Open Journal of Tropical Medicine
Deworming school children in Ethiopia: The importance of a comprehensive approach
Jemal Ali1,7, Allison Polland2, David Adlerstein3, Yirga G Gziabher4, Galia Sabar5, Yonat Liss6 and Zvi Bentwich1,6*
2Department of Urology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
3Department of Family Medicine, Saints Mary and Elizabeth Medical Center, Chicago, IL, USA
4Organization of Social Services for AIDS (OSSA), Mekele City, Ethiopia
5African Studies, Tel Aviv University, Tel Aviv, Israel
6NALA Foundation, Tel Aviv, Israel
7Department of Microbiology Immunology and Parasitology, University of Gondar, College of Medicine and Health Sciences, Gondar, Ethiopia
Cite this as
Ali J, Polland A, Adlerstein D, Gziabher YG, Bentwich Z, et al. (2018) Deworming school children in Ethiopia: The importance of a comprehensive approach. Open J Trop Med 3(1): 001-006. DOI: 10.17352/ojtm.000008Introduction: A quarter of the world’s population, most commonly in developing countries like Ethiopia, are infected with intestinal parasites. School age children are the most affected segment of the population. The World Health Organization (WHO) recommends periodic deworming of all at-risk people living in endemic areas.
Methods: In 2009, Ben Gurion University, in partnership with the NALA Foundation and in collaboration with the Organization for Social Services and AIDS (OSSA), an Ethiopian non-governmental organization, and the health and education bureau of Tigray Regional State, launched a deworming project for school children in Mekele City, Ethiopia. During its four years of implementation (2009-2012), we evaluated the efficacy of this intervention in controlling helminthic infection in participating schools. The program entailed a comprehensive approach, combining intensive health education with water sanitation and mass drug administration and with pre- and post-intervention stool surveys, during each year of the intervention.
Results: A total of 23,214 students were dewormed in four annual deworming campaigns. The results of the sequential stool surveys demonstrated a significant and consistent decrease in the prevalence of both schistosomiasis and soil-transmitted helminthes infection in all the school children who participated in the project, and was sustained throughout the intervention period, decreasing from 52.8% at the beginning to 6.4% at the end.
Conclusions: Results suggest that a comprehensive program, which combines mass drug administration with health education and water sanitation, can lead to sustained control of helminthic infections in school children living in highly endemic areas for these infections. Though continued evaluation of this program is warranted to determine how long and permanent such a control can last, deworming programs of this type are likely to prove useful throughout Ethiopia and other developing countries where these infections are highly common and where their long-lasting control is greatly needed.
Abbreviations
MDA: Mass Drug Administration; NTDs: Neglected Tropical Diseases; SCHi: Schistosoma Mansoni; STH: Soil-Transmitted Helminthes
Introduction
More than 1.5 billion people, or 24% of the world’s population, most commonly in developing countries, are infected with soil-transmitted helminthes (STH) [1] and more than 207 million people, 85% of whom live in Africa, are infected with schistosoma [2]. These worm infections are widespread in most developing countries, like Ethiopia, with extremely high prevalence among school children. Risk factors for these infections include poor hygiene, lack of sanitation and low socioeconomic status. Sanitation coverage varies widely in developing countries. In most of the developing countries, less than half the population uses improved sanitation facilities, with the countries with the lowest coverage concentrated in sub-Saharan Africa and southern Asia [3].
In helminthic infections, light infections are often asymptomatic. However, heavier infections can generate a range of symptoms, including intestinal manifestations (diarrhea and abdominal pain), malnutrition, general malaise and weakness, and impaired cognitive and physical development, resulting in significant school absenteeism. Hookworms cause chronic intestinal blood loss that can result in anemia [4]. Intestinal schistosomiasis can result in abdominal pain, diarrhea and blood in the stool, with complications of chronic infection [5]. Furthermore, helminthic infections are often co-morbid with major infectious diseases, such as malaria, tuberculosis and HIV [6-10].
The impact of helminthic infections extends far beyond these obvious health effects to include economic and social effects resulting from lost school attendance and effective work time [7,11]. Treatment of these infections by MDA, at a cost of three cents (for STH) to fifty cents (for Schistosoma) (in US dollars) per individual, is an extremely cost-effective and attractive way to control these infections [7].
