Open Journal of Tropical Medicine
Toxoplasma Serostatus in Thai Free-Range Chickens: Prevalence and Two Diagnostic Methods
Ruenruetai Udonsom1#, Patcharee Chaichan2#, Aongart Mahittikorn1, Philippe Vignoles2, Aurélien Mercier2, Abdelkrim Aroussi2, Marie-Laure Dardé2,3, Yaowalark Sukthana1*
2INSERM, Univ. Limoges, CHU Limoges, UMR-S 1094, Laboratoire de Parasitologie, 87025 Limoges, France
3Toxoplasma Biological Resource Center, CHU Limoges, 87042 Limoges, France
#Contributed equally to the work
Cite this as
Sukthana Y, Udonsom R, Chaichan P, Mahittikorn A, Vignoles P, et al. (2017) Toxoplasma Serostatus in Thai Free-Range Chickens: Prevalence and Two Diagnostic Methods. Open J Trop Med 1(1): 018-023. DOI: 10.17352/ojtm.000004Toxoplasma seroprevalence in free-range chicken, which directly feed from ground, is a good indicator for detecting an environment contamination by T. gondii oocysts. Many serological methods have been used for diagnosing Toxoplasma infection in animals including chicken. Selection of appropriate methods primarily depend on their sensitivity and specificity as well as laboratory techniques, special reagent or tool requirements and regional prevalence status. The aims of this study were to determine the prevalence of T. gondii antibody in free-range chickens in Thailand and to compare two serodiagnostic methods, indirect fluorescent antibody test (IFAT) and Modified Agglutination Test (MAT). Six hundred free-range chicken sera were collected from 2 villages in Kanchanaburi Province, Thailand, 300 in 2014 and 300 in 2015. The age of chickens was between 3-6 mo, of which chickens of 2015 were older than of 2014. Collected sera were test for T. gondii IgG antibody by both methods. Over all Toxoplasma seroprevalence were 33.0% and 17.7% by IFAT and MAT, respectively. There was poor agreement between the two serological methods (kappa=0.09). IFAT in this study revealed higher positive percentage than MAT. Even though the way of living and environment in the present study was suitable for Toxoplasma transmission, the seroprevalence in Thai free-range chickens was low compared to other tropical regions such as Africa or South America. A low Toxoplasma prevalence was also found in humans, cats and other intermediate hosts in Thailand. Toxoplasmosis in Thailand causes low burden compare to other infectious diseases, however, consume well-cooked free-range chicken meat and proper practice during handling raw meat when cooking should be advocated to prevent and reduce this infection.
Introduction
Toxoplasmosis is a food and water-borne parasitic zoonosis caused by an intracellular protozoan, namely Toxoplasma gondii. The disease is mainly asymptomatic or causes benign clinical manifestations in healthy infected persons, but can have severe consequences in immunocompromised patients or produce congenital toxoplasmosis once primary acquired infection occurs during pregnancy. Cat and other felids are the only definitive hosts, while human, warm-blooded animals, bird and rodent are intermediate host. The infection is transmitted to human by three important routes i.e. 1) ingesting oocysts contaminated in food or water; 2) consuming raw or under-cooked meat containing tissues cysts and 3) vertical transmission from primarily infected mother to fetus.
Due to the asymptomatic character or non-specific signs of this infection in human as well as in other animals, identifying infected hosts to deliver effective prevention measures needs an indirect way such as detection of antibodies. Acting as an intermediate host, free-range chickens mainly feed from bare ground of which T. gondii oocysts, from cat and other felids, are laid. Toxoplasma antibody in chickens, thus, represents a good indicator for T. gondii oocyst contamination in an environment [1]. Evaluation of previously Toxoplasma infection requires accurate diagnostic tools that can rapidly and precisely identify infected hosts, in both definitive and intermediate [2].
Many serological tests are available for Toxoplasma antibody detection, mainly immunoglobulin G (IgG), indicating previous infection. The Sabin-Feldman dye test is the gold-standard method for detecting T. gondii antibody in human, and according to Dubey, it works on many animal species but not on chicken sera since this may be related to fixation of the first component of the complement [3,4]. To date, agglutination tests either direct or modified methods are commonly used for T. gondii antibody detection in animals [5] including free-range chickens [1]. Modified agglutination test (MAT) was recently validated by using cat and mouse bioassays [7]. Toxo-screen DA (Biomérieux®) is one of modified agglutination methods using commercial suspension of T. gondii as antigen [6]. Indirect fluorescent antibody test (IFAT), another highly specific test utilizing species-specific conjugated fluorescein is the gold-standard test for T. gondii detection in pigs [8,9].
