Open Journal of Tropical Medicine
Clinical performance evaluation of five commercial IgM tests for diagnostic of Zika virus infection
Didye Ruiz*, Carlos M Torres, Mayling Alvarez, Pedro A Martinez, Naifi Calzada, Lianna M Garcia and Maria G Guzman
Cite this as
Ruiz D, Torres CM, Alvarez M, Martinez PA, Calzada N, et al. (2020) Clinical performance evaluation of five commercial IgM tests for diagnostic of Zika virus infection. Open J Trop Med 4(1): 007-014. DOI: 10.17352/ojtm.000013Background: Zika Virus (ZIKV) is an emerging mosquito-transmitted flavivirus currently causing large epidemics and represents a global public health. Most ZIKV infections in humans are asymptomatic or mild with self-limiting clinical manifestations. Certainty, an available and sustainable surveillance for risk individual groups to ZIKV infection it is necessary and serological methods offer a good alternative.
Objective: To evaluate the clinical performance of five commercially serologic assays for the detection of anti ZIKV IgM.
Study design: A sera panel of 200 samples, including 100 positive and 100 negative serum to ZIKV infection, all characterized by molecular and serological standard diagnostic assays, was used in the evaluation. Three ELISA (Euroimmun, DiaPro and IBL), one immunochromatographic (Artron) and an immunoblot (Mikrogen) assays were evaluated.
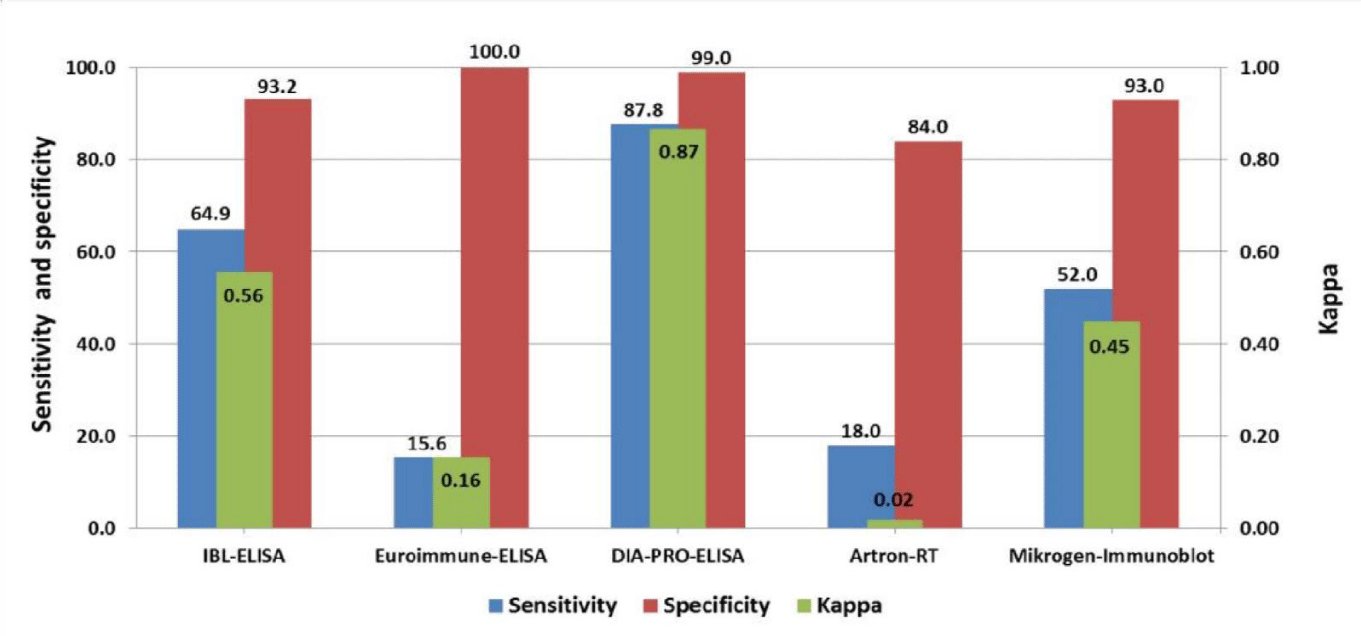
Results: Evaluated assays showed acceptable specificities (>80%) and variable sensitivities. The highest sensitivity was found for DiaPro-ELISA (87.8 %), following by IBL-MAC-ELISA (64.9%) and Mikrogen-Immunoblot (52.0%). Both Euroimmun (15.6%) and Artron (18.0 %) showed a poor sensitivity. The strongest agreement with the molecular reference was found by DiaPro-ELISA (kappa: 0.87), for IBL and Mikrogen was moderate (kappa: 0.56 and 0.45) and poor for Euroimmune and Artron (kappa: 0.16 and 0.02). DiaPro and Euroimmun showed a similar ZIKV IgM detection rate in DENV immune and naïve cases. Cross-reactivity for negative specimens was lower with the exception of Artron-RT assay.
Conclusions: Between the five evaluated assays, DiaPro-ELISA provided the best-assessed parameters for serum samples collected between days 5 to 7 of onset of symptoms.