The World Health Organization (WHO) recommends treating all school children at regular intervals with deworming drugs in areas where helminth infection is common. The WHO states this will improve nutritional status, hemoglobin, and cognition and thus improve health, intellect, and school attendance [12]. For worm infections, morbidity can be significantly reduced through repeated and regular treatment with single-dose MDA delivered through school health programs. The drugs are safe, inexpensive, and simple to administer, and are thus ideally suited for mass administration [13]. However, the rates of reinfection in countries where the infrastructure, sanitation and hygiene have not been improved, are extremely high, raising serious questions about the efficacy of MDA alone.
A number of recent studies have suggested that health education and provision of hygiene and sanitation facilities may impact the prevalence of helminth infections and reinfection [14-18]. Based on our primary experience, we concluded that a more comprehensive approach is needed to achieve behavioral change in school children and reach these goals. Therefore, we developed a program for deworming school children that combines intensive health education, water sanitation, and hygiene with community and parental involvement and commitment, in addition to MDA. We implemented this program in Mekele City, Ethiopia during four years (2009-2012) with the aim of achieving longer lasting control of these infections and a decrease in the rate of reinfection.
Materials and Methods
Study site and study population
Following a pilot project initiated on three sites in collaboration with the Federal Ministry of Health of Ethiopia [19], we implemented the study project, targeting school children in Mekele City. The capital of Tigray Regional State, Ethiopia, Mekele City covers an area of 109 square kilometers. The average projected population between 2009 and 2012, assuming a medium growth rate of 2.7%, was 260,000 [20,21]. According to the last national census in 2007, about 72% housing units lacked bathing facilities in Mekele City and nearly half lacked proper toilet facilities [22]. Most elementary and junior high school compounds in Mekele City did not have properly functioning latrines or running water. With these known risk factors for helminthic infections in mind, we chose to perform our intervention program and study in Mekele City.
During the four years of the program, there were 68 governmental and private elementary and junior high schools in the city, attended by 45,307 students. A total of 38 schools, including roughly half the students in Mekele City (23,214), were involved in the intervention. Schools were selected based on previous studies in the area and existing reports from the city health bureau and in consultation with regional and zonal health bureaus. Priority was given to those schools with a high prevalence of helminth infections, but schools with low prevalence were also included to decrease possible bias of the study. The target age group was 5-15 years and all students present at school during the MDA took the deworming tablets. A total of 1,871 randomly selected students participated in the four annual stool surveys.
Study design and set-up
Between October 2008 and February 2009 (prior to implementation of the deworming project), in collaboration with the health and education bureaus of the regional, zonal and city administrations, we organized and conducted a series of sensitivization and motivation seminars for teachers, principals and workers in the health-care system on neglected tropical diseases (NTDs) and the program we would be initiating. Health education materials were discussed and feedback was received from participants. These materials were ready for distribution at the beginning of February 2009.
In addition, water and sanitation facilities were enhanced in a joint effort with Mashav (Israel’s Agency for International Development & Cooperation); Ben Gurion University’s Center for Emerging Diseases, Tropical Diseases, and AIDS; and the NALA Foundation; and in collaboration with the Mekele City administration. Specifically, 30 six-hole pit latrines (built separately for boys and girls) were constructed and water taps for hand washing were installed in 30 school compounds in Mekele City and prepared for use by November 2009.
From the start, the intervention was planned as a multi-stage deworming program. The comprehensive program entailed training of health-care workers, health education, drug distribution, and improvement of water supply and sanitation. The protocol used on the deworming campaign was based on the WHO guidelines for deworming school children [20] and modified in accordance with resource availability.
Health education program
A comprehensive health education program was initiated in 2009. This entailed training of health-care workers, teachers and community volunteers; the development of health education materials by health professionals and teachers; and the distribution of these materials to children attending all participant schools. Teachers conducted intensive ongoing health education sessions as part of the daily school program, on average a few times a year for the four years of the deworming program.
Health education materials were presented in postcard format, with graphics appearing on the front and messages on the back (Figure 1), culturally adapted and translated to the local language, Tigrigna. The messages were sixfold, including: (1) a general description of worm infections, with a focus on schistosomiasis and STH; (2) signs and symptoms of worm infection; (3) sources of such infections; (4) how these infections are acquired; (5) how these infections are prevented; and (6) how such infections are controlled. In addition, science teachers in the schools took note of the children’s hygienic practices and arranged monthly peer group demonstrations.
