Open Journal of Thyroid Research
Autoimmune Thyroid Diseases and Helicobacter Pylori
Vincenzo Bassi1*, Olimpia Fattoruso2 and Crescenzo Santinelli1
2U.O.C di Patologia Generale, S Giovanni Bosco Hospital, ASL Na-1 Centro, Naples, Italy
Cite this as
Bassi V, Fattoruso O, Santinelli C (2017) Autoimmune Thyroid Diseases and Helicobacter Pylori. Open J Thyroid Res 1(1): 001-006. DOI: 10.17352/ojtr.000001Background: Helicobacter pylori infection is worldwide diffused with up to 50% of incidence in the population of the developed countries and the most virulent strains carrying the cytotoxin-associated gene A antigens. Moreover, bacterial and viral antigens have been suspected to be able to mimic the antigenic profile of the thyroid cell membrane suggesting an important role in the onset of the autoimmune diseases, such as Graves’ disease and Hashimoto’s thyroiditis.
Aims: we reviewed the studies concerning the relationship between the bacterium and the autoimmune thyroid diseases such as Graves’ and Hashimoto’s disease.
Conclusions: the significative association between Helicobacter pylori and Graves’ disease suggests a possible role of this bacterium in the onset and/or the maintenance of the autoimmune disease.
Review
Helicobacter pylori (H. pylori) infection is worldwide spread with an incidence up to 50% of the population in developed countries and 80% in developing countries [1]. The prevalence rate of the infection shows a cohort effect and an increased rate usually is found in elders and males in Italy [2]. The bacterium is a motile, gram-negative microrganism, which typically colonizes the gastric mucosa; serologically it is possible to identify the most virulent strains by the presence of the cytotoxin-associated gene A (Cag-A) antigen [3]. The bacterium is responsible of gastric diseases such as gastritis, gastric/duodenal ulcers and carcinomas [4]. Moreover, extra-digestive diseases, such as immune thrombocytopenic purpura and coronary heart disease, have been reported to be strictly associated with H. pylori [5].
The autoimmune Thyroid Diseases (ATDs) are represented, essentially, by Hashimoto’s Thyroiditis (HT) and its variants (postpartum and sporadic thyroiditis), Graves’Disease (GD) and athrophic thyroiditis [6]. These diseases show a typical marker in the presence of autoantibodies against thyroglobulin (TgAbs), thyreoperoxidase (TPOAbs) and thyrotropin receptor (TRAbs). Both genetic and environmental factors are implicated in the pathogenesis of ATDs and some bacteria and viruses have been suspected to be able to mimic the antigenic profile on thyroid cell membrane, suggesting an important role in the onset of the autoimmune diseases [7]. Furthermore, increased levels of antibodies against some bacteria antigens have been found in the serum of GD patients [8] and, moreover, many gram-positive and gram-negative bacteria show an antigen structure similar to a TSH-binding protein [9].
In the past years different studies have shown a significative correlation between H. pylori presence and HT, although others deny such association [10-16]. The use of different techniques to identify H. pylori infection could explain these conflicting conclusions. For instance, serological detection of H. pylori antibodies is not useful to discriminate between past and ongoing infections. Conversely, 13C-urea breath test and immunoassay test on fresh stool samples detect only ongoing H. pylori infections and are currently considered the preferred not-invasive methods of investigation [17]. Moreover, the presence of similar antigenic sites between a bacterial antigen, such as Cag-A, and TPO structures explains the false positivity of the Abs titers against H. pylori, suggesting a possible bias in the group selection of the enrolled patients in some studies [18]. Also, the different grade of thyroid hypofunction, such as the subclinical or frank hypothyroidism, in the group of HT patients, could be a misleading factor.
Recently, a noteworthy correlation has been demonstrated between an overall H. pylori infection and GD, independently of the hormonal status, using a stool antigen test [19]. Moreover, the correlation with H. pylori has been demonstrated only in hyperthyroid GD patients but not in frank hypothyroid HT patients while, conversely, the Cag-A carrier strains of the bacterium seems to correlate with both thyroid diseases [20]. Furthermore, a recent study showed an increased rate of H. pylori recurrence in GD patients occurring in a short time span, six and twelve months, after successful drug eradication [21]. Suggesting that the H. pylori recrudescence seems to be the more logical explanation vs. a true reinfection of a different bacterium strain. In the future studies of molecular fingerprinting techniques of the involved strains could be useful to clear this hypothesis.