Despite the agreement of agglutination tests and IFAT in terms of sensitivity and specificity in pigs [10] and cats [11], the comparison between these two methods is unclear for chickens. Moreover, the seroprevalence of T. gondii in Thai free-range chickens indicating the situation of contaminated environment is not well established. Therefore, this study aims for detecting the prevalence of Toxoplasma serostatus in free-range chickens in Thailand and to evaluate the performance of the two serological diagnostic methods, IFAT and a commercially available MAT (Toxo-screen DA®, Biomérieux).
Materials and Methods
Study area
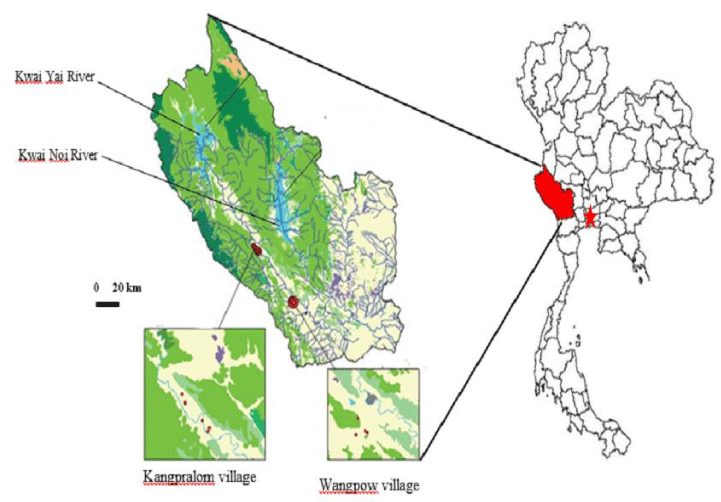
The project was carried out in the westernmost province of Thailand, Kanchanaburi, located at Thai-Myanmar border (Figure 1). Our studied site included 2 villages from Saiyok district, Kangpralom (14°01’ 28.76’’ N, 99°13’ 19.26’’ E) and Wangpow (14°21’ 44.53’’ N, 98°53’ 59.46’’ E). They are situated in the valley of the Kwai River, in a well-preserved rural environment, surrounded by forests with a high biodiversity. Cats and dogs are widely present and freely roam in people’s houses and outside. In Thailand, the majority of chicken husbandry is run by closed-system but there are some areas where naturally free-range chickens still prevail, which our studied sites are among those. Those chickens were free to roam and feed themselves on bare ground around houses together with dogs and cats (Figure 2).
Sera sampling in Thai free-range chickens
From April to August 2014, approximately of 1-2 ml of blood samples of 300 free-range chickens, aged 2-5 months, were collected by puncture of the axillary vein. Blood from another batch of 300 chickens, aged 3-6 months, were collected from May to September 2015. Collected samples were kept in a 4°C container and brought to laboratory at the Department of Protozoology, Faculty of Tropical medicine, Mahidol University for T. gondii IgG antibody.
All experiments with animals were carried out in strict and correct conditions in order to ensure to meet the criteria for approving by animal welfare according to the Animal Ethics Procedures of Faculty of Tropical Medicine–Animal Care and Use Committee (FTM-ACUC), Mahidol University, Bangkok, Thailand (Permit No. FTM-ACUC 007/2014).
Inoculation of chickens for positive control sera
Positive Toxoplasma of chicken serum is needed for the prevalence study. Therefore, 10 free-range chickens from the study sites (6-8 weeks old) were obtained and initially examined by MAT and IFAT to confirm the absence of T. gondii antibody before allocating each 5 chickens into group 1 and group 2. Four chickens from both groups were intraperitoneally inoculated with T. gondii RH strain tachyzoites; 10,000 cells and 40,000 cells, for group 1 and 2, respectively. One chicken from both groups were injected with normal saline as a negative control. All chickens were observed daily and recorded for any clinical sign and symptom of toxoplasmosis. Approximately 1-2 ml of blood samples were collected by puncture of the axillary vein of infected chickens at day 1, 3, 5, 7, 14 and 30 post-inoculation; MAT and IFAT were performed on those collected sera to determine whether these experimental chickens develop T. gondii IgG antibodies.