The intensive health education program was continuously improved during the progress of the intervention, eventually involving community workers, volunteer high school and university students and health extension workers, all of whom promoted the health education program within the schools. Sensitization seminars, as well as comprehensive training on prevention of NTDs in general and helminth infections in particular, were conducted prior to each stool survey.
Stool surveys and intervention
In February 2009, a baseline survey was conducted, involving random sampling of stool specimens for prevalence of schistosomiasis and STH infection, followed by a deworming campaign. In November 2009, a follow-up deworming campaign was undertaken. Thereafter, over the four years of the program, an annual deworming campaign was conducted. Each deworming campaign entailed a follow-up stool survey and MDA.
Students were randomly selected for sampling in each stool survey, and different children were included in each round of surveys. Students were asked to collect their stool during a bowel movement in leakproof, clean stool cups. The stool samples were coded with a number to identify each student. The samples were examined within half an hour and rechecked within two hours, using the Kato-Katz technique [23], in the Department of Microbiology and Parasitology, Mekele University.
All students who were present at school on the days of drug administration were given antihelminthic treatment, which consisted of Albendazole and Praziquantel. Dosing of Praziquantel was based onweight, while a single dose of 400 mg of Albendazole was administered. Drugs were distributed by the city health bureau and delivered by trained volunteers and teachers, accompanied by health professionals. Adverse effects of the MDA throughout the deworming campaign were observed and attended to by nurses.
Ethical considerations
Ethical issues were addressed by obtaining permission from the city’s health and education bureau and from the principals of the participating schools. School children verbally consented to their participation in the survey and the MDA.
Data collection and analysis
Data were collected in a standard record format prepared for the deworming program records. They were entered in Excel format and then converted to SPSS version 16 statistical package. The prevalence of the parasitic infection at the baseline and in subsequent stages was compared.
Results
At baseline and in all the stages of the survey, different parasite species were found. Schistosoma mansoni was the most prevalent parasitic infection across all surveys and Ascaris lumbricoides was the leading STH infection (Table 1). Among the other intestinal parasite species, Hymenolepis nana and Enterobius vermicularis were the most frequent infections. The prevalence of schistosomiasis at baseline was very high in some schools: 100% in FelegDa’ero, 83.1% in Lachi, 81.2% in Gembela, and 43.0% in Aynalem. FelegDa’ero, Lachi and Gembela are adjacent to the Giba river, which is a likely environmental reservoir for Schistosoma mansoni because of the known presence of the snail intermediate host, Biomphalaria pfeifferi [24]. The highest prevalence of STH infection was in Quiha elementary and junior high school (34.2%). Figure 2 displays infection rates over time by school.
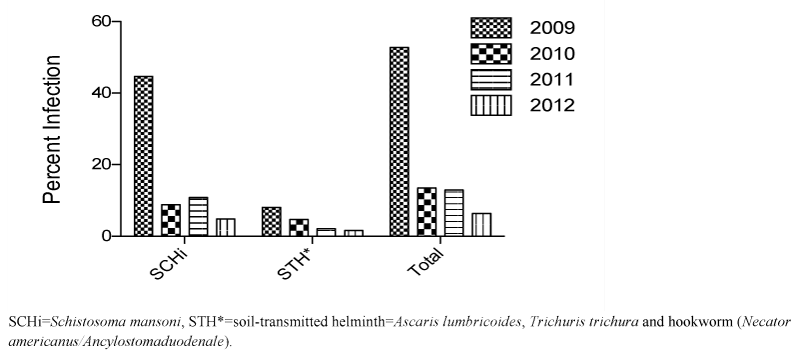
The sequential stool surveys demonstrated a significant and consistent decrease in the prevalence of both schistosomiasis and STH infection in all school children who participated in the project (Figure 3 for cumulative results of all stool surveys). This was sustained throughout the intervention period, decreasing dramatically from 52.8% (44.4% schistosomiasis; 8.1% STH infection) at the beginning to 6.4% (4.8% and 1.6%, respectively) at the end of the four-year, multi-stage deworming program. Moreover, an independent survey carried out by the Ethiopian Federal Ministry of Health indicated that the prevalence of both schistosomiasis and STH in Mekele City remained below 2% in 2014, two years after the conclusion of the intervention [19,25].