Several factors could be considered to explain the different observed results of H. pylori prevalence in GD and HT. Usually, the onset of different ATDs is dependent on numerous autoimmune mechanisms. Cellular autoimmunity with T-Helper (TH) 1 profile of CD4+T helper precursor cells is usually predominant in HT, whereas humoral autoimmunity, expressing in the production of TRAbs or TSH-receptor blocking antibodies, is associated with TH2 profile, prevalent in GD and atrophic thyroiditis. The activated TH profiles induce the expression of different panels of cytokines, such as tumor necrosis factor-alpha (TNF-a), interleukin (IL)-2 and interferon-gamma in HT and IL-4, IL-5, IL-6, IL-9. IL-10 and IL-13 in GD [22]. Moreover, the H. pylori infected patients show a TH2 polarization associated to a TH1 marked reduction suggesting a strong predisposition to humoral autoimmunity onset [23].
Also, the thyroid function, hyperthyroidism vs. hypothyroidism, could be another factor leading to the controversial results on H. pylori prevalence in GD and HT patients.
Anyway, the suggested factors could play through a common pathway, such as the glycoconjugates-mediated adhesion of H. pylori to the gastric mucosa, a crucial step in the establishment of successful bacterial infection. H. pylori glycan receptors include fucosylated ABO blood group antigens and glycans with charged groups, such as sialic acid or sulfate, and neolacto core chains [24-26]. Many H. pylori adhesins have been identified on the basis of their interactions with these receptors: the blood group antigen-binding adhesion (BabA) is specific for H type-1 and Lewis-b antigens, admitting terminal blood groups A and B glycan determinants, whereas the Sialic Acid Binding Adhesin (SabA) recognizes the Sialyl-Lewis a and Sialyl-Lewis x antigens [27-29].
Then, factors such as hyperthyroidism and/or different cytokines induced by humoral immunity, could modify the profile of the adhesion molecules expressed on the gastric mucosa, increasing the overall H. pylori binding in GD and selecting the prevalent Cag-A positive strains in ATDs.
In conclusion, it is possible to suggest a “trigger” role of H. pylori in the onset/maintainance of ATDs?
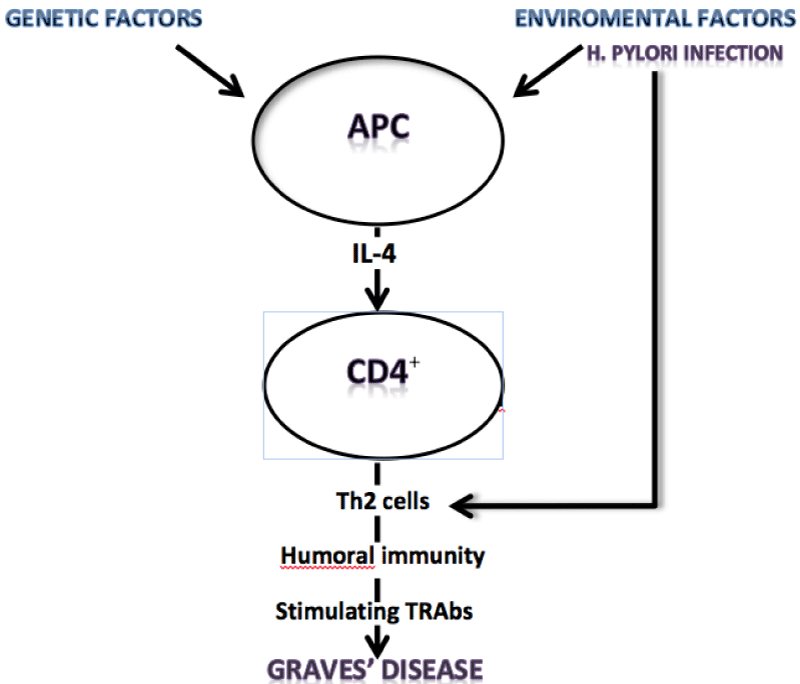
The increased positivity of H. pylori in GD, upon Vfirst diagnosis, and the previous observation that H. pylori infection, usually, starts during childhood [30], suggest that the bacterium is present before the onset of the autoimmune disease. Larizza et al. have proposed that H. pylori infection can induce and/or worsen the course of GD in susceptible young patients showing the human leukocyte DRB1*0301 antigen profile [31]. Then, the authors suggested that H. pylori eradication could prevent GD in these “at high risk” children. Moreover, Cag-A positive H. pylori strains show some nucleotide sequence similarity to thyroid peroxidase (TPO) sequence [32] and a positive linear regression between H. pylori-Abs titers and microsomial autoantibodies with a significant reduction of these antibodies after H. pylori eradication have been demonstrated [33]. Then, cross-reactivity of the antibodies produced against thyroid antigen structures during H. pylori infections could induce a biological effect (Figure 1).
