Rheumatica Acta: Open Access
The Biological Effects of Interleukin-6 and Their Clinical Applications in Autoimmune Diseases and Cancers
Deng-Ho Yang1, 2*
2Division of Rheumatology/Immunology/Allergy, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China
Cite this as
Yang DH (2017) The Biological Effects of Interleukin-6 and Their Clinical Applications in Autoimmune Diseases and Cancers. Rheumatica Acta: Open Access 1(1): 006-016. DOI: 10.17352/raoa.000003Interleukin-6 (IL-6) is one of the pro-inflammatory cytokines involved in pathogenesis of various autoimmune and chronic inflammatory diseases. IL-6 through binding to its cellular receptor can transduce both classical- and trans-signaling pathways. Overproduction of circulating IL-6 can be detected in patients with different autoimmune diseases. Tocilizumab, a humanized monoclonal antibody against IL-6 receptor, can block IL-6-mediated signaling and has been approved for the treatment of rheumatoid arthritis and Castleman’s disease. Besides, expression of IL-6 may promote tumorigenesis and has been detected in various tumors, including multiple myeloma, colorectal cancer, breast cancer, lymphoma, breast cancer and lung cancer. Furthermore, increased levels of circulating IL-6 are associated with poor prognosis and cachexia in cancer patients. Monotherapy with IL-6 blockade or a combination therapy with both IL-6 blockade and conventional chemotherapy may reduce the progression of cancer and improve the status of cachexia in cancer patients. Finally, based upon the known biological effects of IL-6, diseases, other than autoimmune diseases and cancers, potentially to be anti-IL-6 candidates will be briefly discussed.
Introduction
Interleukin-6 (IL-6), composed of 184 amino acids with a molecular weight of 26 kDa, is one of the proinflmmatory cytokine and plays a major role in the progression of systemic and local inflammation. Significant elevation or excessive production of circulating IL-6 usually develops during the disease course of systemic inflammation including infectious or non-infectious diseases [1]. Several studies revealed that IL-6 has multiple functions in immune, inflammation and oncogenesis, and is associated with the promotion of synthesis of acute phase proteins including C-reactive protein (CRP), serum amyloid A, hepatoglobin, fibrinogen and hepcidin [2]. Persistent dysregulation of circulating IL-6 with overproduction may be found in the patients with different autoimmune diseases. Rheumatoid arthritis (RA), one of autoimmune diseases, is a systemic inflammation of polyarthritis, fever, myalgia, malaise and anemia with increasing circulating proinflammatory cytokine including IL-6 [3]. Higher circulating IL-6 is observed in the RA patients and is associated with disease activity, erythrocyte sedimentation rate (ESR), CRP and bony structural damage [4,5]. Increased levels of serum and synovial may be found in the patients with juvenile idiopathic arthritis (JIA) [6,7]. After medication of humanized anti-IL-6 receptor monoclonal antibody (tocilizumab), the clinical manifestations of polyarthritis and serum acute phase proteins are improved. IL-6 targeting therapy serves as a novel therapeutic strategy for the patients with RA and JIA [3,8]. IL-6 can promote osteocalstogensis and induce systemic bone loss with structural bony erosion in the disease course of RA [9]. Among the patients with systemic lupus erythematosus (SLE), increased expression of IL-6 is found, and the clinical manifestations of arthritis and circulating acute phase protein is improved after medication of IL-6 blocker (10). Active systemic inflammation and progressive renal involvement may develop after implication of IL-6 trans-signaling in lupus mice [11]. Elevated concentration of IL-6 is observed in the patients with Castleman’s disease and the level is reduced after clinical improvement [12]. These findings prove that IL-6 plays a major role in the pathogenesis of immunological abnormalities and the development of systemic inflammation in different autoimmune disease.
From the previous studies, IL-6 is also involved in the inflammation related tumorigenesis [13]. These cancers associated with abnormal systemic or local elevation of IL-6 involving different organs include breast, lung, ovarian, pancrea, prostate, colon, kidney and hematology [14]. Serum concentrations of IL-6 and CRP may serve as an excellent predicting biomarker of severity of systemic inflammation in malignancy. In pancreatic cancer patients, tumor associated macrophages may release IL-6 and it up-regulates cytokine expression, promotes proliferation, immune evasion, angiogenesis and resistance to apoptosis of cancer cells [15]. The levels of serum IL-6 are significantly higher in the patients with active weight loss [16]. Higher levels of serum IL-6 is observed in the patients with aggressive metastatic or recurrent breast cancers [17-19]. Among the hematological cancers including lymphoma and multiple myeloma, elevated serum IL-6 levels are found during the disease course [20-23]. In the patients with prostate cancer, higher serum IL-6 levels are associated with clinical metastasis and hormone-refractory [24,25]. Elevated serum IL-6 levels are observed in other types of cancers including colon, lung, ovarin and kidney [26-30]. Therefore, the pathological involvement of IL-6 is not only inflammation but also has the effect of cancer cells related immune response. IL-6 may be one of the pathogenesis in progression and migration of malignancy, and is essential for inducing cancer related inflammation. IL-6 blockade can suppress the differentiation of Th17 cells and can ameliorate chronic systemic inflammation [14]. The overall clinical and biological effects of IL-6 are shown as Table 1. Blocking the effect of functional IL-6 signaling pathway may thus be of benefit in systemic inflammation related different situations. This review is focused to evaluate the pathological role and clinical practice of anti-IL-6 blocker in numerous autoimmune diseases and cancers.