Introduction
Zika virus (ZIKV) is an emerging mosquito-transmitted flavivirus currently causing large epidemics in South and Central America as well as in the Caribbean, representing a global public health emergency [1]. It is closely related to other human pathogenic members of the Flaviviridae family such as Dengue Virus (DENV), West Nile Virus (WNV), Japanese Encephalitis Virus (JEV) and Yellow Fever Virus (YFV). The clinical symptoms associated with ZIKV infection include rash, arthralgia, myalgia and conjunctivitis, and are normally self-limiting. ZIKV infections can lead to major complications and long-term sequelae, including congenital birth defects, neurologic disorders, and prolonged risk for the virus sexual transmission [2,3]. Most ZIKV infections in humans are asymptomatic or mild with self-limiting clinical manifestations [4,5]. In acute phase of ZIKV infection, clinical differentiation can be difficult, mainly respect to DENV infections circulation, therefore the present greatest challenge for the diagnosis of ZIKV be the detection and confirmation of cases in an environment where DENV infection has spread widely and more countries appear every year in endemic conditions of this disease [4,6].
ZIKV disease it is generally considered a mild infection in most cases. Approximately 80% of ZIKV infections are asymptomatic [7]. Various international organizations led by the WHO, evaluated different ways to establish a diagnostic protocol for ZIKV infection based on the established protocols for the diagnosis of other flaviviruses, with the aim of identifying suitable samples and the most reliable technique with greater specificity and sensitivity. Molecular assays for rapid identification of Zika virus RNA were the first evaluated protocols [8,9], however, these methodologies are expensive to support active surveillance throughout the year. So the search for faster, less complex and less expensive methodologies continues to be a current objective.
Serological diagnosis represent a great challenge due to the high cross-reactivity between flavivirus antibodies [10]. Some studies show that monoclonal antibodies (mAbs) against DENV envelope protein and dengue-immune sera can enhance ZIKV infection [11-13] , suggesting the possibility that previous DENV infection may rise the risk and severity of congenital ZIKV infection and consequent fetal microcephaly [14]. To know that ZIKV infections have a clinical relevance in suspected pregnancy women, an available and sustainable laboratory surveillance system based on specific and reliable serological methods is needed [15]. Currently, there is no a reference serological test for the use by evaluating laboratories. Neutralization test as serological gold standard technique has shown cross-reactivity as well as other applied methodologies, making it more difficult the case classification and evaluating process of new commercial assays that come onto the international market.
Here we evaluated 5 commercially available serologic assays for the detection of IgM anti ZIKV with a variety of immunoassay performances, such as: indirect ELISA, Capture ELISA, immunoblot and immunochromatographic.
Material and Methods
Serum specimens
Samples were submitted to the National Reference Laboratories of the Institute of Tropical Medicine “Pedro Kouri”, (IPK) of Havana, Cuba. DENV and ZIKV positive cases were obtained from Arbovirus surveillance established by Public Health Ministry and IPK. DENV sera were collected in a period before 2015, while ZIKV sera were collected from the outbreak presented in our country at 2016. The rest of sample panel, belong to IPK serum bank, were collected before 2015. Ethical statement was obtained from the Ethics Review Committee of IPK Institution.
The sera panel is constituted by 200 samples: a) Group 1: 100 sera collected from ZIKA patients at early convalescent phase of illness (days 5 to 7). Acute phase sera collected of these patients in the first three days of illness were positive by ZIKV RealTime-PCR [8]. ZIKV-positive cases were classified as DENV-immune cases with detectable IgG anti-DENV (IgG ≥ 20) and DENV-naïve cases with no detectable IgG anti-DENV antibodies (IgG < 20), as determined by DENV EIM assay [16,17]. b) Group 2: constituted by sera collected from 30 confirmed DENV infection patients by DENV-PCR [18] and IgM Capture ELISA [19] and classified as 10 DENV-2, 10 DENV-3 and 10 DENV-4 cases. From them, 10 suffered a primary and 20 DENV secondary infection as determined by EIM [17,20]. These sera were collected at days 5 to 7 of fever onset. c) Group 3:constituted by 70 sera collected from 40 healthy blood donors with no detected DENV IgM or IgG antibodies as determined by IgM Capture ELISA and EIM [17,20] and 30 individuals with a positive antibody response to other viral diseases: 8 sera collected two week after vaccination from YF vaccinated individuals and with a positive IgM/IgG anti-YFV antibodies by YF-MAC-ELISA and EIM [21]; 8 sera collected from CHIK confirmed cases (≥ day 5) and with positive IgM anti-CHIKV by MAC-ELISA (PAHO protocol) [22], 7 sera collected from confirmed hepatitis A virus cases (≥ day 5) with positive IgM anti-HAV by MAC-ELISA reference at IPK [23] and 7 sera collected from rubella virus confirmed cases (≥ day 5) by commercial SIEMENS IgM/IgG serology assays [24]. All samples from group 3 were tested by DENV serological references [16-20], showing negative IgM/IgG anti DENV.
Reference Assays
Molecular ReferenceZIKV RealTime-PCR (ZIKV-RT-PCR): Was developed using the second set of primers and probe (primers ZIKV 1086 - ZIKV 1162c, probe ZIKV 1107-FAM) published by Lanciotti and colleagues in 2008 [8].
Serological ReferencesCapture IgM ELISA Assay (MAC-ELISA): MAC-ELISA for the detection of IgM dengue antibody was performed as previously described by Vazquez et al., 2014 [20] . A serum sample was considered positive when the optical density ratio (OD ratio) was ≥2. This value was calculated as P/N where P represents the OD of each serum sample and N represents the mean OD of the negative control wells.
ELISA Inhibition Method (EIM)
EIM for the detection of IgG dengue antibody was performed as previously described by Vazquez et al., for the use of paired sera [17] and monosera [16] . The inhibition percentage was calculated as:
Inhibition%= [1−(OD sample/OD negative control)]×100
The antibody titer of each serum was considered as the highest dilution with a percentage of inhibition ≥50.
A serum with a percentage of inhibition <50 was considered negative for IgG dengue antibodies (<20).
