Open Journal of Tropical Medicine
Mu-opioid receptor expression in multibacillary leprosy
Patrícia Elizabeth Pignataro1,2*, Leonardo Pereira Quintella2, Helen Ferreira1, Adelaide Lopes Amorim1, Roberta Olmo Pinheiro1, Luiz Claudio Ferreira2, Francisco das Chagas de Carvalho Rodrigues2 and Maria Inês Fernandes Pimentel2
2Evandro Chagas National Institute of Infectious Diseases, Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil
Cite this as
Pignataro PE, Quintella LP, Ferreira H, Amorim AL, Pinheiro RO, et al. (2019) Mu - opioid receptor expression in multibacillary leprosy. Open J Trop Med 3(1): 014-019. DOI: 10.17352/ojtm.000010Background: During the clinical course of multibacillary leprosy disease, mainly in Lepromatous Leprosy (LL) patients, acute and systemic inflammatory episodes known as Erythema Nodosum Leprosum (ENL) may be present. The histology of ENL skin lesions initially shows hyperplasia of epidermis followed by a neutrophilic inflammatory process. Sensory neurons and keratinocyte communicate in paracrine way via molecules such as neuropeptides, cytokines, and opioids. Previous studies demonstrated that opioids might cause immunosuppression through Mu Opioid Receptor (MOR).
Objectives: To verify the expression of MOR in leprosy multibacillary patients with and without ENL.
Methods: Cutaneous lesions from fifteen patients with LL with no reaction and 18 patients with ENL from Oswaldo Cruz Foundation, Brazil, were studied. Immunohistochemistry reaction with anti-MOR antibody was performed in paraffin embedded skin lesions biopsy.
Results: Forty-six percent of LL patients without reactional episodes presented positivity for MOR in keratinocytes while in the ENL group 88.9% presented strong staining in epidermis (p=0.02), In lepromatous lesions, almost all macrophages were positive for MOR, in contrast to the rare positivity in ENL macrophages.
Main Conclusions: The data presented here suggest the involvement of MOR in the immunomodulation of ENL.
Introduction
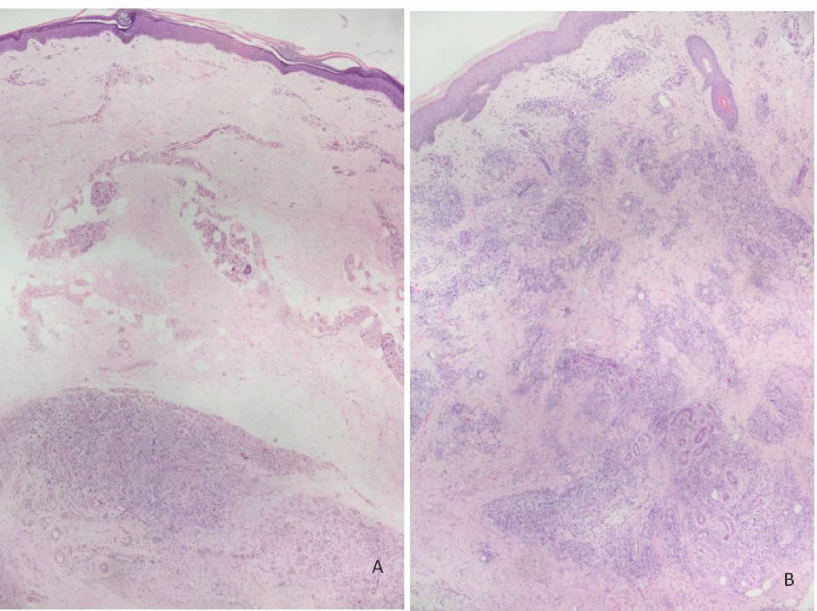
Leprosy is an infectious disease predominantly of skin and peripheral nerves, caused by Mycobacterium leprae. Erythema nodosum leprosum (ENL, type 2 reaction) is a painful inflammatory condition occurring in around 50% Lepromatous Leprosy (LL) and 5% to 10% of Borderline Lepromatous (BL) patients, affecting 13.7% of leprosy patients in hospital-based studies [1]. ENL in the course of the disease occurs particularly in those patients with a bacterial index above 4+ [1]. The histopathological aspects of LL are an atrophic epidermis with a grenz zone of collagen without cells in the superficial dermis between the infiltrate and the epidermis. In the dermis, the infiltrate is composed by macrophages with vacuoles in variable amount and size, multinucleated giant cells, rare lymphocytes and numerous plasma cells is predominantly perivascular, periadnexal and perineural without significant infiltration in perineurium. In ENL reaction, the epidermis becomes hyperplasic, and the grenz zone disappears. The infiltrate is prominently neutrophilic, mainly in the deep layers of the dermis and the subcutaneous tissue among new and pre lepromatous macrophage infiltrate, with a higher percentage of lymphocytes than before. Endothelial swelling, edema and vascular congestion are observed. Micro abscesses can occasionally be observed mainly in deep dermis where lymphocytes and macrophages are newly recruited and spread to the surrounding subcutaneous tissue [2].