Conclusions
The results reported here clearly demonstrate a marked and sustained decrease in the prevalence of both schistosomiasis and STH infections in Mekele City following our 2009-2012 deworming program. The findings of the 2014 independent survey [19,25] further suggest that the decreased prevalence of these infections persisted long after the conclusion of our intervention. This success, which in some locations was truly dramatic, is likely, though not proven, to be accounted for by the comprehensive approach used in our program, which combined MDA with intensive health education and also addressed water and sanitation issues.
To the best of our knowledge, no other intervention targeting NTD control was conducted during the four-year period in question by health or education authorities or by any other NGO or outside agency. Moreover, it was not until 2013, a year after our program was completed, that the Ethiopian Federal Ministry of Health prioritized NTD control. Thus, it can be cautiously argued that the decreased prevalence of schistosomiasis and STH infections in Mekele City can be attributed to our comprehensive deworming program.
One of the main benefits of our comprehensive program was the growing awareness and involvement of the community, as well as of the city administration at all levels, over time. Water and sanitation facilities were improved with the support of the municipality; seminars and training sessions were attended by teachers, principals, community health-care workers and volunteers; and health education sessions were held in all schools participating in the program. The ongoing growth of the circle of involved individuals and groups had a significant influence on the results of the program.
The very impressive decrease in the prevalence of parasitic infections in this study demonstrates the effectiveness of MDA deworming accompanied by sanitation improvements. However, as originally perceived and based on our cumulative experience, we are inclined to conclude that the decreased prevalence of infections would not have been sustained if children had not changed their behavior and retained the information they received about prevention. Considering the unchanged environment over the four years of our program, the decreased prevalence of helminthic infections is probably related to decreased exposure to helminthes, which in turn reflects the changed behavior of the school children.
Limitations and future studies
The study is not without limitations. First, this was part of a programmatic implementation of deworming in which all participants were subjected more or less to the same intervention. Due to ethical considerations, public health authorities did not allow us to have a control group that would not participate in the intervention for comparison.
Second, quantification of the intensity of infection by egg count in the stool (EPG) was regretfully not conducted in all the surveys. This weakens overall observations and should be addressed in future interventions.
Third, the deworming program reached only 50% of school-age children in Mekele City, who received the deworming tablets at least once a year. The sequential stool surveys carried out during the project were likewise performed only in the treatment group. Thus, we lack information on the decrease of infection prevalence among the untreated population of school children and the rest of the population of Mekele City. Such information would have been valuable, as it might have demonstrated a greater effect of our “limited” intervention on the population at large, including the influence of health education. It would be highly informative to collect such data in future interventions of this kind. Moreover, continued evaluation of this program is warranted to determine how long and permanent such control can last.
Finally, we were unfortunately unable to perform a full evaluation of the outcome of the health education program and its efficacy in changing the behavior of school children during this intervention. However, we did conduct such an evaluation previously in a small-scale study in a different area in Ethiopia, which indeed showed the successful and significant effect it had on the knowledge, attitudes and practices of school children. Future studies are needed to evaluate the educational component of the intervention to determine its short- and long-term effects.
Notwithstanding the above limitations, this study has clearly demonstrated a dramatic and sustained decrease in the prevalence of both schistosomiasis and STH infections in school children living in a highly endemic area for these infections. The comprehensive multi-interventional approach, with sequential surveys serving to monitor and evaluate the program, combining MDA with intensive health education and also addressing water and sanitation issues prevalent in the town, may serve as a model that can be applied in other regions in Ethiopia, as well as elsewhere, in areas with high prevalence of these infections.
