangliomas), in addition to the emergence of new methods (eg. microarrays techniques), allowed significant advances in the understanding of rare pediatric forms [43]. These pediatric forms may have a different pathogenesis than those seen in adult GISTs, since they may be lacking of mutations in c-KIT or PDGFRA genes (GIST wild-type) [44]. This feature could suggest that other mechanisms of activation of c-KIT, or oncogenic pathways not related to it, are active within cells. In most of the examined pediatric GIST, there was no indication of any cytogenetic abnormality or alterations in c-KIT exons 9, 11 or 13. Among the few pediatric GIST underwent to a mutational analysis, and reported in the literature, only 11% of cases highlighted mutations in c-KIT or PDGFRA, equally distributed [45]. These evidences are different from those seen in adults sporadic GISTs where mutations in c-KIT are 10 times more common than mutations in PDGFRA [46]. In the pediatric forms these mutations are identified mainly in males. Indeed, only a single case of female patient it has been reported to carry a mutation in PDGFRA. Probably these mutations are random molecular events. It could be assumed the presence of mutations on other c-KIT and PDGFRA exons, different from those found in adults, but when analyzed through direct sequencing no new mutations in c-KIT were identified [47]. Although no KIT mutations are usually found in GIST pediatric forms, such gene is often expressed and its pathway activated [46]. In relation to the small number of cases identified to date, the genetic basis of the onset of a pediatric GIST are still little known and probably are also involved epigenetic changes or hypomorphic mutations in genomic portions that regulate the involved gene. Indeed, the degree of activation measured through the phosphorylation in the corresponding protein in these patients appears to be similar to that found in c-KIT-mutated adult GISTs [48]. Moreover, other c-KIT downstream genes, such as MAPK, Akt, S6 and mTOR, are also activated. These data support the hypothesis that c-KIT and the downstream genes may play a fundamental role as therapeutic targets also in pediatric patients [49]. The majority of pediatric GISTs that show no mutations in c-KIT or PDGFRA are characterized by few chromosomal rearrangements on a large scale [44]. Using microarray technology through SNP analysis, it was observed that almost all the wild-type pediatric GISTs preserve heterozygosity and diploid copy number along the entire genome. This finding agrees with previously evaluated pediatric GISTs by classical cytogenetics, which showed a diploid karyotype [50]. Through subsequent CGH (comparative genomic hybridization) analysis, 60% of the 13 pediatric wild type GISTs showed no chromosomal alterations. In the remaining 40% of tumors with chromosome variations, the most common identified aberration was the deletion 1q, observed in 3 cases. The gene expression profiles of pediatric GISTs are different from those of adult wild type GISTs and are characterized from the upregulation of the following genes: BAALC, IGF1R, FGF4, CRLF1, PLAG1 and NELL1 [45]. Moreover, in a study contucted in 2005, Prakash et al. [51] analyzed the gene expression of GIST tumor samples from children, young adults (under 30 years) and adults,. The authors found a higher expression in the PHKA1, FZD2, NLGN4, IGF1R and ANK3 genes in the first two groups of patients. Young adult GISTs, with similar characteristics to those observed in the pediatric forms, showed gene expression profiles similar to those of wild-type pediatric GISTs. However, the described gene expression experiments were carried out using a small number of tumor samples and the results were not confirmed later because of the difficulty to obtain tissue samples for RNA analysis [51]. The expression of IGF1R (insulin-like growth factor 1 receptor) is 5 times higher in wild-type pediatric GISTs than in wild-type adults. IGF1 and its receptor (IGF1R) play a key role in cell growth, proliferation and development. The analysis of IGF1R protein expression in GISTs, performed by western blotting and immunohistochemistry, showed that the protein is expressed in all GISTs, but the expression levels are much higher than in the wild type GISTs [52]. IGF1R is activated in many GISTs, but its activation levels are not related to its expression. Moreover, it was observed that during stimulation with IGF1, the inhibition of IGF1R by small molecules reduced proliferation of a c-KIT-mutated GIST cell line. These data support the role of IGF1R as a potential therapeutic target in pediatric GISTs [53,54]. As in adult GISTs, the most common clinical manifestations, at the time of diagnosis, include gastrointestinal bleeding and anemia, related to it, and sometimes there is a palpable and bulky abdominal mass that often determine intestinal compression [55]. To the pediatric GISTs are often associated other forms of cancer as osteosarcoma and neuroblastoma. About 10% of pediatric GISTs occur in the context of the Carney triad or dyad, hence other suggestive features of the complex, in particular hyperpigmentation and the syndrome of inappropriate secretion of catecholamines associated to paragangliomas must be carefully sought at the initial evaluation of a pediatric patient suffering from GIST. The