Evaluated Assays: The ZIKV IgM immunoassays (IBL-MAC-ELISA, Euroimmun-ELISA, DiaPro-ELISA, Artron-RT and Mikrogen-Immunoblot) were evaluated using the serum panel of 200 samples. Due to the few availability of IBL-MAC-ELISA kit, was an evaluated using 174 serum sample. Manufacturer`s protocol was followed in each case.
IBL-MAC-ELISA: Zika virus IgM micro-capture ELISA (Ref 30113441; IBL International, Germany) is an enzyme immunoassay for the qualitative determination of IgM class antibodies ZIKV in human serum or plasma. The kit uses an unspecified ZIKV antigen conjugated to horseradish peroxidase. Briefly, in the ZIKV IgM coated microplate, 100 uL standards/controls and diluted samples (1+100) were added into their respective wells, leaving well A1 for the substrate blank (no samples and conjugate). After 1 h incubation at 37°C and three time washers with phosphate-buffered saline 0.2 M, 100 uL of peroxidase labeled ZIKV antigen was added. Plates were incubated at 37°C for 30 min. Washers were repeated as above and 100 uL of TMB substrate solution was dispense into the well, including A1. After 15 min incubation at room temperature (18-25°C) in the dark, the reaction was stopped with 100 uL of 0.2 mol/L sulfuric acid. Optical absorbance was measured using a Microplate Photometer (ChemWell model: 2910, Software 6.4) at 450/620 nm. The cut-off is the mean absorbance value of cut off control determinations. Units (U) were expressed in: sample (mean) absorbance value × 10 /cut-off. U > 11 was considered positive, between 9 and 11 equivocal and <9 negative case.
Euroimmun-ELISA: Anti-Virus Zika IgM ELISA (EI 2668-9601 M; EUROIMMUN, Germany) kit is based on standardized reagents and microtitre plates coated with recombinant ZIKV-NS1. Briefly, sera diluted 1: 101 in sample buffer were added to wells and allowed to react for 1 h at 37 °C. Previously, sera were pre-incubated with sample buffer containing IgG/rheumatoid factor (RF) absorbent to remove class IgM RF from the sample. This step prevents RF-IgM from reacting with specifically bound IgG (leading to false IgM positive results). Bound antibodies were detected by applying goat anti-human IgM peroxidase conjugate for 30min at room temperature, followed by staining with tetramethylbenzidine for 15 min. The enzymatic reaction was stopped by the addition of one volume of 0.5 mol/L sulphuric acid. Colour intensity of the enzymatic reaction was determined photometrically (ChemWell model: 2910, Software 6.4) at 450 nm with reference 620 nm, resulting in extinction values. A signal-to-cut-off ratio (extinctionsample/extinctioncalibrator) was calculated for each sample. Ratio< 0.8 negative cases, between 0.8 and 1.1 equivocal and ≥ 1.1 positive cases.
DiaPro-ELISA: Enzyme ImmunoAssay ZIKV IgM (Ref ZIKVM.CE LOT1116; Diagnostic Bioprobes, Italy) detected qualitative IgM antibodies to ZIKV in human serum and plasma. This kit assay is based on standardized reagents and microtitre plates coated with ZIKV-specific synthetic NS1. Briefly, 50 uL of neutralizing reagent (containing goat anti-IgG) in all wells, leaving well A1 for the substrate blank (no samples and conjugate). Additionally, 100 uL of negative control (in triplicate), single positive control and diluted 1: 101 samples were added. After 1 h at 37°C and 4-5 washing cycles with phosphate-buffered saline 10 mM with 0.05% Tween 20, 100 uL of anti-human IgM polyclonal antibody peroxidase conjugate was added into the wells. After 1 h incubation at 37 °C and similar washing cycles as above, 100 uL of chromogen/substrate were dispense in all wells, including A1 and followed by an incubation period at room temperature for 20 minutes. The reaction was stopped with 100 uL of 0.3M sulphuric acid. Optical absorbance was measured using a Microplate Photometer (ChemWell model: 2910, Software 6.4) at 450/620 nm. Results are expressed as signal to cut-off ratios (S/CO). Ratio <0.9 negative cases, between 0.9 and 1.1 equivocal and >1.1 positive cases.
Artron-RT: Artron One Step ZIKV test (A03-34-322; Artron, Canada) is a qualitative and immunochromatographic assay to the detection of IgG/IgM antibodies to ZIKV in human serum, plasma and whole blood samples. A ZIKV antigen conjugated to a colloidal gold is deposited on the conjugate pad. A unique combination of IgG and IgM antibodies is immobilized on the test zone (line 2 and line 1) of the nitrocellulose membrane, as two individual test lines (IgG line and IgM line) in the test window of the test device. When the sample is added, the gold-antigen conjugate is rehydrate and the ZIK IgG and/or IgM antibodies, if any in the sample, will interact with the gold conjugated antigen. The complex will migrate towards the test window until the test zone, where it will be captured by the relevant anti human IgG and/or IgM, forming a visible pink line, indicating a positive result. If ZIKV antibodies are not present in the sample, no pink line will appear in the test zone, indicating a negative result. After the test is completed, a control line should always appear at the control zone (C). The absence of a pink line in control zone is an indication of an invalid result.