How IL-6-mediated biological effects may lead to the pathogenesis of autoimmune diseases and cancers
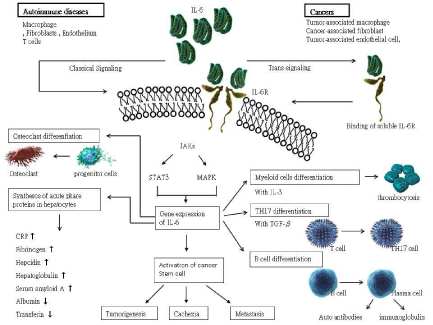
IL-6 is involved in autoimmunity due to influence of different cells, including T cell, B cell, macrophage, neutrophil, hepatocyte, dendritic cell, fibroblast, endothelial cell and different tumor cell. The circulating activity of IL-6 is complex and may induce both pro-inflammatory and anti-inflammatory effects in the immune system. During the progression of systemic inflammation, autoimmune disease, trauma and infection, increased production of pro-inflammatory cytokine and upregulating the expression of IL-6 usually develops [31]. Normal or tumor related macrophage, fibroblast and endothelial cell have the ability to release IL-6 [15]. Then, activation of IL-6 can contribute to anti-inflammatory or pro-inflammatory through the classical and trans-signaling pathways. In the classical signaling pathway, circulating IL-6 can bind to transmembrane, resulting in the activation of downstream signal via Janus kinase (JAK) in target cells. In the trans-signaling pathway, free IL-6 can bind to soluble IL-6 receptor to precede the downstream signal and amplify IL-6 signaling. The JAK signaling is through the activation of gp130. The gene expression of cell differentiation or proliferation may be activated by transcriptional factors of signal transducer and activator factor 3 (STAT3) or mitogen activated protein kinase (MAPK). IL-6 may induce activation and proliferation of B cell [1]. IL-6 with TGF-β can induce T cells for differentiation of Th17 cell and IL-6 overproduced in vivo inhibits the generation of regular T from naive T cells [32,33]. The differentiation of follicular helper T cell from naïve T cell may be contributed by IL-6 and IL-21. Follicular helper T cells migrate to B cell follicle region IL-6 can activate osteoclast leading to increasing bone resorption and is associated with osteoporosis [34]. IL-6 can promote activation and proliferation of hepatocyte through classic signaling to synthesize acute phase proteins, and these acute phase proteins include CRP, fibrinogen, hepatoglobulin and serum amyloid A. From a recent study, the effect of IL-6 can amplify the inflammatory response through a direct involvement of stromal and innate immunity cells [35]. The development of different systemic inflammation related cytokines and chemokines may be induced in this amplifying process. The multiple physiological effects of IL-6 in the immune system are showed as Figure 1.
In the patients with RA, proinflammatory cytokines can stimulate the production of IL-6 in rheumatoid synovial fibroblasts through activation of JAK2/STAT3 [36]. IL-6 may block the cartilage repair by inhibiting the differentiation of mesenchymal stem cells into chondrocytes [37]. Castleman’s disease is a systemic inflammatory disease characterized by general enlarged hyperplastic lymph nodes. This rare lymphoproliferative disorder has numerous systemic manifestations except polyadenopathy including fever, night sweating, fatigue, malaise, anorexia, weight loss, oranomegaly and edema. Thrombocytosis, leukocytosis, anemia, hypoalbuminemia, hypergammaglobulinemia and elevation of CRP are usually found in the clinical laboratory data. These patients with poor control may develop multiple organ failure and lymphoma. The mechanism of Castleman’s disease is not clear, and IL-6 plays a major role in the disease progression [38]. The abnormal expression of other proinflammatory cytokine, such as IL-6, and epidermal growth factor receptor, and vascular endothelial growth factor (VEGF) are observed in these patients. Elevation of circulating IL-6 is observed in the patients with Castleman’s disease and is associated with proliferation of B lymphocytes [12]. Activation and proliferation of B lymphocytes can lead to overproduction of IL-6. Increased IL-6 related immune dysregulation may enhance the production of proinflammatroy protein, such as CRP, and the clinical symptoms of anemia and thrombocytosis. At the same time, VEGF and IL-1 might be stimulated due to responsible for IL-6. Abnormal lymphocytic and vascular proliferation develop during the disease course of Castleman’s disease [39].
Overproduction or overexpression of circulating IL-6 can be found in different types of cancers. There is a direct correlation between IL-6 levels in tumor-associated endothelial cells and the tumorigenicity of cancer stem cells [40]. Tumor associated macrophages can induce IL-6 and may contribute to tumor progression associated with the inflammation related tumorigenesis. Tumor associated macrophages produce IL-6 and it promotes expansion of these caner stem cells. Levels of IL-6 in human hepatocellular carcinoma correlate with tumor stage and markers of caner stem cells [41]. IL-6 signaling may affect inflammation related cancer by modulating the resistance of T cells against apoptosis [42]. The imbalance between regulatory T cells and Th17 cells is observed in this progression. Therefore, the biological effect of IL-6 is not only find in autoimmune disorders, but certain malignancies.
The rationale of developing anti-IL-6 for the clinical therapeutic purposes
IL-6 plays a major role in the systemic and local inflammation of autoimmune disease, including RA, JIA, SLE and Castleman’s disease [3,43]. In other autoimmune disease, increased expression of serum IL-6 is found among the patients with dermatomyositis [44]. The IL-6 blocker may be sometimes useful in these disease, such as adult-onset Still’s disease, amyloidosis, vasculitis, relapsing polychondritis, and systemic sclerosis [43]. These findings suggest that IL-6 is not the only major pathogenesis among these autoimmune diseases. Circulating endothelial cells or progenitor cells are increased in the patient with SLE, and is associated with high INF-α levels and production of autoantibodies. In SLE, the systemic inflammation mediating glomerulonephritis may develop and the IL-6 trans-signaling can exacerbate the systemic inflammation or renal damage. Among SLE patients, plasma IL-6 levels are correlated with CRP and arthritis [10]. During the disease of RA, progressive osteoporosis with bone loss of lumbar spine or femoral neck develops except clinical manifestation of polyarthritis. Circulating IL-6 is positively correlated with the disease activity of RA and negatively correlated with mineral bone density [9]. IL-6 has negative effect on the differentiation of osteoblasts by the inhibition of STAT3 [45]. The medication of anti-IL-6 receptor antibody can prevent the systemic bone loss through inhibiting the migration of osteoclast precursor cells in the model of collagen-induced arthritis [46]. The assays of RA disease activity include DAS28, ESR, CRP and health assessment questionnaire [4], and IL-6 is associated with these clinical assays. IL-6 is important for the development of systemic osteopenia in RA. In autoimmune disease, chronic disease related anemia is due to overproduction of hepcidin through reduction of intestinal iron absorption and blocking the release of iron from macrophages. IL-6 may contribute the induction of hepcidin in anemia of chronic disease. In clinical, the hemoglobin level is increased by the medication of anti-IL-6 receptor antibody in the RA patients [47]. Decreased circulating regular T cells are found among RA patients. After medication of anti-IL-6 receptor antibody, tocilizumb, the clinical disease activity of RA is improved and regular T cells are significantly increased [48,49]. Clinical studies reveal that tocilizumb has effect on control of RA with or without medication of methotrexate. Tocilizumb is still useful for the medication of RA when these patients fail to response TNF-α inhibitor [49]. Monotherapy of tocilizumab is not inferior to tocilizumab combined with methotrexate [50]. The clinical and radiographic effects of tocilizumab may maintain after monotherapy for one year [51]. The subsequent flare will be found after discontinuation of tocilizumab, but the disease can be controlled by re-administration of biologics [52]. Therefore, tocilizumab is one of the most common biological agents for monotherapy [53]. The monotherapy of tocilizumab is superior to monotherapy of TNF-α for reduction of clinical signs and symptoms of RA patients [54].