Previous studies demonstrated that in lepromatous lesions there is a predominance of type 2 macrophages expressing CD163 and interleukin (IL)-10 [3,4]. Elevated serum levels of cytokines as IL-6, IL-10, IL-12, Tumor Necrosis Factor (TNF) and other immunological mediators have been associated with episodes of ENL [5].
Primary sensory neurons, Schwann cells and immune cells act in coordinated patterns and cytokines and chemokines are involved in their signaling pathways [6]. Schwann cells, active resident and infiltrating macrophages, neutrophil granulocytes, and mast cells release prostaglandins and several pro inflammatory cytokines-including IL-1β, IL-6, IL-12, interferon and TNF [6,7]. Skin cells and sensory neurons intercommunicate in autocrine and paracrine ways. There are close functional interactions between skin cells, particularly keratinocytes and neurons, as demonstrated by several molecules (neuropeptides, neurohormones, neurotrophins and their respective receptors) expressed in both skin and neuronal cells [7]. Expression of the Mu-Opioid Receptor (MOR) can be modulated by various cytokines, including IL-1β, IL-4, IL-6 and TNF [8].
Leukocyte chemotaxis, vascular hemodynamics and immune response are directly influenced by neuronal factors released from nociceptor sensory neurons. Noxious stimuli activate afferent signals in sensory nerves, leading to the generation of antidromic axon reflexes and inducing the release of neuropeptides at the peripheral terminals of the neurons. These molecular mediators have several inflammatory actions such as chemotaxis and activation of neutrophils, attraction of macrophages and lymphocytes to the site of injury, and degranulation of mast cells. Neuropeptides such as CGRP can induce dendritic cells toward a T-helper (Th) 2 immunity and reduce Th1 immunity through the production of certain cytokines and inhibition of others, as well as by reduction or increase of dendritic cell migration to local lymph nodes [11].
The opioid peptide endomorphin-2 has immunological effects on the production of cytokines related to inflammation, in Th1 / Th2 balance and in functions related to the innate immune system in macrophages in the murine model. Endomorphin-2 inhibits TNF, IL-10, and IL-12 productions, however, it potentiates IL-1β production by macrophages [12]. These results suggest that endomorphin-2 may modify macrophage functions such as cytokine productions as well as functions related to the innate immune system.
MOR activation results in the enhancement in the activity and also the DNA-binding activity of the transcription factor Nuclear Factor-κB (NF-κB) in primary neurons [13]. NF-κB is responsible for the transcription of inflammatory and anti-inflammatory cytokines in vertebrates.
Cells of the innate immune system respond to NGF through the activation of a variety of responses. NGF activates chemotaxis and enhances the phagocytosis of neutrophils and macrophages, stimulates the cytotoxic activity of eosinophils and induces the degranulation of mast cells [14]. The opioid system is related to MOR and also to NGF and it is implicated in immune and inflammatory reaction, with different responses.