Mikrogen-Immunoblot: RecomLine Tropical Fever IgM immunoblot assay (GARLTF001ES; Mikrogen Diagnostik, Germany) uses recombinant envelope glycoprotein and NS1 antigens. Highly purified recombinant antigens are fixed on nitrocellulose membrane strips. Briefly, test strips are incubated with diluted serum samples and the specific antibodies bind to the pathogen antigens on the strips. Unbound antibodies are then flushed away. In a second step, the strips are incubated with anti-human immunoglobulin antibodies (IgG and/or IgM), which are coupled to horseradish peroxidase. Unbound conjugate antibodies are then flushed away. Specifically bound antibodies are detected with the staining reaction catalyzed by the peroxidase. If an antigen-antibody reaction has taken place, a dark band will appear on the strip at the corresponding point. The reaction control is located under the strip number, and must demonstrate a reaction for each serum sample. If this does not appear the strip is considerate invalid. Cut off control band allows the assessment of the reactivity of each antigen band. Mikrogen assay can defines three different infections (DENV/CHIKV/ZIKV) with the combination of IgM/IgG responses, classifying the cases in: ZIKV positive, Flavivirus positive suspected of ZIKV or DENV infection, Flavivirus positive without differentiation between ZIKV and DENV infection, or CHIKV positive. For this assay we evaluated only the IgM response to ZIKV infection and following an algorithm of positives, negative or invalid test.
Statistical analysis
Sensitivity, specificity and kappa index with 95 % confidence interval were calculated using Epidat version 3.1 (2006, OPS/OMS) in agreement with molecular reference. For the determination of the functional parameters by each commercial test were used the analysis of simple diagnostic tests (sensitivity and specificity), which include a 95% confidence interval. In addition, the analysis of the agreement between the molecular reference and assessed assays, Kappa index (k), was done with similar 95% confidence interval (k<0.40: No acceptable, k 0.40-0.75: Acceptable and k≥ 0.75: Excellent). For IgM detection ratio in cases with or without immunity to DENV and cross-reactivity in samples with infections not related to ZIKV, the comparison of independent proportions analysis was used, determining the significant levels (p) among the different groups of samples (p< 0.05 it is a statistically significant difference, p>0.05 it is not significant difference). Also for tables/pictures EXCEL program was used ( Office 2010).
Results
Results of ZIKV IgM detection by each assays. ELISA commercial tests provided algorithms that resulted in positive, negative, or equivocal cases while immunochromatographic and inmunoblot assays showed positive and negative results. Table 1 showed the IgM detection for each evaluated assays in front to study panel. IBL-MAC-ELISA was the one test that only tested 174 specimens (100 ZIKV positive, 20 DENV positive, 40 healthy donors, 8 YF vaccinated and 6 IgM positive to CHIKV) by insufficient determinations.
IgM detection parameters (sensitivity, specificity and kappa). The specificity of the evaluated systems was higher than 80% while the sensitivity was variable. Figure 1 shows the sensitivity of the ELISA kits: IBL (64.9%, 95% confidence interval: 54.7-75.1), Euroimmun, (15.6%, 95% confidence interval: 7.8-23.4) and DiaPro (87.8 %, 95% confidence interval: 80.8-94.8). Artron-RT and Mikrogen-Immunoblot methods showed sensitivities of 18.0% (95% confidence interval: 9.97-26.03) and 52.0% (95% confidence interval : 41.7-62.3) respectively. Euroimmun (100%, 95% confidence interval: 99.5-100.0) and DiaPro (99.0%, 95% confidence interval: 96.4-100.0) showed the highest specificities, followed by IBL (93.2%, 95% confidence interval: 86.67-99.63) and Mikrogen (93.0%, 95% confidence interval: 87.5-98.5) . Artron showed the lowest specificity (84.0%, 95% confidence interval: 76.3-91.7).
The statistical analysis indicated that Diapro-ELISA was significantly more sensitive than the other assays (p= 0.000) while no differences between IBL and Mikrogen (p= 0.094) and between Euroimmun and Artron assays (p= 0.800) were found. The highest agreement with reference molecular assay was presented by DiaPro-ELISA with a kappa index of 0.87, following by IBL and Mikrogen who showed moderate agreements with kappa values of 0.56 and 0.45 respectively. For Euroimmun and Artron tests, kappa values were poor (kappa: 0.16 and 0.02).
DENV-immune and DENV-naïve IgM detection. The IgM detection in ZIKA confirmed cases exposed (DENV-immune) or not (DENV-naïve) to a previous DENV infection was analysed. The equivocal ZIKV cases were also excluded of this analysis. DiaPro and Euroimmun showed a similar ZIKV IgM detection rate both in DENV immune (87.0 %: 13/15 / 15.7 %: 13/83) and naïve (88.0 %: 73/83/ 15.4 %: 2/13) cases. On the other hand, IBL detected 59.5 %: 47/79 and 93.3 %: 14/15, Mikrogen 45.8 %: 39/85 and 87.0 %: 13/15, and finally, Artron-RT 21.2 %: 18/85 and 0 %: 0/15.