In autoimmune disease, IL-6 related abnormal presentation of hepcidin is central to anemia of chronic diseases including RA and SLE. Significant elevation of serum IL-6 is found in breast cancer patients when compared with healthy controls [55,56]. Breast cancer cells may secrete IL-6 to support the proliferation of cancer, and reduction of proliferation of cancer is observed after IL-6 knockdown [57]. In another study about breast cancer, the expression of IL-6 is associated with the expression of estrogen receptor [58]. However, the abnormal expression of IL-6 also can be detected in cancer cells without expression of estrogen receptor [59]. Therefore, the increased expression of IL-6 plays a critical role in the development of breast cancer independently. Cancer-associated fibroblasts may promote tamoxifen resistance though IL-6-induced degradation of estrogen receptor-α in breast cancer [60]. In colon cancer cells, mucin 2 can increase the secretion of IL-6 and progress the colonic carcinogenesis [61]. The presence of IL-6 is associated with activation of carcinogenesis and DNA damage by upregulation of CYP1B1 and CYP2E2 in the progression of colonic inflammation [62]. In gastric cancer cells, IL-1β may enhance the invasiveness of cancer cells through the IL-6 expression [63]. The immunohistochemical expression of IL-2 receptor and IL-6 is higher in prostate cancer tissue than in normal tissue [64]. IL-6 gene polymorphism is associated with increased risk of bladder cancer [65]. Among female without history of smoking, genetic polymorphism of IL-6 is associated with the risk of lung cancer risk [66]. Increased expression of serum IL-6 is associated with poor survival in lung cancer [67]. After surgical resection of lung cancer cell, the levels of CRP, TNF-α ad IL-6 are decreased [68]. The anti-IL-6 antibody, siltuximab, has more potent effects on tumor inhibition of lung cancer through influence of cancer-associated fibroblasts [69]. The pathogenesis of IL-6 in the development of cancer is about the direct effect from the cancer stem cells or indirect effect from the systemic inflammation. Cancer stem cells may activate the IL-6 signaling pathway and amplify the expression of circulating IL-6 [70]. In the progression of malignancy, IL-6 is associated with angiogenesis and inhibiting apoptosis of tumors. In conclusion, adequate blocking the IL-6 signaling may disturbance the development of tumor related metastasis and migration. Besides, anti-IL-6 therapy severs as a biological effect for improvement of systemic inflammation in cancer and autoimmune disease.
The recent advances of the experience (clinical benefits and adverse events) of applying anti-IL-6 therapeutics
There are numerous cells can release IL-6 and these cells include macrophages, fibroblasts, endothelial cells and myeloid cells. In the patients with autoimmune diseases, IL-6 may be orientated from these different cells and induce immune reaction through classical or trans-IL-6-signalling pathway. Polyarthritis with systemic inflammatory related manifestations develop in the patients with RA. Aggressive synovial swelling and proliferation over joint space may be observed and the pathogenesis of RA is associated with numerous immune cells including T cells, B cells, fibroblasts, macrophages, endothelial cells, chondrocytes and osteoclasts [71]. Active clinical manifestations of synovial angiogenesis and hypertrophy and osteopenia with bony erosion develop in RA [72]. Traditionally, the medications of non-steroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying antirheumatic drugs may be used for RA. For recent years, novel biological agents by blocking TNF-α, IL-1, IL-6, active B cells and T-cell costimulatory are progressing. IL-6 is one of RA related proinflammatory cytokines. Anti-IL-6 therapy may improve the clinical signs of RA or JIA significantly. Except RA, active arthritis, cutaneous presentations and nephropathy are found in the SLE patients. Diffuse lymphoadnopathy and fever with systemic inflammation are observed among the patients with Castleman’s disease. Targeting and blocking IL-6 signaling pathway may improve these clinical systemic inflammations.
In the disease course of malignancy, cachexia is usually found. The biological effect of IL-6 can damage the skeletal muscle, adipose, mucosa of intestine and liver tissue. Therefore, circulating IL-6 may affect the degree of cachexia. At the same time, increased serum IL-6 is observed in different cancers including lung, liver, pancreas, colon, prostate, breast and bladder. Blocking IL-6-signaling pathway can relieve the clinical symptoms of cachexia in cancer patients [73]. IL-6 is one of major transcriptional regulators of hepcidin and chronic anemia with overproduction of hepcidin may contribute to the anemia [74]. Among the multiple myeloma patients with chronic anemia, IL-6 has the activity for acting synergistically with bone morphogenetic protein 2 on the induction of hepcidin [75]. After the blocking medication of IL-6, the anemia is improved by prevention of the induction of hepcidin [74,76]. In summary, different biological effects of IL-6 can be found in autoimmune diseases and cancers. These effects include arthritis, anemia, vascular angiogenesis, autoantibody production, lymphoadenopathy, tumor metastasis and cachexia. The different IL-6 related biological effects are shown as Table 2.
From different studies, IL-6 blockade therapy may be associated with adverse events including infections, infusion reactions, central demyelinating nerve and gastrointestinal perforation [77]. Laboratory abnormalities are found during the medication of IL-6 blockade, and these abnormalities include hyperlipidemia, elevation of liver function and neutropenia [78]. Therefore, adequate evaluation should be performed during anti-IL-6 therapy.
Current knowledge about clinical trials under developing and the aimed illnesses for anti-IL-6
IL-6 is a cytokine with multiple biological functions in the systemic inflammation, and is truly useful in RA, JIA and some clinical symptoms of SLE. Humanized anti-IL-6 receptor antibody is used to treat the patients with Castleman’s disease, and the clinical manifestations and abnormal laboratory data are improved after three months therapy [79]. Siltuximab, a novel anti-IL-6 monoclonal antibody, has therapeutic benefits in the patients with Castelman’s disease. After medication of siltuximab (12 mg/kg every 3 weeks), partial clinical benefit response and tumor response can be found among these patients [80]. In another study, 86% patients with Castleman’s disease have clinical and radiological response with significant decreasing CRP after siltuximab therapy [81].