- World Health Organization (2016) Soil-transmitted helminth infection: Fact sheet, updated. Link: http://bit.ly/32gmxhr
- World Health Organization (2016) Schistosomiasis: Fact sheet, updated. Link: http://bit.ly/2YHnywP
- UNICEF and World Health Organization (2015) Progress on sanitation and drinking water: Updates and MDG assessment. Link: https://uni.cf/2JlQYv7
- Bethony J, Brooker S, Anbonico M, Geiger SM, Loukas A, et al. (2006) Soil-transmitted heminth infections: Ascariasis, trichuriasis and hookworm. Lancet 367: 1521-1532. Link: http://bit.ly/2XD0qOK
- Geyseels B, Polman K, Clerinx J, Kestens K (2006) Human shistosomiasis. Lancet 368: 1106-1118. Link: http://bit.ly/32g2QpP
- Bentwich Z, Horner R, Borkow G (2010) De-worming in developing countries as a feasible and affordable means to fight co-endemic infectious diseases. The Open Biology Journal 3: 97-103. Link: http://bit.ly/2NGG2wh
- Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich SS, et al. (2006) Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med 3: e102. Link: http://bit.ly/30o2VGd
- Borkow G, Bentwich Z (2004) Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: Role of hyporesponsiveness and anergy. Clin Microbiol Rev 17: 1012-1030. Link: http://bit.ly/30qJ0GC
- Borkow G, Weisman Z, Leng Q, et al. (2001) Helminths, human immunodeficiency virus and tuberculosis. Scand J Infect Dis 33: 568-571. Link: http://bit.ly/2XBHdBR
- Hotez PJ, Molyneux DH, Stillwaggon E, Bentwich Z, Kumaresan J (2006) Neglected tropical diseases and HIV/AIDS. Lancet 368: 1865-1866. Link: http://bit.ly/2Jrf7AJ
- Norris J, Adelman C, Spantchak Y, Marano K (2012) Social and economic impact review on neglected tropical diseases. Hudson Institute and Sabin Institute. Link: http://bit.ly/2XEW1zO
- World Health Organization (2002) Prevention and control of schistosomiasis and soil transmitted helminthiasis. Report of a WHO Expert Committee. 912. Link: http://bit.ly/2JlSj57
- World Health Organization (2005) The evidence is in: Deworming helps meet the Millennium Development Goals. Link: http://bit.ly/2JC0w4b
- Freeman MC, Clasen T, Brooker S, Akoko D, Rheingans R (2013) The impact of a school-based hygiene, water quality and sanitation intervention on soiltransmitted helminth re-infection: A cluster-randomized trial. Am J Trop Med Hyg 89: 875-883. Link: http://bit.ly/2NGkaBl
- Steinmann P, Yap P, Utzinger J, Du, Z-W, Jiang, J-Y, et al. (2015) Control of soil-transmitted helminth in Yunnan province, People’s Republic of China: Experiences and lessons from a 5-year multi-intervention trial. Acta Trop 141: 271-280. Link: http://bit.ly/30t8TpB
- Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M (2013) Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: A cluster-randomized controlled trial. PLoS Negl Trop Dis 7: e2397. Link: http://bit.ly/2XvJIAI
- Freeman MC, Ogden S, Jacobson J, Abbott D, Addiss DG, et al. (2013) Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: A rationale for inter-sectoral collaboration. PLoS Negl Trop Dis 7: e2439. Link: http://bit.ly/2XAWliZ
- Asaolu SO, Ofoezie IE (2003) The role of health education and sanitation in the control of helminth infections. Acta Trop 86: 283-294. Link: http://bit.ly/30mMmdN
- Federal Democratic Republic of Ethiopia, Ministry of Health, in Collaboration with World Health Organization Ethiopia and African Regional Office (2013) National master plan on neglected tropical diseases (NTDs), 2013-2015. Link: http://bit.ly/2Lblry1
- Montresor A, Crompton DWT, Gyorkos TW, Savioli L (2011) Helminth Control in School-age Children: A Guide for Managers of Control Programmes. World Health Organization. Link: http://bit.ly/2YIdAeF
- Earth Institute, Columbia University (2012) Research and publications, population data, Mekelle population data. Link: http://bit.ly/2XFOID6
- United Nations Statistics Division (UNSD) (2007) The 2007 population and housing census of Ethiopia: Statistical report for Tigray regional state. Table 8.9, Housing units of towns by type of toilet facility, p 375. Link: http://bit.ly/32gyZ0z
- World Health Organization (1991) Basic Laboratory Methods in Medical Parasitology. Geneva: World Health Organization.
- Brown DS (1964) The distribution of the intermediate host of schistosoma in Ethiopia. Ethiop Med J 2: 250-259.
- Federal Democratic Republic of Ethiopia, Ministry of Health (FMoH) (2016) Second edition of national master plan on neglected tropical diseases (NTDs), 2015-2019. Link: http://bit.ly/2XBmtKi
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