most common histological type is associated with a epithelioid cell or mixed fusiform/epithelioid morphology [56]. The clinical course, generally indolent, however, is usually less predictable than in adults, while considering commonly used prognostic factors (size, mitotic index and tumor site) [44]. Another common feature is the multifocality in the context of the anatomical region site of the localization that affects the onset of local recidives after several years from surgery. Before the introduction of targeted agents (primarily imatinib) in the treatment of GIST, surgery was the only possible treatment and is still the standard when the cancer is radically unresectable [42]. Although lymph nodes dissection is not recommended in adults, in pediatric GISTs, for the relatively higher incidence of lymph node metastases, suspicious nodes are surgically removed [43]. The interval between checks subsequent to surgery should include, beyond physical examination, the use of periodic imaging tests such as ultrasound, CT, MRI and PET, which should be carried out for the first 2 years every 3 months and every 6 months in the following two years, lastly annually as in adults. Before the introduction of mesylate imatinib, radical surgery was offered also to patients with locally advanced disease as well as non totally resectable or metastatic [57]. However, available data showed that complete resection of secondary injuries is not followed by healing and further relapse of disease occurs in almost all cases (90-100%). The framework in pediatric GISTs is similar [55]. The extensive surgery for debulking purpose, particularly used in the past, in the absence of other effective medical therapies, is no longer a viable option in the era of imatinib. GISTs, similar to mesenchymal tumors, are poorly responsive to chemotherapy. Randomized studies with anthracyclines, taxanes, ifosfamide and other various combinations showed response rates below 10%. Imatinib mesylate permitted to have better results than any type of chemotherapy or other treatment [58]. The optimal dose of imatinib in pediatric patients, as initial therapy, ranges from 230 to 400 mg/day and can be increased to 600-800 mg/day at disease progression. Sunitinib or nilotinib can be alternatively used [59].
Heredo-familial GISTs
Hereditary-familial gastrointestinal stromal tumors (GIST), together, represent less than 1% of all GISTs and are characterized 04 by inherited mutations in different susceptibility genes. They can be mainly distinguished in GISTs with c-KIT or PDGFRA mutations, which represent the main pathogenetic events in 90% of sporadic GISTs, and GISTs with mutations in other genes [60].
Heredo-familial GISTs with mutations in c-KIT and PDGFRA
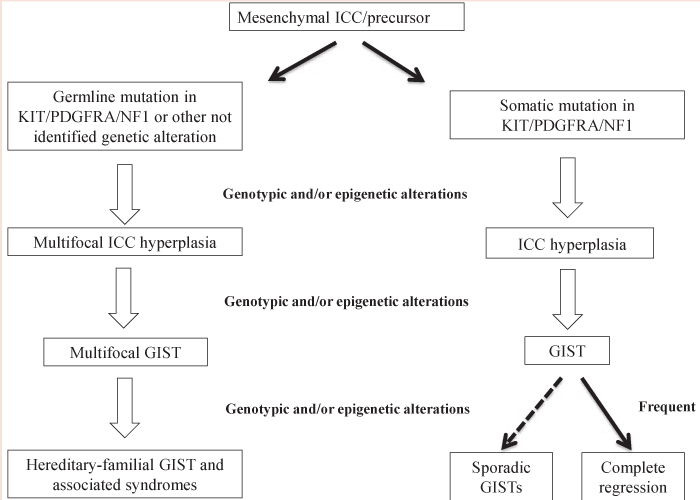
Any activating germline mutation in c-KIT and PDGFRA genes shows autosomal dominant transmission and have been already reported in a small number of families [61]. The clinical manifestations in individuals carrying germline mutations in c-KIT vary in relation to the involved exon. The exon encoding for the membrane receptor juxtamembrane domain in c-KIT is a key and very early event in GIST oncogenesis [62]. When inherited in a germline way, it determine hyperplasia of ICCs that early evolves in the formation of multiple GISTs [63]. It includes also mastocytosis and skin hyperpigmentation, especially at perineum, axillary, hands and face (pigmentary urticaria) level. The presence of pigmentation disorders and mastocytosis are justified by the fact that c-KIT is involved in melanogenesis and in the development of mast cells [58,64]. The association of GIST and ICC generalized hyperplasia supports the origin of these tumors from CD34+ mesenchymal stem cells during ICC differentiation (Figure 2). The c-KIT activation is sufficient to determine an ICC hyperplasia (proliferative stimulus to GIST progenitor cells), but need additional oncogenic events to generate a GIST. Mutations in the tyrosine kinase domain of the receptor determine multiple stomach and small intestine GISTs in the absence of clear skin disorders [61,63] (Figure 2). Also GISTs involving no skin pigmentation or mastocytosis can be associated with abnormalities in the esophageal peristalsis. The onset average age of this group of heredo-familial GISTs is 46 years, slightly lower than the onset average age of sporadic adult GISTs (about 50 years) and out from the indicated range for pediatric GIST [49]. Their initial stages appear biologically similar to those of sporadic GISTs, with similar mechanisms of cytogenetic progression and comparable gene expression profiles. The germline mutations in c-KIT and PDGFRA are mostly similar to those found in sporadic GISTs [11]. However, two families with hereditary GIST showed two mutations never seen in sporadic GISTs, respectively in c-KIT and PDGFRA (Asp419del and Tyr555Cys) [65]. These two missense mutations are located on the extramembrane and juxtamembrane domain, respectively, and are both responsible of the receptor autophosphorylation also in absence of a specific signal. In addition, another study reported the case of a patient who developed lipomas and GIST and showed a germline mutation (Asp561Val) in PDGFRA [66]. The main genotype-phenotype correlations in this heredofamilial GIST group are summarized in Table 3.
