Open Journal of Tropical Medicine
Study of the µ opioid receptor in cutaneous ulcers of leishmaniasis and sporotrichosis according to the complaints of local pain
Patrícia Elizabeth Pignataro1,2*, Leonardo Pereira Quintella2, Luiz Cláudio Ferreira2, Francisco das Chagas de Carvalho Rodrigues2, Liliane de Fátima Antonio Oliveira2, Marcelo Rosandiski Lyra2 and Maria Inês Fernandes Pimentel2
2Evandro Chagas National Institute of Infectious Diseases, Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil
Cite this as
Pignataro PE, Quintella LP, Ferreira LC, de Carvalho Rodrigues FDC, Antonio Oliveira LDF, et al. (2019) Study of the µ opioid receptor in cutaneous ulcers of leishmaniasis and sporotrichosis according to the complaints of local pain. Open J Trop Med 3(1): 007-013. DOI: 10.17352/ojtm.000009Patients with cutaneous leishmaniasis or sporotrichosis with ulcerated lesions may present similar epidemiological and clinical characteristics. Local pain is often referred to in the sporotrichosis lesions, but not in cutaneous leishmaniasis. The µ Opioid Receptor (MOR) is indirectly associated to the production of cytokines, and is related to the epidermal proliferation. The aim of this study was to evaluate MOR expression and the histopathological changes in cutaneous lesions of sporotrichosis and leishmaniasis, and its association with complaints of local pain. Thirty-eight outpatients with sporotrichosis (19), leishmaniasis (14) and unspecific ulcers (5) treated at Rio de Janeiro, Brazil, were submitted to histological and immunohistochemically analysis for MOR according to the complaints of local pain. No association was found among the expression of MOR; the cutaneous histopathological changes of cellular composition of the inflammatory infiltrate tissue, of keratinocyte, of endothelial cells and of nerves; and the presence or absence of local pain in the cutaneous lesions of leishmaniasis, sporotrichosis or unspecific ulcers. A plausible explanation is that nociception is regulated by neurons of the dorsal root ganglion or by descending modulation through noradrenergic inhibitory neurons from central nervous system.
Introduction
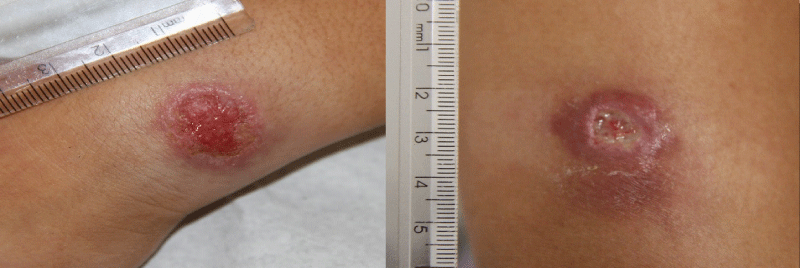
American cutaneous leishmaniasis and sporotrichosis are endemic in the state of Rio de Janeiro, Brazil, with overlapping geographical areas. Both diseases present with similar clinical aspects, usually ulcers in the site of the inoculation of the infectious agent, and it is necessary to establish differential diagnosis between them. One of the distinctive clinical conditions is the presence or absence of local pain, being leishmaniasis ulcers generally painless [1]. On the other hand, sporotrichosis lesions are frequently necrotic painful ulcers with or without lymphatic involvement (Figure 1). Both diseases share similar histopathological aspects [2].
Leishmania (Viannia) braziliensis, the almost exclusive species causing cutaneous leishmaniasis in the state of Rio de Janeiro, Brazil [3], is a parasite with intracellular growth. In leishmaniasis, a few weeks post infection, a nodular lesion develops and displays acute inflammatory response (containing neutrophils, macrophages, and eosinophils). This inflammatory reaction is accompanied by a reduction in parasite numbers in the dermis. Macrophages are the major inflammatory cells at the infection site. However, neutrophils, T and B lymphocytic cells, and Langerhans cells are also present and necrotic areas containing apoptotic neutrophils are observed in the dermis. Keratinocytes proliferate in the granular layer of epidermis with hyperplasia. Following the vigorous inflammatory response caused by macrophages, dermal necrosis develops and the lesion becomes ulcerative. At this stage, CD4+ and CD8+ T cells predominate in the infiltrate and there is an increase in the production of interleukin (IL)-10, Tumor Necrosis Factor (TNF)-α and interferon (IFN)-γ as well as transformation of macrophages from type 1 to type 2. There are variable numbers of parasites, according to the species, and IL-4, IL-10, and Transforming Growth Factor (TGF)-β production increases in non-healing lesions [4,5].