The exact triggers leading to ENL reaction remains a challenging enigma in leprosy immunology. As long as neuroreceptors can lead to the release of neuropeptides by antidromic axon reflex as consequence of Schwann cell inflammatory activity, we postulate that epidermis may be a supporting role in the amplification of the inflammatory process, after vasodilatation and mast cell degranulation [9]. Keratinocytes can interact with infiltrating inflammatory cells, either by release of pro-inflammatory cytokines or via intercellular adhesion reactions [7].
Almost all cutaneous cells express functional receptors for neuropeptides. Skin cells produce neuropeptides and neurotrophins, which in turn stimulate nerve fibers [9]. The expression of MOR can be modulated by various cytokines and by endorphins and previous studies have demonstrated that immune cells can secrete β-endorphins [15]. In addition, immunohistochemical studies in dermatological diseases like psoriasis and rosacea have demonstrated an altered expression of various neuropeptides and of their receptors, as well as marked proliferation of cutaneous nerve in the skin [9]. Cutaneous and autonomic dysfunctions in leprosy are associated with changes in nerve fibers and neuropeptides [16].
Here, in order to evaluate neuroinflammation in the skin, we evaluated the expression of MOR in skin cells from lepromatous patients, known as presenting a low inflammatory profile, and compared to ENL patients, whose lesions display a highest inflammatory profile. Once the nociceptor is inside epidermis, it could shed neuropeptides through antidromic reflex originating pain and starting the inflammatory process from inflamed nerve bundles affected by mycobacteria.
Materials and methods
Study population
Series of 26 patients clinically classified as LL with or without ENL were selected according to the fragments of skin lesions obtained through biopsy at Souza Araújo Outpatient Unit, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil before the start of the leprosy and / or reactional treatment (Table 1).
Histopathology
The histopathology of the cutaneous lesions of non-reactional patients (LL) and those with ENL were evaluated through hematoxylin and eosin (H&E) and Wade staining according to the Ridley & Jopling classification [17].
Immunohistochemistry
Immunohistochemistry was performed in formalin fixed, paraffin embedded tissues, with anti-MOR antibody (AB1580-I Merck Millipore-Sigma-Aldrich, Burlington, Massachussets, USA, batch number #3046772) diluted 1: 4,000.
Tissue samples were deparaffinized in xylene and hydrated in decreasing alcohol series. Antigen retrieval was performed in EDTA, pH 8.0, for 30 min at 98ºC and detected by phosphatase alkaline kit (GBI Labs, Bothel, Washington, USA) with permanent red according protocol [18]. The sections were counterstained with hematoxylin for 1 min, dehydrated in increasing alcohol series, and cleared in xylene. Immunohistochemistry staining was analyzed as positive or negative in epidermis and semi quantitative analysis of dermal macrophages was performed (0 to 3+). Fisher’s exact test was considered at a level of significance of 5%.
Ethical approval
The study was approved by the Research Ethics Committee of the Evandro Chagas National Institute of Infectious Diseases, Oswaldo Cruz Foundation (certificate number CAAE 61115716.5.0000.5262).
Results
We selected 8 females and 18 male leprosy multibacillary patients, with a median age of 40 years-old (minimum 15; maximum 63). In the reactional group, 10 patients presented ENL before, at the first biopsy; 3 patients during; and 5 patients after multidrug therapy (MDT).
