Open Journal of Thyroid Research
Effects of Exogenous Insulin Therapy on Thyroid Nodule Size in Patients with Type 2 Diabetes Mellitus
Samer El-Kaissi1*, Badurudeen Mahmood Buhary2 and Suphia Sherbeeni2
2King Fahad Medical City, Riyadh, Kingdom of Saudi Arabia
Cite this as
El-Kaissi S, Buhary BM, Sherbeeni S (2017) Effects of Exogenous Insulin Therapy on Thyroid Nodule Size in Patients with Type 2 Diabetes Mellitus. Open J Thyroid Res 1(1): 004-006. DOI: 10.17352/ojtr.000002Objectives: Recent evidence suggests that insulin resistance and endogenous hyperinsulinaemia are related to thyroid nodule growth and development, but the effect of exogenous insulin therapy on thyroid nodules is unknown. The objectives of this study are to investigate the effects of exogenous insulin on thyroid nodule size in diabetic patients.
Methods: retrospective analysis of patients with nodular thyroid disease and type 2 diabetes mellitus receiving metformin alone or insulin-and-metformin. Thyroid ultrasonography was used to monitor changes in nodule size: ≥2 mm and ≥20% being statistically significant.
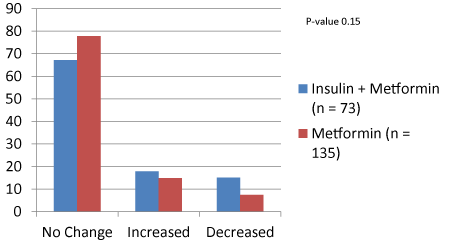
Results: 34 insulin-treated (73 nodules) and 53 metformin-only (135 nodules) patients were followed for 5.9-67.9 months (mean 24.5±1.8). Nodule size ranged from 0.17-5.25 cm (mean 1.29±0.08). There were no significant differences in serum TSH, free-T4 or Vitamin D between the two groups, but haemoglobin A1C was higher in insulin-treated patients at baseline and during follow-up (8.75±0.12% vs. 6.48±0.08%; P-value <0.001). In insulin-treated patients, the number of nodules with no change, increase or decrease in size were 49/73 (67.12%), 13/73 (17.81%) and 11/73 (15.07%) at the end of follow-up. The respective number of nodules in the metformin-only group were 105/135 (77.78%), 20/135 (14.81%) and 10/135 (7.41%). Chi-square test for type of treatment and changes in nodule size was not significant (P-value 0.15).
Conclusions: Compared to metformin alone, treatment with a combination of exogenous insulin and metformin was not associated with thyroid nodule size or thyroid volumes. The effects of exogenous insulin alone, without metformin, on nodular thyroid disease warrant further evaluation.
Introduction
Nodular thyroid disease (NTD) and type 2 diabetes mellitus (T2DM) are common disorders with increasing prevalence throughout the world. While not directly linked to each other, T2DM has been associated with a greater serum TSH, increased thyroid volume and a higher prevalence of NTD [1].
While there are no studies linking exogenous insulin treatment in T2DM and thyroid nodule growth, there is mounting evidence that insulin resistance and endogenous hyperinsulinaemia are associated with nodule development [2] and that reduction of insulin resistance has favourable effects on nodule size [3,4]. Hyperinsulinaemia may also be associated with an increased risk of differentiated thyroid carcinoma [5] and persistence of disease post-operatively [6].
Similarly, acromegalic patients with elevated growth hormone and insulin-like growth factor 1 (IGF-1) are at an increased risk for the development of goitre, thyroid nodules [7], and thyroid malignancy [8]. Growth hormone replacement therapy in growth hormone deficient patients has been associated with increased total thyroid volume and thyroid nodule development [9].
Human insulin and its analogues stimulate IGF-1 receptors potentially leading to cellular proliferation, but the effects of exogenous insulin on thyroid nodule growth are currently unknown. The objective of this study is to compare the effects of metformin alone vs. metformin and exogenous insulin therapy on thyroid nodule size in T2DM patients with non-toxic multinodular goitres.
Methods
This is a retrospective analysis of 87 patients with non-toxic NTD and long-standing T2DM treated with metformin alone (n = 53 patients; total of 135 nodules) or insulin-and-metformin (n = 34 patients; total of 73 nodules) for at least 6-months. Patients were followed for a mean 24.5 ± 1.8 months (range 5.9-67.9 months). A total of 111 nodules underwent fine needle aspiration based on clinical suspicion, and 4/77 (5.2%) insulin-treated and 3/138 (2.2%) metformin-treated nodules were found to be malignant and excluded from the analysis. Nodule size ranged from 0.17-5.25 cm (mean 1.29±0.08). Thyroid ultrasonography was used to monitor changes in thyroid nodule size, with changes ≥2 mm and ≥20% in 2-dimensions deemed statistically significant. Total thyroid volume (TTV) was estimated from the sum of the right and left thyroid lobes. The volume of each lobe was determined using the 3-dimensional elliptical shape volume formula.
All measurements on thyroid ultrasonography throughout the study were performed by one investigator (SEK). Statistical analysis was performed with the software program Minitab 14.12. Proportions were compared with Fisher’s exact test while the sample means were evaluated with the 2-sample t-test. Chi-square test was used to compare the treatment for diabetes and changes in nodule size in the two groups. Significance was set at P value less than 0.05. The study was approved by our institution’s research and ethics committee.