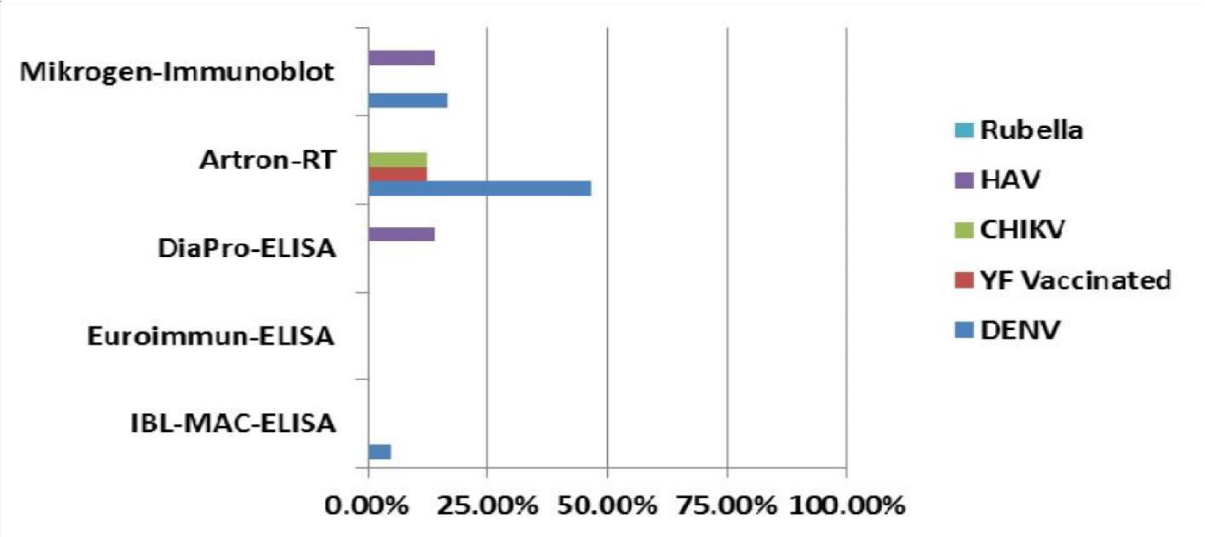
IgM cross-reactivity in non ZIKV specimens. Evaluating the cross-reactive IgM in non ZIKV specimens (Figure 2), we observed the highest reactivity for Artron-RT assay, which presented a 46,7% (14/30) to DENV cases, 12.5% (1/8) to YF vaccinated individual and 12.5% (1/8) to CHIKV cases. Also, According to DENV cases, only in secondary infection cross-reactive IgM was observed. The others four commercial assays had a low reactivity with non ZIKV panel: IBL-MAC-ELISA only showed a 5 % (1/20) to DENV cases, DiaPro a 14.3% (1/7) to HAV cases and Mikrogen a 16.7% (5/30) and 14.3 % (1/7) to DENV (only secondary DENV cases) and HAV cases respectively. Euroimmun-ELISA had not cross-reactive IgM in any negative specimens.
Discussion
Molecular assays for the early and rapid identification of ZIKV RNA have been developed [25], taking into account the low ZIKV viral load and the short period of detectable viraemia [26,27]. The development of commercial immunoassays for serological detection is more challenging due to the high cross-reactivity among the antibodies to flaviviruses [8,11,12].
Here, five commercial serological assays were evaluated in terms of sensitivity and specificity. Of them, DiaPro-ELISA showed the highest value of sensitivity (87.8%) and the best agreement with molecular reference (kappa: 0.87) while Euroimmun ELISA showed the lowest sensitivity (15.6%). Similar results were reported by Pasquier, et al. [28], with highest figure of sensitivity for DiaPro compared to Euroimmun ELISA and IgM detected for longer time. Similarly, L’Huillier, et al. [29] and Granger, et al. [30], reported a low ZIKV IgM sensitivity for Euroimmun assay with figures of (29.8%) and 20.7 % respectively ELISA. In the same way, the sensitivity value of Euroimmun presented by Kikuti, et al. [31], it is comparable to our result (12.5%). In general, specificity was higher than 80% in all tests but indirect ELISA systems presented the highest values (Euroimmun: 100% and DiaPro: 99%), possibly due to the use of specific NS1 antigens. Between them, we found difference in IgM detection. First, we think that conjugate reagents used in both tests are highly specific for the capture of IgM antibodies presented in the serum but the anti-human IgM polyclonal conjugate used as design by DiaPro assay have more opportunity to bind IgM antibodies by several epitopes. Therefore, the number of IgM captured by DiaPro would be more. Second, some studies have been showed the capacity of serum samples antibodies to bind linear peptide sequences and this recognition can be more efficiently if these sequences would be flanked by additional amino acids [32,33] . It would be possible that higher sensitivity of DiaPro could be due to high recognition levels of synthetic NS1 antigen by serum antibodies than recombinant NS1 antigen used by Euroimmun assay.
Respect to MAC-ELISA system (IBL), a sensitivity of 64.9 % and a specificity of 93.2 % were observed. Previous studies demonstrate that this type of system can show higher values of sensitivity and specificity. Safronet, et al. [34], evaluated three MAC-ELISA tests and reported sensitivity values between 57 % to 100 % and specificity values between 60 % -100%. They reported that InBios MAC-ELISA showed comparable results to the CDC-MAC-ELISA [35]. Balmaseda, et al. [36], evaluated two MAC-ELISAs showing sensitivities between 70%-95% and specificities higher than 80%. In the same year, Alison, et al. evaluating three commercially available Zika IgM, found sensitivities between 62.5%-82.8% and similar specificities higher than 80 %. We think that these MAC-ELISA systems would improve in sensitivity parameter working in the ZIKV antigen. Some studies are demonstrated a better specificity recognition of several purified antigens by MAbs and serum antibodies from ZIKV infection cases [13,33,37]. Mishra, et al. [26], working with a highly specific epitope for ZIKV located within the NS2B protein and adapting this NS2B peptide with a biotinylated ELISA system, they obtained excellent sensitivity (96%) and specificity (94%) values from serum of ZIKV infection patients. Also, Rockstroh, et al. [38], using a recombinant protein from mutate highly conserved fusion loop domain of ZIKV envelope (E) protein, found good values of sensitivity (87.5 % ) and specificity (94.2%) measured by ELISA system from ZIKV infection patients.