Multiple myeloma is a malignancy disease associated with B-cell proliferation and elevation of circulating IL-6. In vivo, humanized anti-IL6 monoclonal antibody can inhibit the disease activity of multiple myeloma [82]. In the phase 1 study, siltuximab (anti-IL-6 monoclonal antibody) is used in multiple myeloma for recommend dose of 11 mg/kg once every 3 weeks [83]. The addition of siltuximab in the medication of multiple myeloma has no significant different when compared with traditional chemotherapy; but sustained suppression of circulating CRP is observed in the patients with medication of siltuximab [84].
Chronic prostatitis is associated with the progression and development of prostate cancer. One of the proinflammatory cytokines, IL-6, is important for systemic inflammation and may link the aggressivness of prostate cancer [85]. Elevation of IL-6 level is usually found in prostate cancer patients with metastasis or refractory for hormone or radiation therapy through multiple signal pathways including JAK, STAT and MAPK [86-88]. Inhibition of IL-6 can enhance the radiation sensitivity of prostate cancer [87]. IL-6 has a major role in prostate cancer stem cells and may regulate down signaling of STAT3 activation [89]. In the patients with prostate cancer, bone metastasis may develop during the disease course. IL-6 can increase the expression of RANKL and activate the abnormal bone resorption by osteoclasts in vitro. Blocking the IL-6 signaling by tocilizumab may inhibit skeletal tumor growth in vivo and reduce serum RANKL levels in prostate cancer cells [90]. Therefore, the conogenicity and bone metastasis of prostate cancer can be suppressed by anti-IL-6 antibody. Novel therapy about targeting IL-6 seems to fight against prostate cancer.
IL-6 can promote the breast cancer migration and metastasis with activation of STAT3 signaling pathway [91]. From different clinical studies, increased serum IL-6 concentration is associated with advanced tumor stages of various studies including multiple myeloma, lung cancer, colorectal cancer, prostate cancer and breast cancer [92]. Elevation of serum IL-6 is associated with clinical symptoms of depression, anxiety and tumor progression [93]. The initiation of cancers is associated with the activation of cancer stem cells from normal cells. IL-6 can induce the conversion of normal cells to cancer stem cells through the regulation of OCT-4 gene expression [94]. Blocking IL-6 signaling seems to be a potential therapeutic strategy for some cancers with elevation of circulating IL-6. At the same time, resistant of multiple drugs and targeting cancers develop in some patients with breast cancers. In these patients, abnormal elevation and activation of IL-6 are found. Neutralizing antibodies against IL-6 may reverse resistance of these chemotherapies (paclitaxel and doxorubicin) [95]. Active IL-6 inflammatory feedback loop may lead to aggressive expansion of breast cancer stem cells, and medication of IL-6 receptor antibody can interrupt this inflammatory loop [96].
In colon carcinoma cells, clonogenic proliferation and invasiveness may be induced by IL-6 through the pathways of MAPK and PI3K [97]. This clonogenic effect can be blocked by IL-6 receptor neutralization antibody. The abnormal elevation of IL-6 has the ability to progress the growth of colon cancers. The liver metastasis of colon cancer is associated with the activated macrophages inducing IL-6 secretion of colon cancer cells in vitro [98]. At the same, overexpression of IL-6 is found the metastatic colon carcinoma cells [99]. Therefore, the pathological effect of circulating IL-6 may be amplified by metastatic tumor cells. Chronic colitis related inflammation may induce the tumorgenesis by mediating IL-6/STAT3 signal pathway. In other study, polysaccharide peptidoglycan complex on Lactobacillus has the effect of inhibiting IL-6 production and may block the development of colitis-associated cancer through inhibition of STAT3 signaling [100].
The elevation of circulating IL-6 develops in the patients with lung cancer. The pathogenesis of lung cancer is associated with activation of macrophage with elevation of IL-6 and the development of epithelial to mesenchymal transition [101]. Active migration of lung cancer cells can be induced by IL-6 though JAK/STAT3 pathway [102]. Therefore, IL-6 is important for the metastasis of lung cancer. In vitro, the medication of anti-IL-6 antibody may reverse the induction of epithelial to mesenchymal transition in lung cancer cells [101]. Among the patients with lung cancer, serum IL-6 levels are higher in the patients with cachexia and poor response for chemotherapy [103]. The blocking agent for activity of IL-6 has the potential of improving disease prognosis and cachexia in lung cancer. A humanized anti-IL-6 antibody (ALD518) can improve the lung cancer related anemia and cachexia in Phase I and II clinical trial [104]. A chimeric anti-IL-6 monocolonal antibody, siltuximab, is used in the patients with advanced solid tumors including colorectal, ovarian and pancreatic cancers. Improvement of chronic anemia and systemic inflammation are observed, but adverse events of hepatic function abnormalities, fatigue and neutropenia are also found [76]. The clinical responses of IL-6 blockade in autoimmune diseases and cancers are shown as Table 3.
How to improve the anti-IL-6 therapeutics from both basic and clinical aspects
Tocilizumab is a humanized monoclonal antibody with consistent of complementary determining regions of mouse anti-IL-6 receptor antibody and human IgG1 portion. Tocilizumab may block IL-6-mediated signaling by binding to circulating soluble or transmembrane IL-6 receptors [105]. The disease activity of RA can be controlled by inhibition of Th17 differentiation through targeting IL-6 blocking. Direct down regulation with decreasing concentration of circulating IL-6 is the major target point about the therapy of Castleman’s disease. In the patients with SLE, targeting IL-6 may block the abnormal B cell proliferation and regulate the expression of regular T cells. In the cancer related systemic inflammation, increased levels of circulating IL-6 is a poor prognostic factor and is associated with higher mortality and drug resistance. Targeting IL-6 therapy may improve the drug resistance, chronic anemia and cachexia in cancer patients. The approved indications for anti-IL therapy include RA, systemic juvenile idiopathic arthritis and Castleman’s disease. The off-label applications of anti-IL-6 therapy include SLE, systemic sclerosis, polymyositis, systemic vasculitis and adult onset Still’s disease. The medication of targeting IL-6 therapy is about the systemic inflammation of autoimmune disease. Active systemic autoimmune disease is associated with upregulation of proinflammatory cytokine and IL-6 is one of the most involved common cytokines. Significant improvement of arthritis and circulating parameter of systemic inflammation (including CRP and ESR) are often observed when IL-6 blocker is used in these autoimmune diseases with active inflammation. IL-6 also plays a major role in the pathogenesis of chronic anemia. In the cancer related systemic inflammation, different degree elevation of IL-6 is usually found. Metastasis, active resistant chemotherapy, migration and higher mortality are major issues related upregulation of IL-6 signaling in cancers.