Sporotrichosis in the state of Rio de Janeiro is mainly caused by Sporothrix brasiliensis [6]. In sporotrichosis, dermis presents at an early stage inflammation with infiltration of neutrophils, plasma cells, and lymphocytes that may or may not be intense. Gradually, the usual ulcerated cutaneous lesion typically exhibits a granulomatous dermatitis surrounding a suppurative abscess, with the presence of a central zone composed of neutrophils and a few eosinophils, and an outer zone of lymphocytes and plasma cells. At a later stage, granulomas mainly consist of epithelioid cells. Small abscesses may be seen within granulomas. The histopathological findings are generally nonspecific and variable in different stages of the disease. The histopathological pattern is usually a combination of pyogenic and granulomatous reaction and may display epidermal hyperplasia, papillomatous acanthosis, hyperkeratosis, and intraepidermal microabscesses [7,8].
Experimental immunologic studies in the cutaneous lesions of leishmaniasis and sporotrichosis also show similar in situ profiles with high levels of activated type 2 macrophages and production of IL-4 and IL-10 [9].
Inflammatory mediators are released and tissue acidification activates nociceptive primary afferent neurons that stimulate the sensation of pain causing hyperalgesia [10]. Immunocytes are recruited and release interleukins performing their functions in the process of healing the cutaneous lesion in an orchestrated way. The cytokine cascade results in the activation of COX-2 dependent prostanoid and in the release of catecholamine from sympathetic fibers [11]. Cytokines such as IL-1β, IL-6, TNF-α and IL-8 are related to the pain threshold. On the other hand, opioid peptides render nociceptors less sensitive to excitation and thus inhibit the action of multiple excitatory mediators. Opioid peptides do not bind exclusively to one unique opioid receptor, but instead exhibit affinity for various opioid receptors including μ-, ∂- and κ-opioid receptors [10,12]. Endogenous opioids as endorphins and encephalin act primarily on μ and ∂ opioid receptors. They are synthesized in vivo in order to modulate pain mechanisms and inflammatory pathways, and mediate analgesia in response to painful stimuli by binding to opioid receptors on sensitive cutaneous nerves. Opioids produced by cells of the immune system and keratinocytes are capable of exerting additional effects, such as immunomodulation in cutaneous inflammation. β-endorphin is present in macrophages, monocytes, granulocytes, and lymphocytes, in secretory granules arranged at the cell periphery, ready for exocytose. During the early stages of inflammation, as the leukocytes migrate to the site of infection, they (along with the resident cells) secrete various chemokines such as IL-1, IL-6, IL-8, which lead to hyperalgesia. In the late inflammation stage, macrophages and lymphocytes secrete IL-4, IL-10 and IL-13 inhibiting the hyperalgesic pathways leading to hypoalgesia [13]. Pain perception depends upon the activation of specialized peripheral neurons called Primary Afferent Nociceptors (PANs) from primary afferent fibers (Aβ-, Aδ-, and C-fibers) [14].
The peripheral anti-nociceptive action of μ-opioid receptors (MOR) agonists is greatly increased in inflamed tissues. This is in part due to stimulation of the MOR synthesis in the Dorsal Root Ganglion (DRG) induced by cytokines, especially Neural Growth Factor (NGF), and its transport to peripheral terminals. Furthermore, immune cells, guided to the inflamed region by cell adhesion molecules, are stimulated by local cytokines to secrete endogenous opioid peptides, including β-endorphin and encephalin that bind to the opioid receptors. In addition to peripheral and central pain transmission neurons, MOR agonists produce analgesia by acting on a pain modulation circuit that includes regions of the Central Nervous System. In summary, MOR analgesia depends upon a set of widely distributed neural targets that include NAPs, ascending pain projection neurons and a top-down pain modulatory circuit [14].
Some experimental studies on nociception in leishmaniasis have been performed in order to better understand the profile of cytokines related to pain, without a satisfactory conclusion [15,16]. The purpose of this study was to determine the profile of MOR staining in the well established cutaneous ulcerated lesions of leishmaniasis and sporotrichosis in patients from Rio de Janeiro, Brazil, and to associate the MOR staining profile with the presence or absence of pain in the cutaneous lesions of both diseases.