Heredo-familial GISTs with mutations in other genes
The Carney complex (from the name of the Majo Clinic’s famous anatomist who devoted his activity to the study of these diseases) includes the Triad Carney, described for the first time in 1977, and the Carney/Stratakis Dyad [67]. The Carney triad is an erlier onset syndrome (mean age 20 years) characterized by multiple gastric GISTs with multifocal onset that occurs in association to other tumors: extra-adrenal paragangliomas and pulmonary chondromas [68,69]. A careful follow-up of these patients also showed an increased risk of cortico-adrenal adenomas. The female population is the most affected (85% of all cases) and the disease course is usually slow and indolent (80% of patients alive at 20 years) [68,70]. Local recurrence, as well as liver and peritoneal lymph nodes metastases, are not frequent. In this context c-KIT, PDGFRA and SDH genes are wild-type [71]. The Carney-Stratakis Dyad is characterized by gastric GISTs and multiple paragangliomas. The onset average age is 19 years, and some patients may show, along with GIST, anemia and gastrointestinal haemorrhage, in the absence of evidente paraganglioma. In most patients with the Carney-Stratakis syndrome there is no evidence of mutations in c-KIT and PDGFRA, but germline mutations or deletions in SDH B, C or D genes, coding for the subunit B, C and D of the succinate dehydrogenase (SDH, mitochondrial complex II) were detected [70,72]. The predisposition to the development of these tumors is inherited in an autosomal dominant way with an incomplete penetrance. The succinate dehydrogenase is an enzyme system which has a dual role in the Krebs cycle and in the electrons transport chain in mitochondria [73]. The SDH complex consists of 4 subunits encoded by the genes SDHA, SDHB, SDHC and SDHD, made up of 15, 8, 6 and 4 exons, respectively [74]. The first two genes, located on chromosome 5p15 and on chromosome 1p35, encode for the two catalytic subunits, while the last two for the two transmembrane hydrophilic subunits. Germline mutations in the tumor-suppressor gene SDH were previously associated with hereditary paraganglioma (PGL1) and pheochromocytoma and should be sought in familial GISTs and paragangliomas [69,75] (Table 4). Although no SDH defects have been so far highlighted in Carney Tryad syndrome, the study conducted by Szarek et al. [76], provided new insights into mitochondrial ultrastructure in Carney Tryad tissues and GIST and Paragangliomas from SDH-deficient mice. Indeed, by comparing three different models, the study showed similar abnormalities in mitochondrial structure and function confirming the contribution of the abnormal mitochondria also in Carney Tryad pathogenesis [77]. Moreover, Carney Tryad seems to be also related to the CpG island methylation pattern of SDH subunit loci. Indeed, hypermethylation of SDH subunit C promoter, because of its transcription inactivation, seem to be characteristic of Carney Tryad tumor patients in comparison with Carney-Stratakis Dyad and Paragangliomas [78].
An increased incidence (about 200 times) of GIST in patients affected by type 1 neurofibromatosis (NF1) it has been reported [56,79]. NF1 is one of the most common autosomal dominant disease, and it affects 1/2500-3000 births in the world, with a prevalence of about 1/4000-5000 individuals within the general population. The desease is transmitted in an autosomal dominant way, with a penetrance of 100% at variable expressivity, as segmental or mosaic forms may be detected [80]. The patients’ genetic background influences instead the manifestation of the related phenotypes. The NF1 gene encodes a 220-250 kDa protein, called neurofibromin, which acts by inducing the GTPase function (GAP) of the Ras family proteins. Its action determines the stimulation of the Ras GTPase activity, with the consequent conversion of the mitogenic Ras-GTP complex in the inactive Ras-GDP form [81]. Therefore, the loss of this protein determines the permanent activation of Ras, typical condition of many sporadic tumors in which Ras activating mutations eliminate the catalytic activity [82]. To support this function, it has been shown that NF1-deficient human and mouse cell lines showing a hyperproliferative phenotype can be reverted to wild type with the inhibition of Ras [83,84]. The Ras protein is also involved in signal transduction pathways activated by c-KIT. GIST incidence in this subset of patients is about 5-25%. Even in these cases, these tumors arise in the absence of c-KIT and PDGFRA mutations, mainly in females, and show an indolent clinical course.