Modified agglutination test (MAT)
The suspension of antigen used for MAT technique in this study was an in- house antigen. The test was performed as previously described [12]. Briefly, the sera samples were diluted in a stock of 2-mercaptoethanol performed in U-shaped well microtiter plate by two-fold dilutions starting at 1:10. Killed T. gondii (RH strain) tachyzoites suspension was diluted with BABS buffer and was added to the wells as antigen. The wells were then incubated for at least 7 hr or overnight at room temperature in the absence of light. The diffuse opacity of the Toxoplasma agglutinated form was regarded as a positive result, whereas a central white opaque dot at the well bottom was regarded as a negative result. Positive and negative controls were also analyzed alongside each batch testing. Titers ≥ 10 were defined as positive.
Commercial Modified agglutination test
The suspension of antigen used for MAT serology is a commercial antigen (Toxo-Screen DA®, BioMérieux, Marcy l’Etoile, France) [6]. Chicken sera were diluted in 2-mercaptoethanol (1:10, 1:20, 1:100 and 1:800). Twenty-five µL of antigen suspension were added in each well for the final volume of 50 µl, the antigen was diluted in 1:5 in colored BAB’s albumin buffer (red) pH 8.95. After 5 hours of incubation, sedimentation or agglutination of Toxoplasma was observed. Titers ≥ 10 were defined as positive. Positive and negative controls used were provided in the Toxo-screen DA kit.
Indirect fluorescent antibody test (IFAT)
IFAT was conducted following the protocol of the Animal Health Laboratories, Division of Agriculture, and Western Australia [13]. Killed T. gondii (RH strain) tachyzoites were fixed on a Teflon printed slides and were incubated with 20 μl of the two-fold diluted sera starting at 1:8, for 1 hour at 37 ºC in moist chamber. Goat anti-chicken IgG conjugated to fluorescein isothiocyanate (FITC) (Southern Biotech, USA) was then added to the slides, and incubated for a further 1 hr. Following a rinse, the slides were interpreted under fluorescent microscope at 400x magnification. Positive and negative control were included with each slides, and a titer of ≥ 1:16 is defined as positive result.
Statistical analysis
Statistical analyses were conducted using Fisher’s exact test and Chi Square Test (χ2). The alpha level was set at a standard level of 5% for statistical tests. Results were considered significant when p<0.05. Statistical analyses were performed using the R x 64.3.3.0 software with one or two-tailed significance level of 5%. Furthermore, Kappa coefficient was used to estimate the agreement between serological techniques for the detection of T. gondii antibodies. Binomial model was used to calculate the 95% confidence interval.
Results
T. gondii antibody titer in inoculated chickens
Following T. gondii inoculation, no chickens showed any clinical sign and symptom specific or suggestive to toxoplasmosis, however, one chicken in group 2 (higher dose of inoculation) died before day 30. The earliest detection of T. gondii antibodies was at 7 days post-inoculation in one chicken (C4) in group 1 with titer of 1:32 and another (C9) in group 2 with titer of 1:64 by IFAT. Although, MAT could not detect any positive chicken in group 1 within 7 days post inoculation, one (C9) in group 2 showed seropositive titer at 1:100 (Table 1). Antibodies were detected in all infected chickens by day 14 showing titers ranging from 1:64 to 1:1024 by IFAT and from 1:100 to 1:400 by MAT. At day 30, more than half of seropositive showed stable or lower titer by both IFAT and MAT. All control chicken sera were found negative by both serological methods.
Prevalence of T. gondii infection in free-range chickens and agreement between both techniques
Table 2 showed the number of Toxoplasma seropositive in free-range chickens from two villages, Kangpralom and Wangpow for the two sampling years, 2014 and 2015. Over the 2 periods of sampling, the overall prevalence was 17.7% with MAT vs 33.0% with IFAT. The positive results in the year of 2014 were 11.3% (95% CI=8.0-15.5) and 23.7% (95% CI=19.0-28.9) by MAT and IFAT, respectively. In 2015, the positive results were approximately double, respectively 24.0% (19.2-28.8) and 42.3% (36.7-47.9) by both methods. This may due to older chickens tested in the later than the former year. Toxoplasma IgG antibody prevalence in Wangpow village in 2015 was significantly higher than in Kangprolom (29.0% v.s. 18.1%; p-value = 0.03) by MAT (Table 2). There is poor agreement between the two assays with k = 0.09 (Table 3).
Discussion
The overall prevalence of T. gondii in Thai free-range chickens determined by IFAT and MAT in this study were 33.0% and 17.7%, respectively. The results of the year 2014 revealed approximately 2 times higher than the 2015’s by both methods. This may be because of the older chickens in later year were examined. Our results showed much lower than the previous study in Thailand that reported the prevalence by IFAT of 64% by IFAT in 1-year chickens from Bangkok [14]. A study from Kenya [15] also reported high prevalence as 79.0% in 2-years chickens. These results support the hypothesis that the longer the chickens are exposed to their bare ground environment, the higher Toxoplasma antibody was found.