Materials and methods
Series of 38 patients attended between 2013 and 2019 with a confirmed diagnosis by culture of localized cutaneous ulcers of leishmaniasis (n=14; 6 with local pain) or sporotrichosis (n=19; 9 with local pain), along with five patients with unspecific cutaneous ulcers (2 with pain) that were treated at the Laboratory of Clinical Research and Surveillance in Leishmaniasis, Evandro Chagas National Institute of Infectious Diseases, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil. Fragments of skin lesions previously obtained through biopsy were selected according to the information of presence of pain without gradation of intensity, or absence of it in the cutaneous ulcers at the same day they were submitted to biopsy for diagnostic purposes. Exclusion criteria were the presence of viral (HIV) or metabolic diseases (diabetes) that could alter the perception of pain. The biopsy tissue specimen was fixed in formalin, embedded in paraffin and microtome sections were obtained for histochemistry (hematoxylin & eosin) and immunoreactivity for anti-opioid μ-receptor.
Histopathology
The histopathological analysis focused on changes in the epidermis (hyperplasia or pseudoepitheliomatous hyperplasia), and dermal changes as type of granuloma (compact - clusters of epithelioid cells; or loose – sparse epithelioid cells surrounded by edema), presence and semi quantitative analysis of cells of the mononuclear phagocytic system (analysis of macrophages and epithelioid cells), of polymorphonuclear cells and the presence or absence of necrosis, according to the clinical data of painful or painless cutaneous lesions.
Immunohistochemistry
Anti-opioid μ-receptor (AB1580-I Merk Millipore-Sigma-Aldrich, batch number #3046772) was used for immunohistochemistry analysis. Heat induced epitope retrieval was performed in EDTA, pH 8.0, for 30min at 98ºC. Reaction was detected by phosphatase alkaline kit (GBI Labs, Bothel, Washington, USA) and revealed with permanent red [17]. The sections were counterstained with hematoxylin. Immunohistochemistry staining was analyzed semi quantitatively in epidermis and semi quantitative analysis of dermal macrophages was also performed (0 to 3+).
Fisher’s exact test was used for histochemistry and immunohistochemistry results, and significance was considered at a level of 5%. Analysis was performed using color-based image analysis (with permanent red) to evaluate extent and quantitatively intensity of the staining from 1+ to 3+, (being 3+= strong; 2+=medium; 1+= weak). See the Figure 2. The reading of the slides was performed by two pathologists under light microscopy, with agreement of the results.
Ethical approval
Full consent was obtained from the participants prior to the study on the basis of informed consent and led by experienced professionals in infectious diseases and immunohistochemical techniques.
The study was approved by the Research Ethics Committee of the Evandro Chagas National Institute of Infectious Diseases, Oswaldo Cruz Foundation (certificate number CAAE 61115716.5.0000.5262).
Results
Compact granuloma (mature epithelioid cells in clusters) were present in 50% of the leishmaniasis cutaneous lesions, in 22% of the sporotrichosis cutaneous lesions, and were absent in unspecific ulcers. Cutaneous lesions regardless of the disease presented neutrophils in more than 64% of the cases. Loose granuloma (immature epithelioid cell spread in the dermis) was present respectively in 50% and 88% of cases. Necrosis was present in 27% in the leishmaniasis cases, 69% in the sporotrichosis cases, and it was absent in unspecific ulcers. Pseudoepitheliomatous hyperplasia was more frequent in the leishmaniasis cutaneous lesions (85%) than in sporotrichosis (57%). The finding of a larger proportion of women in the sporotrichosis cases happened out of random. There were no statistically significant results between gender or age and the presence of pain (Tables 1,2).
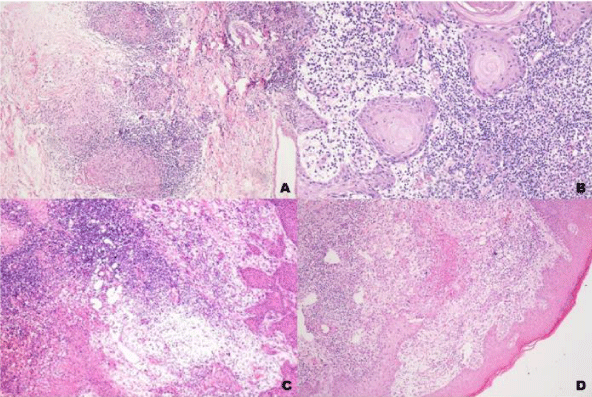
The histopathological presentation of lesions regarding type of granuloma (compact or loose) and the presence of micro abscesses of polymorphonucler cells or necrosis did not correlate with the presence or absence of local pain (Figure 3).