In conclusion, anti-IL-6 therapy has a useful strategy for selective autoimmune inflammation diseases, such as RA, Castelman’s disease, SLE and JIA. Among the patients with cancers, levels of circulating IL-6 are associated with metastasis, prognosis and chronic inflammation. Targeting IL-6 therapy may improve the systemic inflammation related complications due to cancers. Therefore, improvements and benefits of systemic inflammation related clinical manifestations (such as anemia, cachexia and fever) are observed in the cancer patients. Anti-IL-6 therapy may be used for the combination therapy for malignancy. The traditional therapy such as chemotherapy or target therapy cannot be replaced by anti-IL-6 therapy.
Conclusion
The biological effects of IL-6 are multiple functional and are important in the autoimmunity of systemic inflammation. Elevation of systemic or local IL-6 is observed in numerous autoimmune or rheumatic diseases. The administration of targeting anti-IL-6 is useful in RA, Castleman’s disease and juvenile idiopathic arthritis. Limited biologic effects of IL-6 blocking are observed in SLE, systemic sclerosis and polymyositis. The clinical improvements include decreasing serum CRP, arthritis and anemia in these limited responses of autoimmune diseases. Therefore, elevation of circulating IL-6 is not always the major pathogenesis of all autoimmune diseases. IL-6 has a complex role in the pathogenesis of various autoimmune diseases. Sometimes, IL-6 may be the secondary presentation of systemic inflammation in these autoimmune diseases. Except autoimmune diseases related inflammation, cancers related inflammation is usually associated with the systemic effect of circulating IL-6. In almost clinical trial, the serum CRP levels are significant reduced after anti-IL-6 therapy. The clinical manifestations of systemic inflammation including fever, cachexia and anemia are improved. However, the clinical response and disease control are not always seen in some autoimmune inflammation diseases. This phenomenon of substantive reducing CRP is also observed in the cancer patients after the medication of anti-IL-6 therapy. No significant suppression of tumor migration or metastasis is found. The progression of cancer related clinical manifestations is still proceeding. In some studies, the combination of anti-IL-6 therapy may improve the resistance of chemotherapy in malignancy.
In summary, anti-IL-6 therapy is useful in the autoimmune diseases with systemic inflammation, and these autoimmune diseases include RA, Castleman’s disease, JIA and SLE. Significant improvement of circulating acute phase protein, disease activity and anemia is observed in these effective autoimmune diseases. However, the effect of anti-IL-6 therapy is not significant in other autoimmune diseases including polymyositis, systemic sclerosis, psoriasis and systemic vasculitis. In these non-effective autoimmune diseases, decreased circulating CRP and anemia is still found, but no significant improvement of disease activity is observed. Besides, there is no significant improvement of metastasis, prognosis and mortality after anti-IL-6 therapy in the patients with cancers. However, IL-6 still plays a major role in systemic inflammation of numerous cancers including breast cancer, prostate cancer, lung cancer, colon cancer and multiple myeloma. Anti-IL-6 therapy may improve the clinical manifestations of malignancy related systemic inflammation. These effective clinical manifestations of chronic systemic inflammation include fever, anemia and cachexia. Targeting IL-6 antibody for IL-6 inhibition may sensitize tumor cells to irradiation and chemotherapy. These involved organs of malignancy include breast, prostate and colon. Thus, the circulating IL-6 level might severe as a predictor of the radiation or chemotherapy response in these tumor cells.
- Kishimoto T (1989) The biology of interleukin-6. Blood 74: 1-10. Link: https://goo.gl/jgSnfe
- Heinrich PC, Castell JV, Andus T (1990) Interleukin-6 and the acute phase response. Biochem J 265: 621-636. Link: https://goo.gl/IRG5IE
- Yoshida Y, Tanaka T (2014) Interleukin 6 and rheumatoid arthritis. Biomed Res Int 2014: 698313. Link: https://goo.gl/HA3GUA
- Wang J, Platt A, Upmanyu R, Germer S, Lei G, et al. (2013) IL-6 pathway-driven investigation of response to IL-6 receptor inhibition in rheumatoid arthritis. BMJ Open 3: e003199. Link: https://goo.gl/yJ52k9
- Baillet A, Gossec L, Paternotte S, Etcheto A, Combe B, et al. (2015) Evaluation of serum IL-6 level as a surrogate marker of synovial inflammation and as a factor of structural progression in early rheumatoid arthritis: Results from the ESPOIR cohort. Arthritis Care Res. Link: https://goo.gl/uQq2fq
- de Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, (1991) Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum 34: 1158-1163. Link: https://goo.gl/7OTdWr
- De Benedetti F, Pignatti P, Gerloni V, Massa M, Sartirana P, et al. (1997) Differences in synovial fluid cytokine levels between juvenile and adult rheumatoid arthritis. J Rheumatol 24: 1403-1409. Link: https://goo.gl/PkF7pI
- De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, et al. (2012) Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med 367: 2385-2395. Link: https://goo.gl/CD5YFS
- Abdel MMH, Hamad YH, Swilam RS, Barakat MS (2013) Relation of interleukin-6 in rheumatoid arthritis patients to systemic bone loss and structural bone damage. Rheumatol Int 33: 697-703. Link: https://goo.gl/ujlKRQ
- Ball EM, Gibson DS, Bell AL, Rooney MR (2014) Plasma IL-6 levels correlate with clinical and ultrasound measures of arthritis in patients with systemic lupus erythematosus. Lupus 23: 46-56. Link: https://goo.gl/tgxsQl
- Tsantikos E, Maxwell MJ, Putoczki T, Ernst M, Rose-John S, et al. (2013) Interleukin-6 trans-signaling exacerbates inflammation and renal pathology in lupus-prone mice. Arthritis Rheum 65: 2691-2702. Link: https://goo.gl/e6apqV
- Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, et al. (1989) Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood 74: 1360-1367. Link: https://goo.gl/EFpSzK
- Trikha M, Corringham R, Klein B, Rossi JF (2003) Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res 9: 4653-4665. Link: https://goo.gl/OX3ccS