Artron-RT showed a very low sensitivity. The immunochromatographic tests may improve their sensitivity and specificity by the use of specific antigens and nanotechnology. An study by Bosch, et al. [39], showed an immunochromatographic test that differentiate between the four DENV serotypes and ZIKV, using a pair of specific MAbs to detect NS1 protein and conjugated gold nanoparticles. They do not observe cross-reaction between viral agents and observed values of sensitivity and specificity of 81% and 86% respectively. To develop specific NS1 MAbs they performed linear peptide epitopes. In a recently study, Soren, et al. [40], using ZIKV overlapping oligopeptides by microarray chips found 22 antibody target regions reacted exclusively with one or more ZIKV sera pools, 13 recognized by IgM and 9 by IgG, representing a truly ZIKV-specific region for serological diagnostic.
Mikrogen assay could also be improved in the IgM detection. We observed slight bands in the recognition of IgM by ZIKV NS1 recombinant, bands that were visible but sometimes don’t overcome the intensity of the cut off band, which speak about of lowers IgM detection levels (low recognition) by the used NS1 recombinant protein or manufacturer must readjust cut off detection for insure the positivity of ZIKV cases.
Analyzing the IgM detection in ZIKV cases immune or not to a previous DENV infection, the best results were observed for the indirect ELISA tests, with no differences between them. This result suggest that the system sensitivity is not affected by the presence of antibodies to a previous flavivirus infection as DENV, although in case of Euroimmun ELISA the presented problem was in the sensitivity levels of IgM detection. Contrary, IBL and Mikrogen kits presented the higher IgM detection for ZIKV cases DENV naïve than ZIKV cases DENV immune, which could be due to higher levels of cross-reactive IgG antibodies presented in serum of ZIKV cases DENV immune, representing interference in the recognition between ZIKV IgM antibodies and antigens used in the protocols. Respect to Artron-RT, only detected a 21.2% in ZIKV cases with previous immunity and this suggest that system has a low specificity to ZIKV IgM and high to IgM from previous flavivirus infection.
Some studies have showed evidences about the antibody cross reactivity associated to different serological diagnostic systems. Dejnirattisai, et al. [11], worked a sera panel from patients with DENV secondary infection, found IgM cross reactivity in all cases using a ZIKV IgM capture ELISA. Felix et al., 2017 [41], using three DENV ELISA systems (2 Capture and 1 Indirect) showed IgM cross-reactivity in sera from ZIKV confirmed patients, between 4.9% and 16.4% in samples collected before day 7 of symptom onset and between 13.1% and 37.7% in samples collected after day 14. Steinhagen, et al. 2016 [42], evaluating Euroimmun IgM ELISA with a sera panel that included confirmed DENV, YFV, WNV, JEV and CHIKV cases, observed only a cross-reactivity of 2.9% for WNV samples. Maeki, et al. 2019 [43], found in sera of patients with JEV infection cross reactivity to WNV, DENV and TBEV in IgM and /or IgG ELISA. E Souza, et al. 2019 [44], evaluating three different DENV commercial assays (Focus Diagnostic, Euroimmun and Abbot) against a sera panel with post-yellow fever vaccinated samples found cross reactivity of 3.9% in one of them, Focus Diagnostic.
In the present study, we found a negative cross-reactivity to Flavivirus immunity by indirect ELISAs (Euroimmun and DiaPro), may be by the use of NS1 improved antigen in these serological protocols.
Although DiaPro insert only presented specificity assessment with a panel with normal individuals and blood donors, it wasn’t showed reactivity to Flavivirus cases in our study. For Euroimmun-ELISA, similar to previous studies [10,34], Flavi cross-reactivity was not presented. Mikrogen test had reactivity in five DENV cases (16.7%) but higher IgM responses to recombinant envelope glycoprotein than recombinant NS1 protein were observed. In agree to specificity manufacturer assessment (Mikrogen insert) crossreactivity to other Flavivirus cases was not found. In relation to IBL-MAC-ELISA assay had a low reactivity (5%) to DENV cases, acceptable to ZIKV serological diagnostic. For Artron-RT, the high cross-reactive found to DENV confirmed cases limits its use as serological diagnostic.
In general, the ELISA systems showed the lowest percentages of cross reactivity in the study, although the indirect ELISAs (Euroimmun and DIAPRO) not revealed IgM cross reactivity against samples with serological immunity to other flavivirus (DENV confirmed cases and YF vaccinated individuals). This could be due to the use of highly purified and specific NS1 antigens. It is to know that IgM antibodies elicited to the E proteins of DENV and other flaviviruses are highly cross-reactive with ZIKV E protein, contrary to antibody response against non-structural proteins that is more viruses specific. However, the sensitivity levels for Euroimmun system were the lowest in the study and its usefulness for ZIK serological diagnostic is not good. DIAPRO system showed the best functional parameters from the present study. Respect to IBL assay, we think that the low cross reactivity in DENV samples could be improve, working in the unspecified ZIKV antigen conjugated used in the protocol. Mikrogen system should improve in the IgM cross reactivity in samples with DENV infection.
Conclusions
This evaluation used 200 serum samples to determine the ability of five commercial tests to detect anti-ZIKV IgM in serum samples from confirmed ZIKV infection cases and other with different viral infections that could often co-circulate with it, as DENV. The specificity of evaluated tests was acceptable, although the sensitivity was variable, representing a limitation to the use of some of them in the diagnostic routine practice. An available and sustainable surveillance system for risk individual groups to ZIKV infection, mainly pregnancy woman, it is necessary and serological devices offer a good alternative. DiaPro-ELISA showed the most acceptable assessed parameters for this use, with a sensitivity of 87.8 % and a specificity of 99.0 %. Also, the best agreement with reference molecular standard for IgM detection (kappa: 0.87) was showed. This assay can be recommended as a serological tool in serum samples collected between days 5 and 7 of symptoms onset, identifying a recent infection case of ZIKV. Furthermore, it could be recommender a small evaluation in asymptomatic and symptomatic pregnancy woman samples group because we don’t include this type of specimens in our study. Although, we don’t evaluated IgG detection in the present work, it is important to have in account the ability of IgG detection for serological confirmation. All producers have a protocol for ZIKV IgG detection.