The 33.0%-42.3% prevalence by IFAT observed in this study were similar to the 40.5% prevalence reported in Costa Rica [16], but lower than in Brazil (53.6%) [17] and in Kenya (79%) [15]. The difference might be due to higher cat density in those areas, or suitable temperature for T. gondii oocysts survival. Toxoplasma seropositive in human and other warm-blooded animals in those regions were high compared to Thailand, suggesting a higher level of soil contamination by oocysts and in a general way a more important circulation of the parasite in the environment from these areas. Actually, a higher prevalence was expected in the present study as the study sites were suitable for T. gondii transmission among its definitive and intermediate hosts including cats, dogs, chickens and human, which shared the same bare-ground environment. However, in Thailand in general Toxoplasma seroprevalence was about 30% in human [18] and very low as 7.3% in stray cats [19]. Sukthana [20] suggested that may be because of the high temperature and strong sunlight which inactivate oocysts. Furthermore, even though Thai domestic and stray cats are kept outdoors, they are fed on rice and well cooked fish.
When using MAT, T. gondii antibody were found 11.3% in 2014 and 24.0% in 2015. This was relatively similar to other countries where the seropositivity of T. gondii in free-range chickens determined by MAT were 17.9% in India [21], 18.8% in Northeastern China [22], 24.2% in Vietnam, 24.4% in Indonesia [23]. For comparison, seroprevalence in chickens seems higher in Africa and South America with 30.5% in Central Ethiopia [24], 40.4% in Nigeria [25], 38.8% in Brazil as well as 50.3% and 51.1% in rural and urban environments from Gabon [26].
There are several serological methods that are commonly used in Toxoplasma laboratories; the Sabin-Feldman dye test is recommended for human toxoplasmosis diagnosis, whereas indirect immunofluorescent test (IFAT) and modified agglutination test (MAT) are amongst the preferably used methods for T. gondii antibodies detection in various animal species. Those techniques are, however, present with some limitations for diagnosing toxoplasma infection [27]. Such as IFAT requires special equipment or appropriate anti-species serum. MAT is simple, easy to perform and does not require those [7], however, some articles seems to call into question the fact that this technique can be applied to all animal species [28]. In this study, both IFAT and MAT were used and able to detect T. gondii antibodies in all inoculated chickens. IFAT detected Toxoplasma IgG antibody with the titer of 1:32 at the earliest on day 7 in one chicken even in the group inoculated with lower concentration (Group 1 = 10,000 tachyzoites/ml), while MAT was unable to detect any. One chicken, inoculated with the higher concentration at 400,000 tachyzoites/ml, was seropositive at the titer of 1:64 by IFAT and 1:100 by MAT at day 7. This might be due to storage and travel conditions that affected in-house MAT antigen. Thus, we designed to use Toxo-Screen DA (BioMérieux®) instead of MAT to detect Toxoplasma prevalence in naturally free-range chickens. The principle of Toxo-Screen DA (BioMérieux®) is identical to MAT technique, but the condition of storage of this commercially available antigen was better than that of in-house MAT. The results from the present study bear some resemblance to other experimental chicken studies, who demonstrated that IFAT was able to detect T. gondii antibodies in chickens at first to second week post-inoculation, when infected with 1.5 x 107 T. gondii tachyzoites (RH strain) injected muscularly [14], or fed with 103 or 105 T. gondii oocysts K7 (avirulent) strains [29]. Similarly, after chickens was fed with 103 or 105 of ME49 oocysts, MAT was able to detect antibody at 1:100 serum dilutions at day 15 post-inoculation [30].
Although IFAT was defined as the gold standard test for T. gondii antibody detection in cow and pig [9,31], this technique however has not been validated in chicken. The higher seroprevalence in free-range chickens detected by IFAT than MAT in this study might be due to some explanations: firstly, false positives IFAT reactions due to non-specific fluorescent labelling with limited number of T. gondii tachyzoite or to variations in the brightness of fluorescein [31,32]. Secondly, agglutination test is less sensitive than IFAT, as shown by our experimental chickens results, therefore, MAT may have detected less positive cases in naturally chickens [30], comparing the sensitivity and specificity of serological tests with bioassay in mice, found 48.0% of false positive rate for IFAT and 32.0% of false positive rate by MAT. This suggests that there is no reliable serological technique as shown by the low kappa coefficient found in our study.