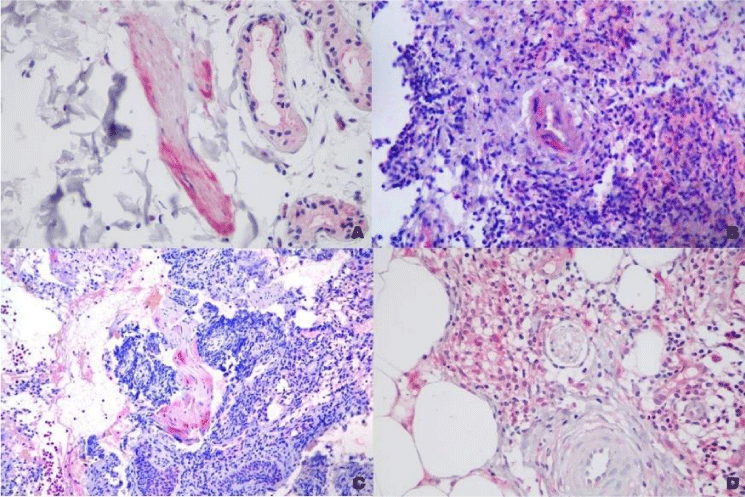
MOR staining had irregular distribution in epidermis with higher intensity in the areas with pseudoepitheliomatous hyperplasia. It was also present, although in lower intensity, in macrophages and epithelioid cells. It was present in some nerves and in the endothelial cells (Figures 3-5).
There was no association between the clinical complaint of pain in the lesions and the intensity of the MOR staining.
Discussion
A comparison between histopathological changes and immunohistochemical expression of MOR in painful and painless cutaneous ulcers of leishmania, sporotrichosis and also in unspecific ulcers was performed. Ulcerated cutaneous lesions of leishmaniasis and sporotrichosis did not show significant differences between histopathological aspects or intensity of μ-opioid receptor staining when comparing lesions with presence or absence of local pain. However, there was stronger staining in the areas with pseudoepitheliomatous hyperplasia, a finding not yet described in the literature (Table 2).
The keratinocytes are surrounded by inflammatory infiltrate being stimulated and releasing cytokines and growing factors, including NGF and opioids. Probably the high intensity of protease and peptidase from the local inflammatory activity destroyed opioids in those patients who referred to painful lesions. On the other hand, in those patients with painless lesions, a possible explanation is that the anti-nociception came from the DRG neurons.
Opioids from Immunocytes interacting with receptors on sensory nerves inhibit nociception in inflammation sites. Increased amounts of opioid peptides in immune cells infiltrating the inflamed tissue include macrophages, mast cells, lymphocytes, plasma cells and polymorphonucler leukocytes [18].
Opioids are also released from the inflammatory cells acting at paracrine way at the site of injury, while opioid receptors are activated in the periphery and DRG neurons in painful conditions. Opioid receptors have a key role in wound healing and homeostasis [19].
Up regulation of opioid receptors and accumulation of opioid peptide-producing immune cells are detected at the site of nerve injury, accompanied by enhanced antinociceptive activity of opioid agonists. Immune cell-derived opioid peptides can block the excitation of nociceptors in nerves within injured tissue, as demonstrated in surgical patients [17].
The murine model is an excellent system for the analysis of Th1/Th2 differentiation in leishmaniasis. Resistance to Leishmania (L.) major depends on the development of a L. major specific Th1 response, while Th2 differentiation results in susceptibility to this pathogen. The epidermis is the major source of immunomodulatory mediators that triggers Th1 to Th2 differentiation. This epidermal gene induction was significantly stronger in resistant mice especially regarding genes known to promote Th1. Mice with a selective IL-6 deficiency in non-hematopoietic cells (including keratinocytes) showed a Th2 switch and dramatic deterioration of cutaneous leishmaniasis. These data suggest that epidermal cytokine expression may be a decisive factor in the generation of protective Th1 immunity and resolution of the inflammatory process [20].