Data availability
All datasets on which the conclusions of the manuscript rely are attached with the manuscript.
Acknowledgements
The authors wish to thank IBL International, Euroimmun Medizinische Labordiagnostika AG, Diagnostic Bioprobes, Artron and Mikrogen Diagnostik for donating the kits for this study. We thank to all authors for their suggestions and reviewing this manuscript.
Funding Information
This research was supported by European Commission [grant number.1801046] and ZikaPlan Research Project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
- ECDC. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. In. Stockholm:: European Centre for Disease Prevention and Control (ECDC); 2015.
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, et al. (2009) Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360: 2536-2543. Link: https://bit.ly/2WBuz2i
- Gould EA, Solomon T (2008) Pathogenic flaviviruses. Lancet 371: 500-509. Link: https://bit.ly/3ftQWiY
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, et al. (2016) Zika Virus: History, Emergence, Biology, and Prospects for Control. Antiviral Res 130: 69-80. Link: https://bit.ly/2WwZywB
- Grossi-Soyster EN, LaBeaud AD (2017) Clinical aspects of Zika virus. Curr Opin Pediatr 29: 102-106. Link: https://bit.ly/3bcmre7
- Wikan N, Smith DR (2016) Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis 16: e119-e126. Link: https://bit.ly/3fsxMtL
- Chua A, Prat I, Nuebling CM, Wood D, Moussy F (2017) Update on Zika Diagnostic Tests and WHO's Related Activities. PLoS Negl Trop Dis 11: e0005269. Link: https://bit.ly/3dnrmKv
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, et al. (2008) Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232-1239. Link: https://bit.ly/2YII9U7
- Charrel RN, Leparc-Goffart I, Pas S, de Lamballerie X, Koopmans M, et al. (2016) Background review for diagnostic test development for Zika virus infection. Bull World Health Organ 94: 574-584D. Link: https://bit.ly/2xLTM1O
- Steinhagen K, Probst C, Radzimski C, Chanasit JS, Emmerich P, et al. (2016) Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill 21. Link: https://bit.ly/35C8IvI
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, et al .(2016) Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 17: 1102-1108. Link: https://go.nature.com/2WbPk5D
- Priyamvada L, Hudson W, Ahmed R, Wrammert J (2017) Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology. Emerg Microbes Infect 6: e33. Link: https://bit.ly/3dihW2S
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, et al. (2016) Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Sci 353: 823-826. Link: https://bit.ly/3dsLFpN
- Castanha PMS, Nascimento EJM, Braga C, Cordeiro MT, de Carvalho OV, et al. (2017) Dengue Virus-Specific Antibodies Enhance Brazilian Zika Virus Infection. J Infect Dis 215: 781-785. Link: https://bit.ly/2YNhhmh
- Granger D, Hilgart H, Misner L, Christensen J, Bistodeau S, et al. (2017) Serologic Testing for Zika Virus: Comparison of Three Zika Virus IgM-Screening Enzyme-Linked Immunosorbent Assays and Initial Laboratory Experiences. J Clin Microbiol 55: 2127-2136. Link: https://bit.ly/3bcnc6X
- Vazquez S, Acosta N, Ruiz D, Calzada N, Alvarez AM, et al. (2009) Immunoglobulin G antibody response in children and adults with acute dengue 3 infection. J Virol Methods 159: 6-9. Link: https://bit.ly/3dse3IX
- Vazquez S, Bravo JR, Perez AB, Guzman MG (1997) Inhibition ELISA. Its utility for classifying a case of dengue. Rev Cubana Med Trop 49: 108-112. Link: https://bit.ly/35EaUTp
- Rosario D, Alvarez M, Diaz J, Contreras R, Rodriguez R, et al. (1998) Polymerase chain reaction for rapid detection and serotyping of dengue virus in clinical samples. Rev Panam Salud Publica 4: 1-5. Link: https://bit.ly/3fywP3h
- Vazquez S, Perez AB, Ruiz D, Rodriguez R, Pupo M, et al. (2005) Serological markers during dengue 3 primary and secondary infections. J Clin Virol 33: 132-137. Link: https://bit.ly/2xFMdcL
- Vazquez S, Lozano C, Perez AB, Castellanos Y, Ruiz D, et al. (2014) Dengue specific immunoglobulins M, A, and E in primary and secondary dengue 4 infected Salvadorian children. J Med Virol 86: 1576-1583. Link: https://bit.ly/2zl2OTW
- Vazquez S, Valdes O, Pupo M, Delgado I, Alvarez M, et al. (2003) MACELISA and ELISA inhibition methods for detection of antibodies after yellow fever vaccination. J Virol Methods 110: 179-184. Link: https://bit.ly/2SMwUXg
- Mardekian SK, Roberts AL (2015) Diagnostic Options and Challenges for Dengue and Chikungunya Viruses. Biomed Res Int 834371. Link: https://bit.ly/2YKYCHx
- Rodriguez-Lay L, Quintana A, Montalvo-Villalba M, Lemos G, Bello M, et al. (2008) Dual Infection With Hepatitis A and E Viruses in Outbreaks and in Sporadic Clinical Cases: Cuba 1998–2003. J Med Virol 80: 798-802. Link: https://bit.ly/3dpB27c
- Marina A, Yermalovich G, Semeiko E, Samoilovich E, Svirchevskaya C, et al. (2014) Etiology of Maculopapular Rash in Measles and Rubella Suspected Patients from Belarus. PLOS ONE 9: e111541. Link: https://bit.ly/2YNlIgV
- Landry ML, St George K (2017) Laboratory Diagnosis of Zika Virus Infection. Arch Pathol Lab Med 141: 60-67. Link: https://bit.ly/2YEULMc
- Fourcade C, Mansuy JM, Dutertre M, Delpech M, Marchou B, et al. (2016) Viral load kinetics of Zika virus in plasma, urine and saliva in a couple returning from Martinique, French West Indies. J Clin Virol 82: 1-4. Link: https://bit.ly/2YKYesz
- Pessoa R, Patriota JV, de Souza Mde L, Abd El Wahed A, Sanabani SS (2016) Detection of Zika virus in Brazilian patients during the first five days of infection - urine versus plasma. Euro Surveill 21. Link: https://bit.ly/3drXn4m
- Pasquier C, Joguet G, Mengelle C, Chapuy-Regaud S, Pavili Lynda PN, et al. (2017) Kinetics of anti-ZIKV antibodies after zika infection using two commercial enzyme-linked immunoassays. Diagn Microbiol Infect Dis S0732-8893 30275-4.