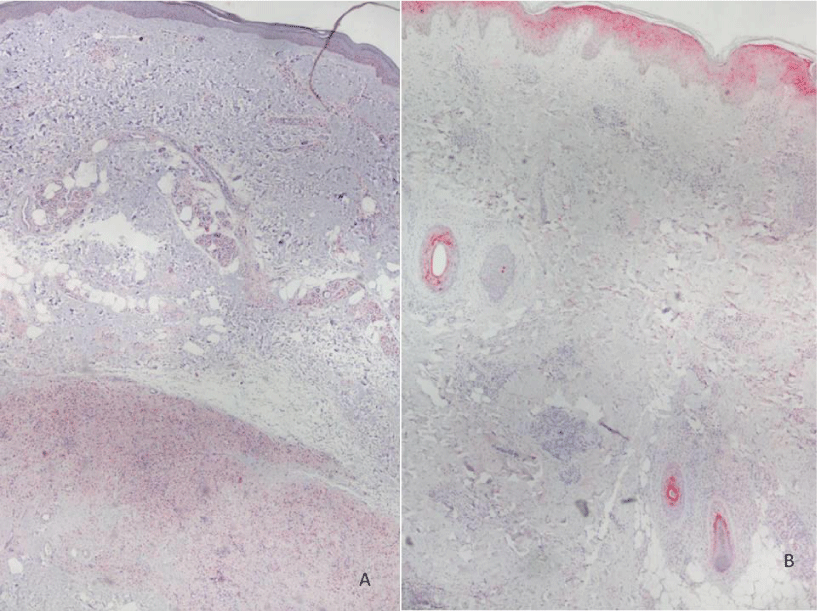
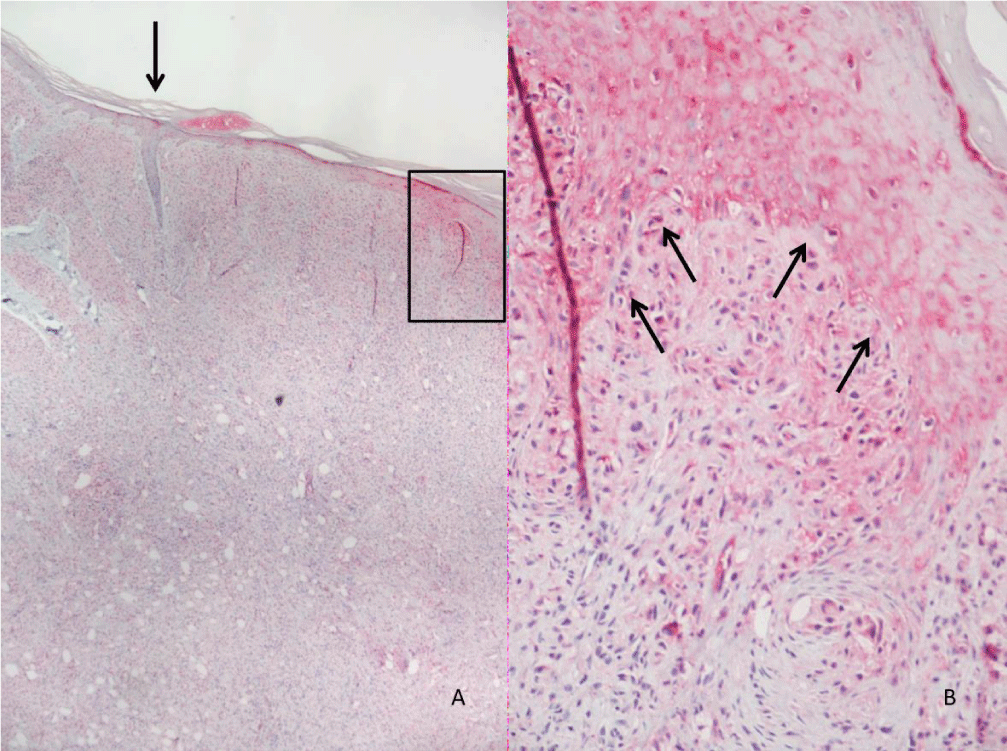
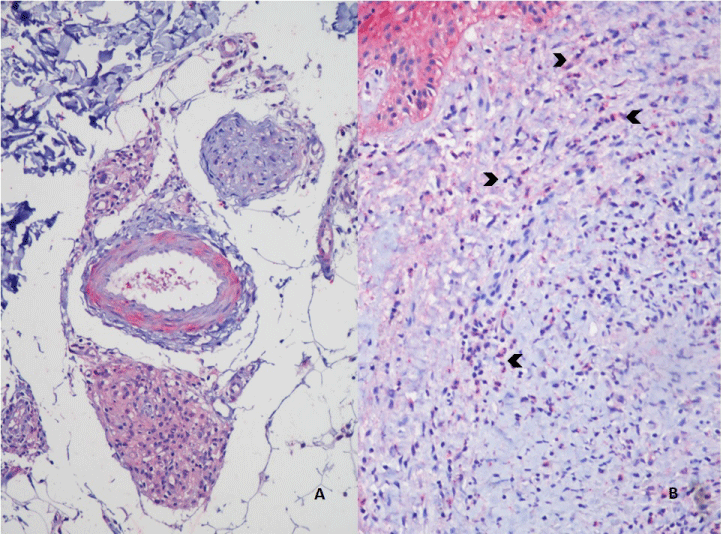
Histopathological evaluation is shown in Figure 1. Analysis of MOR expression in skin cells through immunohistochemistry revealed that in the LL group, a positive stain of 1+ to 3+ was observed in macrophages (Figures 2-5). Forty-six percent of the patients without reactional episodes presented positivity in the epidermis (Figures 2-4). Figure 3 shows a grading transition in the positivity throughout the epidermis in a LL patient.