Results
A total of 34 insulin-and-metformin treated patients (73 nodules) and 53 metformin-only patients (135 nodules) were included in the analysis. Apart from a higher haemoglobin A1C in the insulin-treated patients, there were no significant differences at baseline in the age, serum TSH, free-T4, vitamin D levels, mean nodule size or TTV of the two groups (Table 1). During follow-up, there were no significant differences in the serum TSH, free-T4, or vitamin D levels between the two groups, although the haemoglobin A1C remained higher in the insulin-treated patients suggesting less stringent glycaemic control compared to metformin-only patients (Table 2).
In insulin-treated patients, the number of nodules with no change, increase or decrease in size were 49/73 (67.12%), 13/73 (17.81%) and 11/73 (15.07%) at the end of follow-up. The respective number of nodules in the metformin-only group were 105/135 (77.78%), 20/135 (14.81%) and 10/135 (7.41%). Chi-square analysis for the type of treatment and changes in nodule size was not statistically significant (P-value 0.15 – Figure 1).
Changes in TTV were also not significantly different between the two groups (P-value 0.15). The respective number of patients with no change, increase or decrease in TTV were 30, nil and 4 out of 34 insulin-treated patients, and 51, 2 and nil out of 53 metformin-treated patients.
Discussion
In this study, type 2 diabetic patients treated with an insulin-metformin combination regimen did not have significant changes in thyroid nodule size or thyroid volume over a mean follow-up duration of 24-months. These findings are reassuring as many insulin-treated patients with T2DM often have co-existing NTD. However, it remains to be determined if exogenous insulin therapy, without metformin, does not impact the development and progression of NTD.
Treatment with metformin has been shown to reduce insulin resistance and has been associated with a decrease in thyroid nodule size [3,4]. In a 6-months study of 66 women with insulin resistance and NTD, metformin therapy was associated with a 30% median reduction in nodule size while the combination of levothyroxine and metformin was associated with 55% reduction [10]. In our study, we could not evaluate the effects of metformin on nodule size as both groups were treated with metformin.
The relationship between exogenous insulin therapy and thyroid malignancy was not examined in this study. A total of seven patients with confirmed thyroid malignancy (n=7) were excluded from the analysis. However, in a large database study, Tseng et al., did not find an increased risk of thyroid malignancy in insulin-treated diabetic patients [11].
Limitations of this study include its retrospective design, small sample size and relatively short follow-up duration as changes in thyroid nodule size may take years to occur. The unavailability of baseline thyroid nodule measurements before starting insulin therapy is another limitation, as changes in thyroid nodule size may occur within the first few weeks of insulin therapy. Moreover, the insulin group were also treated with metformin which may have masked the effects of insulin on nodule growth. Lastly, the total number of insulin units per kilogram of body weight was not available. This may be important to determine if patients on a high dose of insulin, e.g. > 2 units/kg, are at a greater risk for thyroid nodule growth.
Conclusions
Compared to metformin alone, there was no association between insulin-metformin combination therapy and changes in nodule size of diabetic patients. Prospective studies are needed to examine this relationship further, especially in patients treated only with insulin rather than insulin and metformin.
- Anil C, Akkurt A, Ayturk S, Kut A, Gursoy A (2013) Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metabolism 62: 970-975.Link: https://goo.gl/1QBcgc
- Heidari Z, MA Mashhadi, Nosratzehi S. (2015) Insulin Resistance in Patients with Benign Thyroid Nodules. Arch Iran Med 18(9): 572-576. Link: https://goo.gl/0cm6QG
- Anil C, Kut A, Atesagaoglu B, Nar A, Bascil Tutuncu N, et al. (2016) Metformin Decreases Thyroid Volume and Nodule Size in Subjects with Insulin Resistance: A Preliminary Study. Med Princ Pract 25: 233-236. Link: https://goo.gl/XE9nNZ
- Karimifar M, Aminorroaya A, Amini M, Mirfendereski T, Iraj B, et al. (2014) Effect of metformin on thyroid stimulating hormone and thyroid volume in patients with prediabetes: A randomized placebo-controlled clinical trial. J Res Med Sci 19: 1019-1026. Link: https://goo.gl/Io2rFJ
- Bae MJ, Kim SS, Kim WJ, Yi YS, Jeon YK, et al. (2016) High prevalence of papillary thyroid cancer in Korean women with insulin resistance. Head Neck 38: 66-71. Link: https://goo.gl/hc1Pa2
- Pitoia F, Abelleira E, Bueno F, Urciuoli C, Schmidt A, et al. (2015) Insulin resistance is another factor that increases the risk of recurrence in patients with thyroid cancer. Endocrine 48: 894-901. Link: https://goo.gl/YOJUrw
- Cheung NW, Boyages SC (1997) The thyroid gland in acromegaly: an ultrasonographic study. Clin Endocrinol (Oxf) 46: 545-549. Link: https://goo.gl/HJOHON
- Rogozinski A, Furioso A, Glikman P, Junco M, Laudi R, et al. (2012) Thyroid nodules in acromegaly. Arq Bras Endocrinol Metabol 56: 300-304. Link: https://goo.gl/e7xZDt
- Curto L, Giovinazzo S, Alibrandi A, Campennì A, Trimarchi F, et al. (2015) Effects of GH replacement therapy on thyroid volume and nodule development in GH deficient adults: a retrospective cohort study. Eur J Endocrinol 172: 543-552. Link: https://goo.gl/31NyLf
- Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H (2011) Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord 9: 69-75. Link: https://goo.gl/eMkaTn
- Tseng CH (2014) Treatment with human insulin does not increase thyroid cancer risk in patients with type 2 diabetes. Eur J Clin Invest 44: 736-742. Link: https://goo.gl/wkoQs1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