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is involved in cellular responses and plays a key role in regulating the immune response to infection. It has been reported that the MOR gene transcription in various immune cells, including human T lymphocytes, leukocytes, and mature dendritic cells, induced the proinflammatory cytokine TNF. NF-κB was identified to be responsible for TNF-induced MOR gene expression on the human MOR gene promoter [21].
Keratinocytes probably are very active and may produce many cytokines that hinder the resolution of the inflammatory process, mainly IL-8 that sustain continuous recruitment of neutrophils. These cells produce others substances that maintain the inflammation down-regulated by stimulating Th1 cells such as chemokines CXCL9, CXCL10, CXCL11 and CXCL1 and chemokines as CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8(IL8) which are among those of angiogenic family includes chemokines CXCL1, CXCL2, CXCL3, CXCL5, CCLX6, CXCL7 and CXCL8 (IL8). On the other hand, neutrophils-derived cytokines lead to the persistence of the inflammatory process, expressing pro-inflammatory (TNF, IL-1α, IL-1β, IL-6, IL-8, IL-l7) or anti-inflammatory cytokines, besides angiogenic or fibrogenic factors [22].
In the cutaneous lesions of sporotrichosis and leishmaniasis the observation of neutrophils and necrosis in the wound is very usual even in deeper dermis [9]. Many times there is no complaint of pain, even with a lot of enzymes and peptidases acidifying the environment. So another explanation besides local opioid production should be postulated. Some authors reported experiments with rats pursuing explanations for neuropathic pain, and found out that MOR comes from DRG through the peripheral nerves and blocks the local pain. NGF up regulates MOR in DRG. This is followed by enhanced axonal MOR transport towards peripheral nerve terminals and subsequent increase of MOR in nerve fibers within skin. NGF-induced effects occurring through DRG to peripheral nerve fibers and the potentiating of antinociception were abrogated by NGF neutralization. This suggests that NGF not only contributes to inflammatory pain but also governs the MOR up regulation, resulting in enhanced opioid susceptibility towards better pain control [23].
Intense tissue damaging stimuli activates primary afferent nociceptors in cell bodies in the DRG. The central terminals of the nociceptors contact second-order neurons in the dorsal horn of the spinal cord gray matter. Agonists of MOR directly inhibit pain transmitting neurons in the periphery and central nervous system. They also act on descending pain modulatory circuits that control spinal cord pain transmission provided by noradrenergic innervations of the dorsal horn [14].
A weakness of this study is to be a retrospective study. Further studies with larger number of patients and with gradation of local pain sensation would allow a better understanding on the issue of local pain in granulomatous infectious diseases of the skin.
In conclusion, the general concept that leishmaniasis presents mainly painless lesions or sporotrichosis presents painful lesions could not be directly demonstrated in this study. There was no association between MOR staining and the presence or absence of local pain in the cutaneous lesions of both diseases. Certainly, there must be pain modulation by local opioids, but the main modulation seems to be originated from the peripheral and central nervous system.
We thank to the Leishmaniasis Clinical Research and Surveillance Laboratory and the Pathology Department from Evandro Chagas National Institute of Infectious Diseases, Oswaldo Cruz Foundation.
Financial Support
Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) through code 001.