The oncogenetic counselling in heredo-familial GISTs
The identification of susceptibility genes involved in various hereditary cancer syndromes provided the molecular basis of genetic tests that allow to identify, among high risk individuals, germline mutation carriers considered for the “high risk” of developping a specific cancer. This new acquisition of molecular genetics has revealed the need to employ the oncogenetic counselling to meet the needs of all those cancer or healthy individuals, who want to understand the risk of recurrence of a genetic disease within the family for planning a proper clinical management [85]. The number of research laboratories and experts in the field which dedicate their activities to oncogenetic counselling is growing tremendously. This is mainly due to the increased demand from oncologists and other physicians who daily see patients with high risk factors for heredo-familial tumors, which are consequently directed to genetic counseling. The first risk factor for an hereditary cancer is the early age of onset, even without a family history [86]. In the case of heredo-familial GISTs, the reference age is about 45 years. The presence of the same type of tumor in different members from a side of the family (paternal or maternal) or the association of a group of various cancers caused by mutations in c-KIT, PDGFRA and SDH (e.g. paragangliomas and chondromas) represent high risk factors for identifying family candidates for the genetic counseling [87]. The oncogenetic counseling (CGO) takes place within specialized centers and provides a multidisciplinary and integrated approach among various specialists such as medical oncologists, geneticists and psychologists. Each of these figures plays a well-established role and acts at a different level of the various phases of a counseling (pre-test phase, the genetic test phase, posttest, follow-up) [88]. The multidisciplinary approach takes account of the different aspects and needs of the subject at risk of an hereditary cancer. Individuals at risk of developping a tumor are subjected to analysis of the personal and family history through the reconstruction of a pedigree during the pre-test. The main features of the personal history that suggest hereditary GIST include early age GIST diagnosis, primitive multiple GISTs, or GIST associated with another tumor (eg paragangliomas, adenomas, chondromas, neurofibromas) [69]. The reconstruction of the family history of a proband represents a fundamental step, since the detailed description of the pedigree allows specialists to make a correct diagnosis, predict prognosis more accurately and, thus, help the experts in making decisions. It should be fundamental to collect personal and clinical information for at least three generations for the family members of first (children, siblings, parents), second (grandparents, uncles, grandchildren) and third degree (cousins, great-uncles) of the proband to facilitate the identification of the hereditary pattern possibly present in the family [89]. For the correct construction of a pedigree you need to collect as many information as possible, considering:
a) either the paternal or the maternal side;
b) Parenthood, consanguinity, the use of assisted reproductive technologies.
It is important to gather information on family members both suffering or unaffected from tumors. For each family member with cancer is necessary to evaluate:
a) the type and the primary tumor site;
b) the age of primary tumor onset to the first diagnosis;
c) the current age if the individual is still alive, or the age of death and the cause of death;
d) the exposure to carcinogens (e.g., tobacco, exposure to radiation);
e) other significant health problems (e.g., presence of known genetic diseases which can predispose to the development of tumors); For each family member unaffected from cancer is necessary to examine:
a) the current age or age death;
b) the cause of death (if applicable);
c) surgical interventions that can have reduced the risk of developing cancer;
d) screening performed for early detection of cancer;
e) the exposure to carcinogens;
f) other diseases;
In particular, the characteristics that may suggest the genetic predisposition for the GIST onset include the presence of:
- Two or more relatives with first degree familiarity with GIST;
- A family member who has been diagnosed with GIST and another member with an another rare primary tumor;
- A family member with GIST who have a personal or family history for the typical cutaneous manifestations, multifocality or results a carrier of the NF1 gene mutations.