Conversely, hyperthyroidism in GD patients could just be a predisposing factor to H. pylori infection, and the gastric colonization of the microorganism could represent an epiphenomenon, not involved in the onset of the autoimmune disease (Table 1).
In conclusion, further studies will be necessary to investigate the correlations between the bacterium and ATDs but the demonstrated increased infection rate of H. pylori in ATDs suggests that it is possible in the future to add new findings in the puzzling factors underlying the mechanism of ATDs onset.
- Pounder RE, Ng D (1995) The prevalence of helicobacter pylori infection in different countries. Alimen Pharmacol ther 9: 33-39. Link: https://goo.gl/JUAaqh
- Ponzetto A, Pellicano R, Morgando A, Cirillo D, Marchiaro G, et al. (2001) Seroprevalence of Helicobacter pylori infection among blood donors in Torino, Italy. Minerva Gastroenterol. Dietol 47: 3-7. Link: https://goo.gl/FGFgPL
- Atherton JC (1998) H. Pylori virulence factors. Br Med Bull 54: 105-120. Link: https://goo.gl/JMiH9y
- Blaser MJ (1998) Helicobacter pylori and gastric diseases. BMJ 316: 1507-1510. Link: https://goo.gl/9LJoUn
- Tsang KW, Lam SK (1999) Helicobacter pylori and extra-digestive diseases. J Gastroenterol Hepatol 14: 844-850. Link: https://goo.gl/aNzuMx
- Pearce EN, Farwell AP, Braverman LE (2003) Thyroiditis. N Engl J Med 348: 2646–2655. Link: https://goo.gl/uKwvVb
- Valtonen VV, Ruutu P, Varis K, Ranki M, Malkamaki M, et al. (1986) Serological evidence for the role of bacterial infections in the pathogenesis of thyroid diseases. Acta Med Scand 219: 105-111. Link: https://goo.gl/uojH8v
- Joasoo A, Robertson P, Murray IP (1975) Letter: Viral antibodies in thyrotoxicosis. Lancet 2: 125-131. Link: https://goo.gl/9fnwNw
- Tomer Y, Davies TF (1993) Infection, Thyroid Disease, and Autoimmunity. Endocr Rev 14: 104-120. Link: https://goo.gl/5vkqb2
- De Luis DA, Varela C, de la Calle H, Canton R, de Argila CM, et al. (1998) Helicobacter Pylori infection is markedly increased in patients with autoimmune atrophic thyroiditis. J Clin Gastroenterol 26: 259-263. Link: https://goo.gl/uPEKDd
- Figura N, Di Cairano G, Lorè F, Guarino E, Gragnoli A, et al. (1999) The infection by Helicobacter pylori strains expressing CagA is highly prevalent in women with autoimmune thyroid disorders. J Physiol Pharmacol 50: 817-826. Link: https://goo.gl/WdH4pi
- Arslan MS, Ekiz F, Deveci M, Sahin M, Topaloglu O, et al. (2015) The relationship between cytotoxin-associated gene A positive Helicobacter pylori infection and autoimmune thyroid disease. Endocr Res 40: 211-214. Link: https://goo.gl/C0Jm0v
- Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ (2009) Helicobacter pylori infection and endocrine disorders: is there a link? World J Gastroenterol 15: 2701-2707. Link: https://goo.gl/qbuZv7
- Tomasi PA, Dore MP, Fanciulli G, Sanciu F, Realdi G, et al. (2005) Is there anything to the reported association between Helicobacter pylori infection and autoimmune thyroiditis? Dig Dis Sci 50: 385- 388. Link: https://goo.gl/eGKjGT
- Franceschi F, Satta MA, Mentella MC, Penland R, Candelli M, et al. (2005) Helicobacter pylori infection in patients with Hashimoto’s thyroiditis. Helicobacter 9: 369-372. Link: https://goo.gl/qPKofq
- Shmuely H, Shimon I, Gitter LA (2016) Helicobacter pylori infection in women with Hashimoto thyroiditis: A case-control study. Medicine (Baltimore) 95. e4074. Link: https://goo.gl/J4BqXr
- Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, et al. (2012) Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. European Helicobacter Study Group. Gut 61: 646-664. Link: https://goo.gl/Ehtu34