- L'Huillier AG, Hamid-Allie A, Kristjanson E, Papageorgiou L, Hung S, et al. (2017) Evaluation of Euroimmun Anti-Zika Virus IgM and IgG Enzyme-Linked Immunosorbent Assays for Zika Virus Serologic Testing. J Clin Microbiol 55: 2462-2471. Link: https://bit.ly/2yzUteT
- Granger D, Leo YS, Lee LK, Theel ES (2017) Serodiagnosis of dengue virus infection using commercially available antibody and NS1 antigen ELISAs. Diagn Microbiol Infect Dis 88: 120-124. Link: https://bit.ly/3co5nms
- Kikuti M, Tauro LB, Moreira PSS, Campos GS, Paploski IAD, et al. (2018) Diagnostic performance of commercial IgM and IgG enzyme-linked immunoassays (ELISAs) for diagnosis of Zika virus infection. Virol J 15: 108. Link: https://bit.ly/2xHSW6b
- Buus S, Rockberg J, Forsström B, Nilsson P, Uhlen M, et al. (2012) High-resolution mapping of linear antibody epitopes using ultrahigh-density peptide microarrays. Mol Cell Proteomics 11: 1790-1800. Link: https://bit.ly/35IjmBo
- Mishra N, Caciula A, Price A, Thakkar R, Ng J, et al. (2018) Diagnosis of Zika Virus Infection by Peptide Array and Enzyme-Linked Immunosorbent Assay. MBio 9. Link: https://bit.ly/2yrIQqx
- Safronet D, Sloan A, Stein D, Mendoza E, Barairo N, et al. (2017) Evaluation of 5 Commercially Available Zika Virus Immunoassays. Emerg Infect Dis 23: 1577-1580. Link: https://bit.ly/2W8q3JI
- Lindsey NP, Staples JE, Powell K, Rabe IB, Fischer M, et al. (2018) Ability To Serologically Confirm Recent Zika Virus Infection in Areas with Varying Past Incidence of Dengue Virus Infection in the United States and U.S. Territories in 2016. J Clin Microbiol 56. Link: https://bit.ly/2LbocNK
- Balmaseda A, Zambrana JV, Collado D, Garcia N, Saborio S, et al. (2018) Comparison of Four Serological Methods and Two Reverse Transcription-PCR Assays for Diagnosis and Surveillance of Zika Virus Infection. J Clin Microbiol 56 pii: e01785-17. Link: https://bit.ly/2YHC5LV
- Cleton NB, Godeke GJ, Reimerink J, Beersma MF, Doorn HR, et al. (2015) Spot the difference-development of a syndrome based protein microarray for specific serological detection of multiple flavivirus infections in travelers. PLoS Negl Trop Dis 9: e0003580. Link: https://bit.ly/2Wx2vNK
- Rockstroh A, Moges B, Barzon L, Sinigaglia A, Palu G, et al.(2017) Specific detection of dengue and Zika virus antibodies using envelope proteins with mutations in the conserved fusion loop. Emerg Microbes Infect 6: e99. Link: https://bit.ly/2Wff9lD
- Bosch I, de Puig H, Hiley M, Carre-Camps M, Perdomo-Celis F, et al. (2017) Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci Transl Med 9. Link: https://bit.ly/2La5JBr
- Hansen S, Hotop SK, Faye O, Ndiaye O, Böhlken-Fascher S, et al. (2019) Diagnosing Zika virus infection against a background of other flaviviruses: Studies in high resolution serological analysis Sci Rep 9: 3648. Link: https://bit.ly/2WzmYBp
- Clara Felix A, Santiago Souza NC, Figueiredo WM, Costa AA, Inenami M, et al. (2017) Cross reactivity of commercial anti-dengue immunoassays in patients with acute Zika virus infection. J Med Virol 89: 1477-1479. Link: https://bit.ly/35EF8pq
- Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, et al. (2016) Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill 21. Link: https://bit.ly/3dsO9V9
- Maeki T, Tajima S, Ikeda M, Kato F, Taniguchi S, et al. (2019) Analysis of cross-reactivity between flaviviruses with sera of patients with Japanese encephalitis showed the importance of neutralization tests for the diagnosis of Japanese encephalitis. J Infect Chemother 1-5. Link: https://bit.ly/2SIpS5z
- Souza NCSE, Félix A, de Paulaa A, Levia J, Pannutia C, et al. (2019) Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int J Infect Dis 81: 4-5. Link: https://bit.ly/2Wx1BRm
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