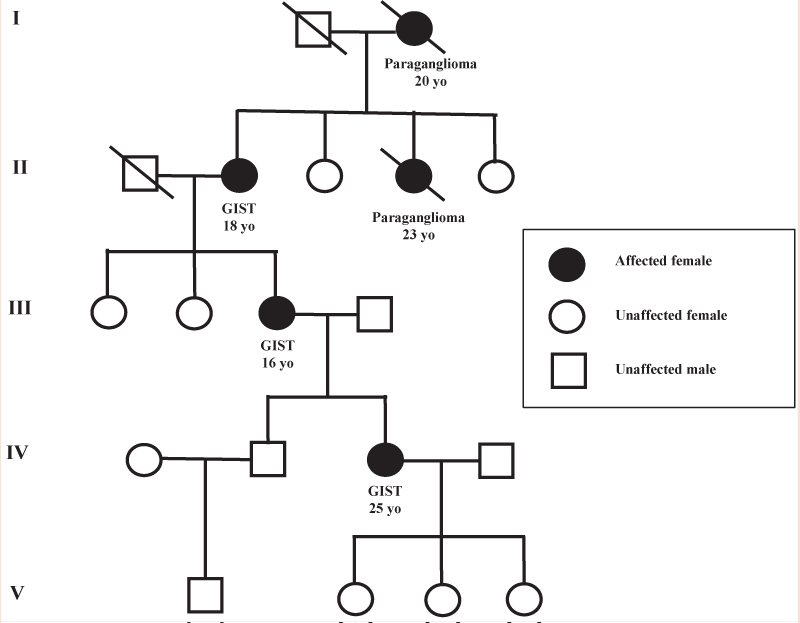
The Figure 3 shows the pedigree of a family affected by CarneyStratakis Dyad, characterized by the verticality of the hereditary transmission for 4 generations and by the association of GIST cases with paragangliomas. Moreover, noteworthy, the early onset age of the disease respect the sporadic cases. After the evaluation of the personal and family history follows the phase of the genetic test. The identification of a genetic mutation may allow, thanks to the genotype-phenotype correlations associated with the syndrome, to frame the clinical manifestations of the syndromes previously described. The post-test involves the delivery of the report, and for mutation carriers, contemplate the follow-up in order to adopt individualized surveillance programs [90,91].
Conclusions
GIST represent the most common mesenchymal tumors of the gastrointestinal tract. In adulthood, they mainly occur in the sporadic form but there is a small percentage of heredo-familial GISTs characterized by germline mutations in different susceptibility genes. In fact, the majority of sporadic GISTs show high frequency of activating mutations of the c-KIT oncogene. Moreover, a smaller percentage of GIST cases present mutually exclusive alterations in PDGFRA oncogene. Both gene sequences encode for a molecule with tyrosine kinase activity with a pivotal role in many pathways such as PI3K/AKT, RAS/RAF/MAPK and JAK/STAT. Genetic alterations in c-KIT and PDGFRA represent both the main pathogenetic events responsible of 90% of sporadic GISTs [92]. Moreover, the GIST pediatric variant is described only in 1 and 2% of all studied cases and they probably have a different pathogenesis if compared to adult GISTs. Indeed, they often did not show any mutations in c-KIT or PDGFRA as demonstrated in the examined pediatric GISTs where only 11% of all cases highlighted mutations in c-KIT or PDGFRA. Furthermore, heredo-familial GIST, represent less than 1% of all GISTs and are characterized by inherited mutations in different susceptibility genes. Their initial stages appear biologically similar to those of sporadic GIST as well as the germline mutations in c-KIT and PDGFRA are mostly similar to those found in sporadic GISTs. In individuals carrying germline mutations in c-KIT, the clinical manifestations tightly depend on the involved exon as the one encoding for the juxtamembrane domain which is implicated in the early stage oncogenesis. Indeed, it determine hyperplasia of ICC that early evolves in the formation of multiple GISTs as also mastocytosis and skin hyperpigmentation. However, not all GIST variants, although they appear hereditary, show detectable mutations in susceptibility genes. Indeed, the Carney Triad is characterized by multiple gastric GISTs and occurs often in association to other tumors: extra-adrenal paragangliomas and pulmonary chondromas. In this context, c-KIT, PDGFRA and SDH genes are often wild type. On the contrary, in the Carney-Stratakis Dyad, characterized by gastric GISTs and multiple paragangliomas even if there is not apparently evidence of mutations in c-KIT and PDGFRA, germline mutations or deletions in SDH B, C or D genes were found. Moreover, an increased GIST incidence has been also reported in patients affected by type 1 neurofibromatosis with no mutations in c-KIT and PDGFRA [93]. Indeed, these patients often show multifocal GISTs in the small intestine as well as in stomac even if at lower frequency. The interest in studying the role of susceptibility genes involved in several hereditary cancer syndromes has dramatically risen in the last decade. Indeed, the genetic counseling appear to be fundamental in order to meet the needs of cancer patients as well as healthy subjects in understanding the risk of recurrence of a genetic disease within their family. It provides a multidisciplinary and integrated approach among various specialists such as medical oncologists, geneticists and psychologists, each of them playing a well-established role at the different levels of a counseling. The reconstruction of the family history of a proband represents a fundamental step, since the detailed description of the pedigree allows to specialists to make a correct diagnosis, predict prognosis more accurately and, thus, plan a proper and personalized clinical management.
- Joensuu H (2006) Gastrointestinal stromal tumor (GIST). Ann Oncol 10: x280-286.
- Gheorghe M, Predescu D, Iosif C, Ardeleanu C, Bacanu F, et al. (2014) Clinical and therapeutic considerations of GIST. J Med Life 7: 139-149.
- Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, et al. (2015) Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 40: 39-46.
- Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RM, et al. (2005) Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 41: 2868-2872.
- Connolly EM, Gaffney E, Reynolds JV (2003) Gastrointestinal stromal tumours. Br J Surg 90: 1178-1186.
- Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, et al. (1999) Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 23: 377-389.
- Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM (1998) Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 152: 1259-1269.
- Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC (2001) The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol 54: 96-102.
- Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF (2000) Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 7: 705-712.
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, et al. (2002) Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 33: 459-465.
- Patil DT, Rubin BP (2015) Genetics of Gastrointestinal Stromal Tumors: A Heterogeneous Family of Tumors? Surg Pathol Clin 8: 515-524.
- Parkin B, Chugh R (2011) Molecular Pathology of Gastrointestinal Stromal Tumors and Implications for Treatment and Prognosis. Curr Prob Cancer 35: 245-254.
- Dow N, Giblen G, Sobin LH, Miettinen M (2006) Gastrointestinal stromal tumors: differential diagnosis. Semin Diagn Pathol 23: 111-119.
- Corless CL, Heinrich MC (2006) Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3: 557-586.
- de Silva CM, Reid R (2003) Gastrointestinal stromal tumors (GIST): C-kit mutations, CD117 expression, differential diagnosis and targeted cancer therapy with Imatinib. Pathol Oncol Res 9: 13-19.
- Yang J, Du X, Lazar AJ, Pollock R, Hunt K, Chen K et al. (2008) Genetic aberrations of gastrointestinal stromal tumors. Cancer 113: 1532-1543.
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, et al. (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279: 577-580.
- Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, et al. (2013) The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci 9: 435-443.
- Hirano K, Shishido-Hara Y, Kitazawa A, Kojima K, Sumiishi A, et al. (2008) Expression of stem cell factor (SCF), a KIT ligand, in gastrointestinal stromal tumors (GISTs): a potential marker for tumor proliferation. Pathol Res Pract 204: 799-807.
- Coindre JM, Emile JF, Monges G, Ranchere-Vince D, Scoazec JY (2005) Gastrointestinal stromal tumors: definition, histological, immunohistochemical, and molecular features, and diagnostic strategy. Ann Pathol 25: 358-385.
- Ali S (2007) Role of c-kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST). Gene 401: 38-45.
- Patel S (2013) Exploring novel therapeutic targets in GIST: focus on the PI3K/ Akt/mTOR pathway. Curr Oncol Rep 15: 386-395.
- Corless CL, Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal stromal tumors. J Clin Oncol 22: 3813-3825.
- Lasota J, Miettinen M (2008) Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 53: 245-266.
- Xu CW, Lin S, Wang WL, Gao WB, Lv JY, et al. (2015) Analysis of mutation of the c-Kit gene and in gastrointestinal stromal tumors. Exp Ther Med 10: 1045-1051.
- Barnett CM, Corless CL, Heinrich MC (2013) Gastrointestinal stromal tumors: molecular markers and genetic subtypes. Hematol Oncol Clin North Am 27: 871-888.
- Yamamoto H, Oda Y (2015) Gastrointestinal stromal tumor: recent advances in pathology and genetics. Pathol Int 65: 9-18.
- Schaefer IM, Delfs C, Cameron S, Gunawan B, Agaimy A, et al. (2014) Chromosomal aberrations in primary PDGFRA-mutated gastrointestinal stromal tumors. Hum Pathol 45: 85-97.
- Nannini M, Biasco G, Maleddu A, Pantaleo MA (2011) New molecular targets beyond KIT and PDGFRA in gastrointestinal stromal tumors: present and future. Expert Opin Ther Targets 15: 803-815.
- Tarn C, Godwin AK (2005) Molecular research directions in the management of gastrointestinal stromal tumors. Curr Treat Options Oncol 6: 473-486.
- Burger H, den Bakker MA, Kros JM, van Tol H, de Bruin AM, et al. (2005) Activating mutations in c-KIT and PDGFRalpha are exclusively found in gastrointestinal stromal tumors and not in other tumors overexpressing these imatinib mesylate target genes. Cancer Biol Ther 4: 1270-1274.
- Hornick JL, Fletcher CD (2007) The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol 38: 679-87.
- Nannini M, Biasco G, Astolfi A, Pantaleo MA (2013) An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST). J Med Genet 50: 653-6361.
- Andersson J, Bumming P, Meis-Kindblom JM, Sihto H, Nupponen N, et al. (2006) Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology 130: 1573-1581.
- Boikos SA, Stratakis CA (2014) The genetic landscape of gastrointestinal stromal tumor lacking KIT and PDGFRA mutations. Endocrine 47: 401-408.
- Kang W, Zhu C, Yu J, Ye X, Ma Z (2015) KIT gene mutations in gastrointestinal stromal tumor. Front Biosci (Landmark Ed) 20: 919-926.
- Du CY, Shi YQ, Zhou Y, Fu H, Zhao G (2008) The analysis of status and clinical implication of KIT and PDGFRA mutations in gastrointestinal stromal tumor (GIST). J Surg Oncol 98: 175-178.