- Mesquida M, Leszczynska A, Llorenc V, Adan A (2014) Interleukin-6 blockade in ocular inflammatory diseases. Clin Exp Immunol 176: 301-309. Link: https://goo.gl/qzqKYr
- Lesina M, Wormann SM, Neuhofer P, Song L, Algul H (2014) Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol 26: 80-87. Link: https://goo.gl/wKYfHN
- Okada S, Okusaka T, Ishii H, Kyogoku A, Yoshimori M, et al. (1998) Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol 28: 12-15. Link: https://goo.gl/ixRkWo
- Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, et al. (2002) Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer 2: 311-315. Link: https://goo.gl/ifQs22
- Zhang GJ, Adachi I (1999) Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 19: 1427-1432. Link: https://goo.gl/TveFgp
- Yokoe T, Iino Y, Takei H, Horiguchi J, Koibuchi Y, et al. (1996) Changes of cytokines and thyroid function in patients with recurrent breast cancer. Anticancer Res 17: 695-699. Link: https://goo.gl/AD2IoJ
- Seymour JF, Talpaz M, Hagemeister FB, Cabanillas F, Kurzrock R (1997) Clinical correlates of elevated serum levels of interleukin 6 in patients with untreated Hodgkin's disease. Am J Med 102: 21-28. Link: https://goo.gl/ND8w9I
- Preti HA, Cabanillas F, Talpaz M, Tucker SL, Seymour JF, et al. (1997) Prognostic value of serum interleukin-6 in diffuse large-cell lymphoma. Ann Intern Med 127: 186-194. Link: https://goo.gl/VdO2E7
- Wierzbowska A, Urbanska-Rys H, Robak T (1999) Circulating IL-6-type cytokines and sIL-6R in patients with multiple myeloma. Br J Haematol 105: 412-419. Link: https://goo.gl/bB8Kl0
- Pulkki K, Pelliniemi TT, Rajamaki A, Tienhaara A, Laakso M, Lahtinen R (1996) Soluble interleukin-6 receptor as a prognostic factor in multiple myeloma. Finnish Leukaemia Group. Br J Haematol 92: 370-374. Link: https://goo.gl/IMrq3a
- Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP (1999) Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate 41: 127-133. Link: https://goo.gl/Hb7RQ6
- Akimoto S, Okumura A, Fuse H (1998) Relationship between serum levels of interleukin-6, tumor necrosis factor-alpha and bone turnover markers in prostate cancer patients. Endocr J 45: 183-189. Link: https://goo.gl/AME37r
- Kinoshita T, Ito H, Miki C (1999) Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer 85: 2526-2531. Link: https://goo.gl/QdM6UC
- Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, et al. (1997) Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol 66: 27-30. Link: https://goo.gl/Q4gL9Z
- De Vita F, Orditura M, Auriemma A, Infusino S, Roscigno A, et al. (1998) Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep 5: 649-652. Link: https://goo.gl/gbPVgq
- Nakano T, Chahinian AP, Shinjo M, Tonomura A, Miyake M, et al. (1998) Interleukin 6 and its relationship to clinical parameters in patients with malignant pleural mesothelioma. Br J Cancer 77: 907-912. Link: https://goo.gl/8MvQPC
- Walther MM, Johnson B, Culley D, Shah R, Weber J, et al. (1998) Serum interleukin-6 levels in metastatic renal cell carcinoma before treatment with interleukin-2 correlates with paraneoplastic syndromes but not patient survival. J Urol 159: 718-722. Link: https://goo.gl/oCLKNQ
- Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM (2001) The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 15: 43-58. Link: https://goo.gl/kQnGYu
- Miossec P, Korn T, Kuchroo VK (2009) Interleukin-17 and type 17 helper T cells. N Engl J Med 361: 888-898. Link: https://goo.gl/lfZMgX
- Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, et al. (2011) The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol 186: 32-40. Link: https://goo.gl/QfBwZZ
- Park HJ, Kim DH, Lim SH, Kim WJ, Youn J, et al. (2014) Insights into the role of follicular helper T cells in autoimmunity. Immune Netw 14: 21-29. Link: https://goo.gl/WxBbJv
- Caiello I, Minnone G, Holzinger D, Vogl T, Prencipe G, et al. (2014) IL-6 amplifies TLR mediated cytokine and chemokine production: implications for the pathogenesis of rheumatic inflammatory diseases. PLoS One 9: e107886. Link: https://goo.gl/wCYvZA
- Muraoka S, Kusunoki N, Takahashi H, Tsuchiya K, Kawai S (2012) Leptin stimulates interleukin-6 production via janus kinase 2/signal transducer and activator of transcription 3 in rheumatoid synovial fibroblasts. Clin Exp Rheumatol 31: 589-595. Link: https://goo.gl/cZNT7n
- Wei H, Shen G, Deng X, Lou D, Sun B, et al. (2013) The role of IL-6 in bone marrow (BM)-derived mesenchymal stem cells (MSCs) proliferation and chondrogenesis. Cell Tissue Bank 14: 699-706. Link: https://goo.gl/KTA2L9
- Bonekamp D, Hruban RH, Fishman EK (2014) The great mimickers: Castleman disease. Semin Ultrasound CT MR 35: 263-271. Link: https://goo.gl/gPak1e
- El-Osta HE, Kurzrock R (2011) Castleman's disease: from basic mechanisms to molecular therapeutics. Oncologist 16: 497-511. Link: https://goo.gl/L2vsND
- Krishnamurthy S, Warner KA, Dong Z, Imai A, Nor C, et al. (2014) Endothelial interleukin-6 defines the tumorigenic potential of primary human cancer stem cells. Stem Cells 32: 2845-2857. Link: https://goo.gl/XFmzGw
- Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, et al. (2014) Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 147: 1393-1404. Link: https://goo.gl/hbhqNy
- Neurath MF, Finotto S (2011) IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 22: 83-89. Link: https://goo.gl/dJ10HE
- Alten R, Maleitzke T (2013) Tocilizumab: a novel humanized anti-interleukin 6 (IL-6) receptor antibody for the treatment of patients with non-RA systemic, inflammatory rheumatic diseases. Ann Med 45: 357-363. Link: https://goo.gl/Xbchgt
- Yang M, Cen X, Xie Q, Zuo C, Shi G, et al. (2013) Serum interleukin-6 expression level and its clinical significance in patients with dermatomyositis. Clin Dev Immunol 2013: 717808. Link: https://goo.gl/Zxja7n
- Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, et al. (2014) IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab 32: 378-392. Link: https://goo.gl/LVKpW5
- Tanaka K, Hashizume M, Mihara M, Yoshida H, Suzuki M, et al. (2014) Anti-interleukin-6 receptor antibody prevents systemic bone mass loss via reducing the number of osteoclast precursors in bone marrow in a collagen-induced arthritis model. Clin Exp Immunol 175: 172-180. Link: https://goo.gl/Thz0km