In the ENL group, 88.9% presented strong staining in epidermis and weak staining in most macrophages (Figures 2,4-5). Among 7 ENL patients in which we evaluated their previous LL biopsy, the MOR staining revealed that three patients that did not present MOR staining in epidermis in the pre-reactional period became positive during ENL diagnosis. Analysis of MOR expression in the epidermis revealed a significant difference between the LL and ENL groups (Figure 2, p=0,02).
Discussion
We evaluated the expression of MOR in skin lesion cells from both non-reactional and reactional lepromatous leprosy patients. Epidermal cells from LL patients presented lower percentages of staining for MOR comparing to the ENL patients. These data suggest that neural activity from epidermal neuroreceptors, probably through antidromic axon reflexes, produces the release of neuropeptides at the peripheral terminals of the neurons, possibly exacerbating inflammatory responses. This hypothesis is suggested by the demonstration of a transition between atrophy of epidermis without MOR staining, and the epidermis with hyperplasia and MOR staining in the skin lesion of one of the LL patients. This aspect could be interpreted as indirectly showing the in situ release of neuropeptides by neurogenic inflammation activity.
Only few LL patients demonstrated MOR positivity in epidermis. We hypothesize that the positivity for MOR at epidermis of LL was a subclinical reactional episode. In most of them there was association with edema or paresthesia on limbs, possibly reflecting vascular neuropathy by commitment of autonomous nervous system by neurogenic inflammation. While in ENL patients presented strong positivity in the epidermis, probably due to the release of neuropeptides by nociceptors.
Usually, type 2 macrophages, which secrete endorphins, are more conspicuous in LL lesions. Therefore, it cannot be established with certainty if there was a switch of the phenotype of the macrophages, but the intensity of MOR expression is suggestive of a changing of the metabolic profile or at least a switch of the cytokine pattern in ENL as compared to LL without clinical reaction. It is possible that during ENL newly recruited macrophages spread in dermis and subcutaneous, with no pre-formed detectable MOR.
TNF in normal skin is pre-formed and stored in dermal mast cells and, following stimulation, may be produced by a variety of cutaneous cells, including keratinocytes, dermal dendritic cells and Langerhans cells. TNF is a potent inflammatory cytokine and has long been considered to be a key mediator in the local and systemic inflammatory symptoms observed during leprosy reactions [3].
Cutaneous primary sensory afferents release SP and CGRP in the epidermis. These neuropeptides then diffuse through the interstitial space to bind to their receptors on the keratinocyte cell surface. Keratinocyte neurokinin 1 receptor (NK1) stimulates cellular proliferation, SP expression and secretion of CGRP, that up-regulate TNF, IL-6, and NGF expression and secretion. Keratinocyte-secreted inflammatory cytokines and NGF can induce inflammatory mediators that can activate various cellular components of the innate and adaptive immune systems, thus supporting the development of inflammatory skin diseases [8].