- Blay JY, Le Cesne A, Cassier PA, Ray-Coquard IL (2012) Gastrointestinal stromal tumors (GIST): a rare entity, a tumor model for personalized therapy, and yet ten different molecular subtypes. Discov Med 13: 357-367.39.Haller F (2010) [Molecular biological evaluation of prognostic parameters in GIST. Development of an integrative model of tumor progression]. Pathologec 31: 161-166.
- Agaimy A, Terracciano LM, Dirnhofer S, Tornillo L, Foerster A, et al. (2009) V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol 62: 613-616.
- Miranda C, Nucifora M, Molinari F, Conca E, Anania MC, et al. (2012) KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res 18: 1769-1776.
- Janeway KA, Pappo A (2012) Treatment guidelines for gastrointestinal stromal tumors in children and young adults. J Pediatr Hematol Oncol 34: S69-72.
- Cypriano MS, Jenkins JJ, Pappo AS, Rao BN, Daw NC (2004) Pediatric gastrointestinal stromal tumors and leiomyosarcoma. Cancer 101: 39-50.
- Miettinen M, Lasota J, Sobin LH (2005) Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with longterm follow-up and review of the literature. Am J Surg Pathol 29: 1373-1381.
- Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res 14: 3204-3215.
- Janeway KA, Liegl B, Harlow A, Le C, Perez-Atayde A, et al. (2007) Pediatric KIT wild-type and platelet-derived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res 67: 9084-9088.
- Price VE, Zielenska M, Chilton-MacNeill S, Smith CR, Pappo AS (2005) Clinical and molecular characteristics of pediatric gastrointestinal stromal tumors (GISTs). Pediatr Blood Cancer 45: 20-24.
- Pappo AS, Janeway K, Laquaglia M, Kim SY (2011) Special considerations in pediatric gastrointestinal tumors. J Surg Oncol 104: 928-932.
- Li FP, Fletcher JA, Heinrich MC, Garber JE, Sallan SE, et al. (2005) Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in a kindred. J Clin Oncol 23: 2735-2743.
- Verschuur A, Andre N, Blay JY (2011) [Gastrointestinal stromal tumours in pediatrics: a summary of the literature on this orphan disease]. Bull Cancer 98: 79-86.
- Prakash S, Sarran L, Socci N, DeMatteo RP, Eisenstat J, et al. (2005) Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol 27:179-187.
- Janeway KA, Zhu MJ, Barretina J, Perez-Atayde A, Demetri GD, et al. (2010) Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer 127: 2718-2722.
- Braconi C, Bracci R, Bearzi I, Bianchi F, Sabato S, Mandolesi A, et al. (2008) Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol 19: 1293-1298.
- Tarn C, Rink L, Merkel E, Flieder D, Pathak H, et al. (2008) Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A 105: 8387-8392.
- Chiarugi M, Galatioto C, Lippolis P, Zocco G, Seccia M (2007) Gastrointestinal stromal tumour of the duodenum in childhood: a rare case report. BMC Cancer 7: 79.
- Miettinen M, Lasota J(2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130: 1466-1478.
- Benesch M, Wardelmann E, Ferrari A, Brennan B, Verschuur A (2009) Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr Blood Cancer 53: 1171-1179.
- Hartmann K, Wardelmann E, Ma Y, Merkelbach-Bruse S, Preussner LM, et al. (2005) Novel germline mutation of KIT associated with familial gastrointestinal stromal tumors and mastocytosis. Gastroenterology 129: 1042-1046.
- Kuroiwa M, Hiwatari M, Hirato J, Suzuki N, Tsuchida Y, et al. (2005) Advanced-stage gastrointestinal stromal tumor treated with imatinib in a 12-year-old girl with a unique mutation of PDGFRA. J Pediatr Surg 40: 17981801.
- Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, et al. (1998) Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet 19: 323-324.
- O’Riain C, Corless CL, Heinrich MC, Keegan D, Vioreanu M, et al. (2005) Gastrointestinal stromal tumors: insights from a new familial GIST kindred with unusual genetic and pathologic features. Am J Surg Pathol 29: 16801683.
- Tarn C, Merkel E, Canutescu AA, Shen W, Skorobogatko Y, et al. (2005) Analysis of KIT mutations in sporadic and familial gastrointestinal stromal tumors: therapeutic implications through protein modeling. Clin Cancer Res 11: 3668-3677.
- Isozaki K, Terris B, Belghiti J, Schiffmann S, Hirota S, et al. (2000) Germlineactivating mutation in the kinase domain of KIT gene in familial gastrointestinal stromal tumors. Am J Pathol 157: 1581-1585.
- Carballo M, Roig I, Aguilar F, Pol MA, Gamundi MJ, et al. (2005) Novel c-KIT germline mutation in a family with gastrointestinal stromal tumors and cutaneous hyperpigmentation. Am J Med Genet A 132A: 361-364.