- Hashimoto M, Fujii T, Hamaguchi M, Furu M, Ito H, et al. (2014) Increase of hemoglobin levels by anti-IL-6 receptor antibody (tocilizumab) in rheumatoid arthritis. PLoS One 9: e98202. Link: https://goo.gl/ZUxgqC
- Kikuchi J, Hashizume M, Kaneko Y, Yoshimoto K, Nishina N, et al. (2015) Peripheral blood CD4 + CD25 + CD127 low regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther 17: 10. Link: https://goo.gl/EP7PNd
- Kaneko A (2013) Tocilizumab in rheumatoid arthritis: efficacy, safety and its place in therapy. Ther Adv Chronic Dis 4: 15-21. Link: https://goo.gl/drFy04
- Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, et al. (2013) Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 72: 43-50. Link: https://goo.gl/L4hMim
- Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, et al. (2014) Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis 73: 803-809. Link: https://goo.gl/xNle5O
- Huizinga TW, Conaghan PG, Martin-Mola E, Schett G, Amital H, et al. (2014) Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis 74: 35-43. Link: https://goo.gl/zq3cWK
- Kaufmann J, Feist E, Roske AE, Schmidt WA (2013) Monotherapy with tocilizumab or TNF-alpha inhibitors in patients with rheumatoid arthritis: efficacy, treatment satisfaction, and persistence in routine clinical practice. Clin Rheumatol 32: 1347-1355. Link: https://goo.gl/zpT56n
- Gabay C, Emery P, van VR, Dikranian A, Alten R, et al. (2013) Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381: 1541-1550. Link: https://goo.gl/iuur4h
- Al-Youzbaki WB, Al-Youzbaki NB, Telfah MM (2014) Tissue polypeptide antigen & interleukin-6: Are their serum levels a predictor for response to chemotherapy in breast cancer?. Pak J Med Sci 30: 1108-1112. Link: https://goo.gl/ntceMd
- Alokail MS, Al-Daghri NM, Mohammed AK, Vanhoutte P, Alenad A (2014) Increased TNF alpha, IL-6 and ErbB2 mRNA expression in peripheral blood leukocytes from breast cancer patients. Med Oncol 31: 38. Link: https://goo.gl/8No6Yq
- Itou J, Tanaka S, Sato F, Akiyama R, Kawakami Y, et al. (2015) An optical labeling-based proliferation assay system reveals the paracrine effect of interleukin-6 in breast cancer. Biochim Biophys Acta 1853: 27-40. Link: https://goo.gl/PzZl02
- Fontanini G, Campani D, Roncella M, Cecchetti D, Calvo S, et al. (1999) Expression of interleukin 6 (IL-6) correlates with oestrogen receptor in human breast carcinoma. Br J Cancer 80: 579-584. Link: https://goo.gl/lT70qY
- Schillace RV, Skinner AM, Pommier RF, O'Neill S, Muller PJ, et al. (2014) Estrogen receptor, progesterone receptor, interleukin-6 and interleukin-8 are variable in breast cancer and benign stem/progenitor cell populations. BMC Cancer 14: 733. Link: https://goo.gl/Fvb6Pg
- Sun X, Mao Y, Wang J, Zu L, Hao M, et al. (2014) IL-6 secreted by cancer-associated fibroblasts induces tamoxifen resistance in luminal breast cancer. Oncogene. Link: https://goo.gl/4NzXmQ
- Shan YS, Hsu HP, Lai MD, Yen MC, Fang JH, et al. (2014) Suppression of mucin 2 promotes interleukin-6 secretion and tumor growth in an orthotopic immune-competent colon cancer animal model. Oncol Rep 32: 2335-2342. Link: https://goo.gl/WvdnG6
- Patel SA, Bhambra U, Charalambous MP, David RM, Edwards RJ, et al. (2014) Interleukin-6 mediated upregulation of CYP1B1 and CYP2E1 in colorectal cancer involves DNA methylation, miR27b and STAT3. Br J Cancer 111: 2287-2296. Link: https://goo.gl/EoLvtU
- Xia Y, Khoi PN, Yoon HJ, Lian S, Joo YE, et al. (2015) Piperine inhibits IL-1beta-induced IL-6 expression by suppressing p38 MAPK and STAT3 activation in gastric cancer cells. Mol Cell Biochem 398: 147-156. Link: https://goo.gl/grJytp
- Engelhardt PF, Seklehner S, Brustmann H, Lusuardi L, Riedl CR (2015) Immunohistochemical expression of interleukin-2 receptor and interleukin-6 in patients with prostate cancer and benign prostatic hyperplasia: Association with asymptomatic inflammatory prostatitis NIH category IV. Scand J Urol 1-7. Link: https://goo.gl/iFtzZ6
- Ebadi N, Jahed M, Mivehchi M, Majidizadeh T, Asgary M, et al. (2014) Interleukin-12 and interleukin-6 gene polymorphisms and risk of bladder cancer in the Iranian population. Asian Pac J Cancer Prev 15: 7869-7873. Link: https://goo.gl/WYoxII
- Su M, Zhou B (2014) Association of genetic polymorphisms in IL-6 and IL-1beta gene with risk of lung cancer in female non-smokers. Zhongguo Fei Ai Za Zhi 17: 612-617. Link: https://goo.gl/AyWUnG
- Ryan BM, Pine SR, Chaturvedi AK, Caporaso N, Harris CC (2014) A combined prognostic serum interleukin-8 and interleukin-6 classifier for stage 1 lung cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J Thorac Oncol 9: 1494-1503. Link: https://goo.gl/JlpHRu
- Liu Y, Zhao H, Liu J, Wu Y, Xu S, et al. (2014) Influence of thoracoscopic surgery on inflammatory reaction of the body for early peripheral lung cancer patients. Zhongguo Fei Ai Za Zhi 17: 730-733. Link: https://goo.gl/bxXvPA
- Song L, Smith MA, Doshi P, Sasser K, Fulp W, et al. (2014) Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J Thorac Oncol 9: 974-982. Link: https://goo.gl/0gMknm
- Naugler WE, Karin M (2008) The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 14: 109-119. Link: https://goo.gl/SF7CeJ
- Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376: 1094-1108. Link: https://goo.gl/O7ZA9d
- Jutley G, Raza K, Buckley CD (2015) New pathogenic insights into rheumatoid arthritis. Curr Opin Rheumatol 27: 249-255. Link: https://goo.gl/hhynFc
- Narsale AA, Carson JA (2014) Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care 8: 321-327. Link: https://goo.gl/jR9xmj
- Sharma S, Nemeth E, Chen YH, Goodnough J, Huston A, et al. (2008) Involvement of hepcidin in the anemia of multiple myeloma. Clin Cancer Res 14: 3262-3267. Link: https://goo.gl/UacVfD
- Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, et al. (2010) In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood 116: 3635-3644. Link: https://goo.gl/WoJqGG
- Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, et al. (2014) A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 20: 2192-2204. Link: https://goo.gl/bf6H3l
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, et al. (2014) Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 41: 15-23. Link: https://goo.gl/OKo4Aq