- Johansen HK, Norgaard A, Andersen LP, Jensen P, Nielsen H, et al. (1995) Cross-reactive antigens shared by Pseudomonas aeruginosa, Helicobacter pylori, Campylobacter jejuni and Haemophilus influenzae may cause false positive titers of antibody to H. pylori. Clin Diagn Lab Immunol 2: 149-155. Link: https://goo.gl/Q8fDu8
- Bassi V, Santinelli C, Iengo A, Romano C (2010) Identification of a correlation between Helicobacter pylori infection and Graves’ Disease. Helicobacter 15: 558-562. Link: https://goo.gl/mD64r1
- Bassi V , Marino G , Iengo A , Fattoruso O , Santinelli C (2012) Autoimmune thyroid diseases and Helicobacter pylori: the correlation is present only in Graves's disease. World J Gastroenterol 18: 1093-1097. Link: https://goo.gl/mNMFER
- Bassi V, Fattoruso O, Polistina MT, Santinelli C (2014) Graves' disease shows a significant increase in the Helicobacter pylori recurrence. Clin Endocrinol (Oxf) 81: 784-785. Link: https://goo.gl/5YMmm2
- Roura-Mir C, Catalfamo M, Sospedra M, Alcalde L, Pujol-Borrell R, et al. (1997) Single-cell analysis of intrathyroidal lymphocytes shows differential cytokine expression in Hashimoto’s and Graves’ disease. Eur J Immunol 27: 3290–3302. Link: https://goo.gl/QsKx8a
- Satoh Y, Ogawara H, Kawamura O, Kusano M, Murakami H (2012) Clinical Significance of Peripheral Blood T Lymphocyte Subsets in Helicobacter pylori-Infected Patients. Gastroenterol Res Pract 2012: 819842. Link: https://goo.gl/0fImYL
- Aspholm-Hurtig M, Dailide G, Lahmann M (2004) Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305: 519–522. Link: https://goo.gl/Kc8TOK
- Boren T, Falk P, Roth KA, Larson G, Normark S (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262: 1892–1895. Link: https://goo.gl/6I5WdR
- Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, et al. (2002) Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297: 573–578. Link: https://goo.gl/gQs0QP
- Guzman-Murillo MA, Ruiz-Bustos E, Ho BOW, Ascencio F (2001) Involvement of the heparan sulphate-binding proteins of Helicobacter pylori in its adherence to HeLa S3 and Kato III cell lines. J Med Microbiol 50: 320–329. Link: https://goo.gl/hU5QQ2
- Miller-Podraza H, Weikkolainen K, Larsson T, Linde P, Helin J, et al. (2009) Helicobacter pylori binding to new glycans based on N-acetyllactosamine. Glycobiology 19: 399–407. Link: https://goo.gl/W5SJy0
- Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, et al. (1998) Helicobacter pylori adhesin binding fucosylated histo blood group antigens revealed by retagging. Science 279: 373–377. Link: https://goo.gl/UmHNoe
- Ko GH, Park HB, Shin MK, Park CK, Lee JH, Youn HS (1997) Monoclonal antibodies against helicobacter Pylori cross-react with human tissue. Helicobacter 2: 210-215. Link: https://goo.gl/D9vFOD
- Larizza D, Calcaterra V, Martinetti M, Negrini R, De Silvestri A, et al. (2006) Helicobacter Pilori infection and autoimmune thyroid disease in young patients: the disadvantage of carrying the human leukocyte antigen-DRB1*0301 allele. JCEM 91: 176-179. Link: https://goo.gl/mDHkZg
- Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, et al. (1997) The complete genome sequence of the gastric pathogen Helicobacter Pylori. Nature 388: 539-547. Link: https://goo.gl/df0mfh
- Bertalot G, Montresor G, Tampieri M, Spasiano A, Pedroni M, et al. (2004) Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin. Endocrinol 61: 650-652. Link: https://goo.gl/wa7sEv
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