It has been reported that the MOR gene transcription in various immune cells, including human T lymphocytes, leukocytes, and mature dendritic cells, induce the proinflammatory cytokine TNF. NF-κB was identified to be responsible for TNF-induced MOR gene expression on the human MOR gene promoter [8]. However, MOR seems not to be directly responsible for the switch of the profile of the immune cells. NF-κB-dependent genes are critical for the opioid-induced biological responses of neuronal, keratinocyte and immune cells. MOR gene promoter may not be sequentially activated by NF-κB, but it seems that the opioid system and NF-κB are connected to NGF activation [8].
Cytokines such as IL-1β, TNF and IL-6, involved in inflammation, are promoters of NGF synthesis in a variety of cell types as neurons, Schwann cells, mast cells, and keratinocytes. Inflammatory cytokines can induce the synthesis of NGF in neuronal and glial as well as in epithelial and endothelial cells. On the other hand, NGF induces MOR production [19].
The opioid system has been studied along with immune cells but with controversial in vitro results, perhaps depending on the opioid concentration. Our results suggest that MOR appears mainly in exacerbated inflammatory conditions (ENL), probably generated by neuroreceptors in epidermis due to antidromic activity. It may cause endothelial stimulus and trigger the inflammatory process.
A possible explanation for the demonstration that MOR is more intensely found in ENL than in LL lesions of patients without reaction may be a higher level of NGF due to the damage of fibers during the reactional process. Moreover, the low expression of MOR in the epidermis of the lesions of LL patients without reaction episodes may reflect a high concentration of M. leprae in Schwann cells that somehow suppress NF-κB activation through downregulation of the inflammatory process [20].
Neurochemical and physiological alterations in the skin are usually accompanied by activation of dorsal root ganglion (DRG) neuron and glial cells. Agonists as β-endorphin and encephalin reduce the excitability of peripheral nociceptor terminals and inhibit the peripheral release of vasoactive peptides from these terminals [21]. The peripheral action of MOR agonists is greatly increased in inflamed tissues [22]. This is due in part to the stimulation of MOR synthesis in the dorsal root ganglia by cytokines and their transport to peripheral terminals. In addition, immune cells, guided to the inflamed region by cell adhesion molecules in inflammatory cells, endothelial cells and keratinocytes are stimulated by local cytokines to secrete endogenous opioid peptides that bind to MOR [23,24].
The authors report immunohistochemistry studies in order to verify the expression of MOR in leprosy multibacillary patients with and without Erythema Nodosum Leprosum (ENL). Data suggest that keratinocytes with nociceptors participate in the ENL episodes, and the opioid system (indirectly shown through MOR demonstration) may act as modulator of neuroinflammation, integrated with nociceptors, Schwann cell, keratinocytes, and endothelial cells probably by the release of neuropeptides, TNF and NGF in an orchestrated way.
We would like to thank the leprosy pathology laboratory team for the aid in the immunohistochemical staining.
Sponsorship
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior, Brasil (CAPES), through finance code 001.
- Polycarpou A, Walker SL, Lockwood DN (2017) A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol 8: 233. Link: http://bit.ly/2QVAAUV
- Cuevas J, Rodríguez-Peralto JL, Carrillo R, Contreras F (2007) Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg 26: 126-130. Link: http://bit.ly/2FrES0J
- Moura DF, de Mattos KA, Amadeu TP, Andrade PR, Sales JS, et al. (2012) CD163 favors Mycobacterium leprae survival and persistence by promoting anti-inflammatory pathways in lepromatous macrophages. Eur J Immunol 42: 2925-2936. Link: http://bit.ly/2SYnhFD
- Fachin LR, Soares CT, Belone AF, Trombone AP, Rosa PS, et al. (2017) Immunohistochemical assessment of cell populations in leprosy-spectrum lesions and reactional forms. Histol Histopathol 32: 385-396. Link: http://bit.ly/2N14Lsw
- Moraes MO, Sarno EN, Almeida AS, Saraiva BC, Nery JA, et al. (1999) Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and interleukin-12 in reactions (RR and ENL). Scand J Immunol 50: 541-549. Link: http://bit.ly/36vwAkt
- Moalem G, Tracey DJ (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 51: 240-264. Link: http://bit.ly/2Qsfqyt
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M (2006) Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86: 1309-1379. Link: http://bit.ly/2ZV9GAB
- Kraus J (2009) Regulation of mu-opioid receptors by cytokines. Front Biosci 1: 164-170. Link: http://bit.ly/2QsUWWu
- Stricker (2018) Mikroskopische Studien iiber Wachstum und Wechsel der Haai'e,. In: Ebner V (ed) manual of human embriology 24. Sitz. Her. d. K. Akad. d. Wiss., Wien. Cyted by: Choi JE, di Nardo A. Skin neurogenic inflammation. Semin Immunopathol 40: 249-259.