- Campbell L, Chen C, Bhagat SS, Parker RA, Ostor AJ (2011) Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 50: 552-562. Link: https://goo.gl/U2RNz2
- Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, et al. (2000) Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood 95: 56-61. Link: https://goo.gl/NPoKpO
- Van RF, Fayad L, Voorhees P, Furman R, Lonial S, et al. (2010) Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman's disease. J Clin Oncol 28: 3701-3708. Link: https://goo.gl/vqnNoc
- Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, et al. (2013) A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res 19: 3659-3670. Link: https://goo.gl/lCkHaZ
- Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, et al. (2009) A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res 15: 7144-7152. Link: https://goo.gl/B1fTDV
- Suzuki K, Ogura M, Abe Y, Suzuki T, Tobinai K, et al. (2015) Phase 1 study in Japan of siltuximab, an anti-IL-6 monoclonal antibody, in relapsed/refractory multiple myeloma. Int J Hematol 101: 286-294. Link: https://goo.gl/31p7Th
- Orlowski RZ, Gercheva L, Williams C, Sutherland H, Robak T, et al. (2015) A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am J Hematol 90: 42-49. Link: https://goo.gl/Vu40Lu
- Ben Jemaa A, Sallami S, Ramarli D, Colombatti M, Oueslati R (2013) The proinflammatory cytokine, IL-6, and its interference with bFGF signaling and PSMA in prostate cancer cells. Inflammation 36: 643-650. Link: https://goo.gl/Pd8lHR
- Nguyen DP, Li J, Tewari AK (2014) Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int 113: 986-992. Link: https://goo.gl/vvQ4Gk
- Wu CT, Chen MF, Chen WC, Hsieh CC (2013) The role of IL-6 in the radiation response of prostate cancer. Radiat Oncol 8:159. Link: https://goo.gl/RpNLL8
- Kutikov A, Makhov P, Golovine K, Canter DJ, Sirohi M, et al. (2011) Interleukin-6: a potential biomarker of resistance to multitargeted receptor tyrosine kinase inhibitors in castration-resistant prostate cancer. Urology 78: 968 e7-11. Link: https://goo.gl/kLG9g9
- Kroon P, Berry PA, Stower MJ, Rodrigues G, Mann VM, et al. (2013) JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res 73: 5288-5298. Link: https://goo.gl/Bub7KP
- Zheng Y, Basel D, Chow SO, Fong-Yee C, Kim S, et al. (2014) Targeting IL-6 and RANKL signaling inhibits prostate cancer growth in bone. Clin Exp Metastasis 31: 921-933. Link: https://goo.gl/kaQ0Xs
- Snyder M, Huang XY, Zhang JJ (2011) Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J Biol Chem 286: 38886-38893. Link: https://goo.gl/UUiNIu
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z (2012) Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 38: 904-910. Link: https://goo.gl/PF0Eh9
- Jehn CF, Flath B, Strux A, Krebs M, Possinger K, et al. (2012) Influence of age, performance status, cancer activity, and IL-6 on anxiety and depression in patients with metastatic breast cancer. Breast Cancer Res Treat 136: 789-794. Link: https://goo.gl/Hm22Wj
- Kim SY, Kang JW, Song X, Kim BK, Yoo YD, et al. (2013) Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal 25: 961-969. Link: https://goo.gl/XbTs46
- Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q, et al. (2012) Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 135: 737-747. Link: https://goo.gl/ssBHl8
- Korkaya H, Kim GI, Davis A, Malik F, Henry NL, et al. (2012) Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell 47: 570-584. Link: https://goo.gl/xcg3TZ
- Hsu CP, Chen YL, Huang CC, Chou CC, Liu CL, et al. (2011) Anti-interleukin-6 receptor antibody inhibits the progression in human colon carcinoma cells. Eur J Clin Invest 41: 277-284. Link: https://goo.gl/QCXijk
- Li YY, Hsieh LL, Tang RP, Liao SK, Yeh KY (2009) Interleukin-6 (IL-6) released by macrophages induces IL-6 secretion in the human colon cancer HT-29 cell line. Hum Immunol 70: 151-158. Link: https://goo.gl/ZcTz7x
- Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, et al. (2000) Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett 151: 31-38. Link: https://goo.gl/6e65vj
- Matsumoto S, Hara T, Nagaoka M, Mike A, Mitsuyama K, et al. (2009) A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology 128: e170-180. Link: https://goo.gl/ilARyO
- Dehai C, Bo P, Qiang T, Lihua S, Fang L, et al. (2014) Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6. Immunol Lett 160: 1-10. Link: https://goo.gl/OPsJEu
- Liu RY, Zeng Y, Lei Z, Wang L, Yang H, et al. (2014) JAK/STAT3 signaling is required for TGF-beta-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol 44: 1643-1651. Link: https://goo.gl/69Cp8n
- Ando K, Takahashi F, Kato M, Kaneko N, Doi T, et al. (2014) Tocilizumab, a proposed therapy for the cachexia of Interleukin6-expressing lung cancer. PLoS One 9: e102436. Link: https://goo.gl/YChJFw
- Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR (2011) A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 11: 1663-1668. Link: https://goo.gl/Ux9HyH
- Tanaka T, Kishimoto T (2012) Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci 8: 1227-1236. Link: https://goo.gl/ubDyAY
- Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, et al. (2014) Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis Link: https://goo.gl/bByhBM
- Schoels MM, van DHD, Breedveld FC, Burmester GR, Dougados M, et al. (2013) Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statement. Ann Rheum Dis 72: 583-589. Link: https://goo.gl/GgGOMv
- Munoz J, Dhillon N, Janku F, Watowich SS, Hong DS (2014) STAT3 inhibitors: finding a home in lymphoma and leukemia. Oncologist 19: 536-544. Link: https://goo.gl/W7xvoI
- Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, et al. (2010) Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 62: 542-552. Link: https://goo.gl/jrbimg
- Smith PC, Keller ET (2001) Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate 48: 47-53. Link: https://goo.gl/14P6gX
- Guo Y, Nemeth J, O'Brien C, Susa M, Liu X, et al. (2010) Effects of siltuximab on the IL-6-induced signaling pathway in ovarian cancer. Clin Cancer Res 16: 5759-5769. Link: https://goo.gl/9Mn8nn
- Li J, Lan T, Zhang C, Zeng C, Hou J, et al. (2015) Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget Link: https://goo.gl/Q2M8Mj
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