- Antonio LF, Pimentel MIF, Lyra MR, Madeira MF, Miranda LF, et al. (2017) Sporothrix schenkii Sensu Lato identification in fragments of skin lesion cultured in NNN medium for differential diagnosis of cutaneous leishmaniasis. Diag Microbiol Infect Dis 87: 118-120. Link: http://bit.ly/2upk0Fh
- Quintella LP, Passos SR, Miranda LH, Cuzzi T, Barros MB, et al. (2012) Proposal of a histopathological predictive rule for the differential diagnosis between American tegumentary leishmaniasis and sporotrichosis skin lesions. Br J Dermatol 167: 837-846. Link: http://bit.ly/2MZhHiB
- Baptista C, Schubach AO, Madeira MF, Leal CA, Pires MQ, et al. (2009) Leishmania (Viannia) braziliensis genotypes identified in lesions of patients with atypical or typical manifestations of tegumentary leishmaniasis: evaluation by two molecular markers. Exp Parasitol 121: 317-322. Link: http://bit.ly/2sHws2M
- Nylén S, Eidsmo L (2012) Tissue damage and immunity in cutaneous leishmaniasis. Parasite Immunol 34: 551-561. Link: http://bit.ly/2QRbLsV
- Abdoli A, Maspi N, Ghaffarifar F (2017) Wound healing in cutaneous leishmaniasis: A double edged sword of IL-10 and TGF-β. Comp Immunol Microbiol Infect Dis 51: 15-26. Link: http://bit.ly/2SYtrWy
- Almeida-Paes R, Oliveira MME, Freitas DFS, do Valle AC, Zancopé-Oliveira RM, et al. (2014) Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Neglect Trop Dis 8: 284-287. Link: http://bit.ly/2MZnbtE
- Zhang YQ, Xu XG, Zhang M, Jiang P, Zhou XY, et al. (2011) Sporotrichosis: clinical and histopathological manifestations. Am J Dermatopathol 33: 296-302. Link: http://bit.ly/2QPrTv1
- Quintella LP, Passos SR, do Vale AC, Galhardo MC, Barros MB, et al. (2011) Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J Cutan Pathol 38: 25-32.Link: http://bit.ly/2QtwB2B
- Morgado FN, Schubach AO, Pimentel MI, Lyra MR, Vasconcellos ÉC, et al. (2016) Is there any difference between the in situ and systemic IL-10 and IFN-γ production when clinical forms of cutaneous sporotrichosis are compared? PLoS One 11: e0162764. Link: http://bit.ly/35y4yUh
- Hua S, Cabot PJ (2010) Mechanisms of peripheral immune-cell-mediated analgesia ininflammation: clinical and therapeutic implications. Trends Pharmacol Sci 31: 427-433. Link: http://bit.ly/35rHM00
- Sommer C, Kress M (2004) Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361: 184-187. Link: http://bit.ly/2T7YgIr
- Busch-Dienstfertig M, Stein C (2010) Opioid receptors and opioid peptide-producing leukocytes in inflammatory pain-basic and therapeutic aspects. Brain Behav Immun 24: 683-694. Link: http://bit.ly/2Fr8uv7
- Ninković J, Roy S (2013) Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 45: 9-24. Link: http://bit.ly/37Q0Ir9
- Fields HL (2011) Mu opioid receptor mediated analgesia and reward. In: The opiate receptors. Gavril W. Pasternak, editor. New York Dordrecht Heidelberg London: Humana Press Springer 239-264. Link: http://bit.ly/2SWyRBb
- Cangussú SD, Souza CC, Castro MS, Vieira LQ, Cunha FQ, et al. (2013) The endogenous cytokine profile and nerve fibre density in mouse ear Leishmania major-induced lesions related to nociceptive thresholds. Exp Parasitol 133:193-200. Link: http://bit.ly/35sru7d
- Karam MC, Merckbawi R, El-Kouba JE, Bazzi SI, Bodman-Smith KB (2013) In Leishmania major-induced inflammation, interleukin-13 reduces hyperalgesia, down-regulatesIL-1β and up-regulates IL-6 in an IL-4 independent mechanism. Exp Parasitol 134: 200-205. Link: http://bit.ly/36pi8dE
- Stein C (2018) New concepts in opioid analgesia. Expert Opin Investig Drugs 27: 765-775.Link: http://bit.ly/2ZWt8ww
- Stein C, Hassan AH, Przewłocki R, Gramsch C, Peter K, et al. (990) Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A 87: 5935-5939. Link: http://bit.ly/2Frglch
- Wang Y, Gupta M, Poonawala T, Farooqui M, Li Y, et al. (2017) Opioids and opioid receptors orchestrate wound repair. Transl Res 185: 13-23. Link: http://bit.ly/37LGsH7
- Ehrchen JM, Roebrock K, Foell D, Nippe N, von Stebut E, et al. (2010) Keratinocytes determine Th1 immunity during early experimental leishmaniasis. PLoS Pathog 6: e1000871. Link: http://bit.ly/2ZVg5LX
- Kraus J, Börner C, Giannini E, Höllt V (2003) The role of nuclear factor kappa B in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol Pharmacol 64: 876-884. Link: http://bit.ly/2Qrgoes
- Tecchio C, Micheletti A, Cassatella MA (2014) Neutrophil-derived cytokines: facts beyond expression. Front Immunol 5: 508. Link: http://bit.ly/36vJQFF
- Mousa SA, Cheppudira BP, Shaqura M, Fischer O, Hofmann J, et al. (2007) Nerve growth factor governs the enhanced ability of opioids to suppress inflammatory pain. Brain 130: 502-513. Link: http://bit.ly/2tAycKY
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