- Bigliardi-Qi M, Sumanovski LT, Büchner S, Rufli T, Bigliardi PL (2004) Mu-opiate receptor and beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology 209: 183-189. Link: http://bit.ly/2QQngB3
- Chiu IM, von Hehn CA, Woolf CJ (2012) Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15: 1063-1037. Link: http://bit.ly/2sSOJdo
- Azuma Y, Ohura K (2002) Endomorphins 1 and 2 inhibit IL-10 and IL-12 production and innate immune functions, and potentiate NK-kappaB DNA binding in THP-1 differentiate to macrophage-like cells. Scand J Immunol 56: 260-269. Link: http://bit.ly/2sJHjt1
- Hou YN, Vlaskovska M, Cebers G, Kasakov L, Liljequist S, et al. (1996) A mu-receptor opioid agonist induces AP-1 and NF-kappa B transcription factor activity in primary cultures of rat cortical neurons. Neurosci Lett 212: 159-162. Link: http://bit.ly/2QxcnVY
- Minnone G, de Benedetti F, Bracci-Laudiero L (2017) NGF and its receptors in the regularion of inflammatory response. Int J Mol Sci 18: pii: E1028. Link: http://bit.ly/36qGa89
- Pannell M, Labuz D, Celik MÖ, Keye J, Batra A, et al (2016) Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation 13: 262. Link: http://bit.ly/2MZnAwk
- Karanth SS, Springall DR, Lucas S, Levy D, Ashby P, et al. (1989) Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J Pathol 157: 15-26. Link: http://bit.ly/2sJIuZv
- Ridley DS, Jopling WH (1966) Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34: 255-273. Link: http://bit.ly/36Hky7q
- The IHC-plus™ Immunohistochemistry Protocol. LSBio. Link: http://bit.ly/2sWu8VB
- Mousa SA, Cheppudira BP, Shaqura M, Fischer O, Hofmann J, et al. (2007) Nerve growth factor governs the enhanced ability of opioids to suppress inflammatory pain. Brain 130: 502-513. Link: http://bit.ly/2tAycKY
- Pereira RM, Calegari-Silva TC, Hernandez MO, Saliba AM, Redner P, et al. (2005) Mycobacterium leprae induce NF-kappaB-dependent transcription repression in human Schwann cells. Bioche Biophys Res Commun 335: 20-26. Link: http://bit.ly/37FTc1O
- Fields HL (2011) Mu opioid receptor mediated analgesia and reward. In: The opiate receptors. Gavril W. Pasternak, editor. New York Dordrecht Heidelberg London: Humana Press Springer 239-264. Link: http://bit.ly/2SWyRBb
- Zhou L, Zhang Q, Stein C, Schafer M (1998) Contribution of opioid receptors on primary afferent versus sympathetic neurons to peripheral opioid analgesia. J Pharmacol Exp Ther 286: 1000-1006. Link: http://bit.ly/2QylmWV
- Smith EM (2003) Opioid peptides in immune cells. Adv Exp Med Biol 521: 51-68. Link: http://bit.ly/2NhhW91
- Stein C, Schafer M, Machelska H (2003) Attacking pain at its source: new perspectives on opioids. Nat Med 9:1003-1008. Link: http://bit.ly/39ITxCE
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