- de Raedt T, Cools J, Debiec-Rychter M, Brems H, Mentens N, et al. (2006) Intestinal neurofibromatosis is a subtype of familial GIST and results from a dominant activating mutation in PDGFRA. Gastroenterology 131: 1907-1912.
- Pasini B, Matyakhina L, Bei T, Muchow M, Boikos S, et al. (2007) Multiple gastrointestinal stromal and other tumors caused by platelet-derived growth factor receptor alpha gene mutations: a case associated with a germline V561D defect. J Clin Endocrinol Metab 92: 3728-3732.
- Carney JA (1999) Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc 74: 543-552.
- Stratakis CA, Carney JA (2009) The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med 266: 43-52.
- Carney JA, Stratakis CA (2002) Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet 108: 132-139.
- McWhinney SR, Pasini B, Stratakis CA (2007) Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med 357: 1054-1056.
- Alrashdi I, Bano G, Maher ER, Hodgson SV (2010) Carney triad versus Carney Stratakis syndrome: two cases which illustrate the difficulty in distinguishing between these conditions in individual patients. Fam Cancer 9: 443-447.
- Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, et al. (2008) Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 16: 79-88.
- Postow MA, Robson ME (2012) Inherited gastrointestinal stromal tumor syndromes: mutations, clinical features, and therapeutic implications. Clin Sarcoma Res 2: 16.
- Oudijk L, Gaal J, Korpershoek E, van Nederveen FH, Kelly L, et al. (2013) SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors. Mod Pathol 26: 456-463.
- Bolland M, Benn D, Croxson M, McCall J, Shaw JF, et al. (2006) Gastrointestinal stromal tumour in succinate dehydrogenase subunit B mutation-associated familial phaeochromocytoma/paraganglioma. ANZ J Surg 76: 763-764.
- Szarek E, Ball ER, Imperiale A, Tsokos M, Faucz FR, et al. (2015) Carney triad, SDH-deficient tumors, and Sdhb+/- mice share abnormal mitochondria. Endocr Relat Cancer 22: 345-352.
- Yan L, Zou L, Zhao W, Wang Y, Liu B, et al. (2015) Clinicopathological significance of c-KIT mutation in gastrointestinal stromal tumors: a systematic review and meta-analysis. Sci Rep 5:13718.
- Haller F, Moskalev EA, Faucz FR, Barthelmess S, Wiemann S, et al. (2014) Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer 21: 567-577.
- Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, et al. (2006) Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet 15: 1015-1023.
- Agaimy A, Vassos N, Croner RS (2012) Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen’s disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol 5: 852-862.
- Afsar CU, Kara IO, Kozat BK, Demiryurek H, Duman BB, et al. (2013) Neurofibromatosis type 1, gastrointestinal stromal tumor, leiomyosarcoma and osteosarcoma: four cases of rare tumors and a review of the literature. Crit Rev Oncol Hematol 86: 191-199.
- Abramowicz A, Gos M (2014) Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med 18: 297-306.
- Wu M, Wallace MR, Muir D (2005) Tumorigenic properties of neurofibromindeficient Schwann cells in culture and as syngrafts in Nf1 knockout mice. J Neurosci Res 82: 357-367.
- Khalaf WF, Yang FC, Chen S, White H, Bessler W, et al. (2007) K-ras is critical for modulating multiple c-kit-mediated cellular functions in wild-type and Nf1+/- mast cells. J Immunol 178: 2527-2534.
- Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, et al. (2005) Consensus meeting for the management of gastrointestinal stromal tumors.
- Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 16: 566-578.
- Bleiker EM, Aaronson NK, Menko FH, Hahn DE, van Asperen CJ, et al. (1997) Genetic counseling for hereditary cancer: a pilot study on experiences of patients and family members. Patient Educ Couns 32: 107-116.
- Agarwal R, Robson M (2009) Inherited predisposition to gastrointestinal stromal tumor. Hematol Oncol Clin North Am 23: 1-13.
- Lewis KM (2014) Identifying hereditary cancer: genetic counseling and cancer risk assessment. Curr Probl Cancer 38: 216-225.
- Sifri R, Gangadharappa S, Acheson LS (2004) Identifying and testing for hereditary susceptibility to common cancers. CA Cancer J Clin 54: 309-326.
- Agaimy A, Hartmann A (2010) [Hereditary and non-hereditary syndromic gastointestinal stromal tumours]. Pathologe 31: 430-437.
- Cummings S (2000) The genetic testing process: how much counseling is needed? J Clin Oncol 18: 60S-64S.
- von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, et al. (2014) Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw 12: 853-862.
- Valencia E, Saif MW (2014) Neurofibromatosis type 1 and GIST: is there a correlation? Anticancer Res 34: 5609-5612.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