Most conventional serological tests require whole-cell of T. gondii tachyzoites from mice or cell culture, which lacks of standardization between laboratories, therefore, may give inaccurate and non-specific results [33]. Recently, the recombinant antigens of secretory microneme proteins (MIC3), rhoptry proteins (ROPs), rhoptry neck proteins (RONs), and dense granule antigens (GRA) were developed as an alternative method for Toxoplasma sero-diagnosis for both humans and animals, and demonstrated to be more sensitive and specific at detecting T. gondii antibodies in multiple animal species compared to MAT and IFAT [33-38]. Specifically, Sun and colleagues reported >90% agreement between recombinant GRA1, GRA7 and Toxoplasma soluble antigens (TSA) for T. gondii antibody detection in chickens, with GRA7 showed higher sensitivity and specificity than GRA1 and TSA [40]. To establish good epidemiological data of toxoplasmosis, additional other serological tests such as ELISA or recombinant antigen based diagnostic technique, bioassays and polymerase PCR based assays was recommended. Some reports found that using ELISA to detect T. gondii antibody in free-range chickens is seemingly more specific and sensitive than IFAT and MAT [30,41].
In the present study, both IFAT and MAT were able to detect T. gondii antibody in chickens as an indicator for contamination in environment. Despite comparatively different prevalence determined by the two diagnostic methods, the data indicates contamination of T. gondii in chickens and ultimately in the environment in rural area of Thailand. Even though chicken meat is normally consumed well-cooked in Thailand, care should be taken when handling undercooked meat to reduce the spreading of T. gondii infection to other animal hosts.
This study was partially supported by the ICTM grant of the Faculty of Tropical Medicine, Mahidol University. The authors are grateful to the veterinarian assistant staff of the Faculty of Veterinary Science, Kanchanaburi Campus, Mahidol University for their help taking care of the chickens.
- Dubey JP (2010) Toxoplasma gondii Infections in Chickens (Gallus domesticus): Prevalence, Clinical Disease, Diagnosis and Public Health Significance. Zoonoses and Public Health 57: 60-73. Link: https://goo.gl/AT7P3q
- Zhu CH, Cui LL, Zhang LS (2012) Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma gondii antibodies in sera of naturally infected dogs and cats. Iranian J Parasitol 7: 89-95. Link: https://goo.gl/N741o1
- Udonsom R, Buddhirongawatr R, Sukthana Y (2010) Is Sabin-Feldman dye test using T. gondii tachyzoites from animal inoculation still the best method for detecting Toxoplasma gondii antibodies? Southeast Asian J Trop Med Public Health 41: 1059-1064. Link: https://goo.gl/86KXwF
- Reiter-Owona I, Petersen E, Joyson D, Aspork H, Darde ML, et al. (1999) The past and present role of Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull WHO 77: 929-935. Link: https://goo.gl/v3UmG6
- Dubey JP, Fair PA, Bossart GD, Hill D, Fayer R, et al. (2015) Comparison of several serologic tests to detect antibodies to Toxoplasma gondii in naturally exposed bottlenose dolphins (Tursiops truncates). J Parasitol 91: 1074-1081. Link: https://goo.gl/E3ZMTJ
- Desmonts G, Remington JS (1980) Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol 11: 562–568. Link: https://goo.gl/dG9TXY
- Dubey JP, Lauri E, Kwowk OC (2016) Validation of the modified agglutination test for the detection of Toxoplasma gondii in free-range chickens by using cat and mouse bioassay. Parasitol 143: 314-319. Link: https://goo.gl/T6dZw7
- Garcia JL, Navarro IT, Vidotto O, Gennari SM, Machado RZ, et al. (2006) Toxoplasma gondii: Comparison of a rhoptry-ELISA with IFAT and MAT for antibody detection in sera of experimentally infected pigs. Exp Parasitol 113: 100-105. Link: https://goo.gl/v6xMTQ
- Sroka J, Cencek T, Ziomiko I, Karamon J, Zwolinski J (2008) Preliminary assessment of ELISA, MAT, and LAT for detecting Toxplasma gondii antibodies in pigs. Bull Vet Inst Pulawy 2: 545-549. Link: https://goo.gl/D966nX
- Minho A, Freire R, Vidotto O, Gennari S, Marana E, et al. (2004) Evaluation of the indirect fluorescent antibody test and modified agglutination test for detection of antibodies modified agglutination test for detection of antibodies against Toxoplasma gondii in experimentally infected pigs. Pesq Vet Bras 24: 199-202. Link: https://goo.gl/EUbuaW
- Macrì G, Sala M, Linder A, Pettirossi N, Scarpulla M (2009) Comparison of indirect fluorescent antibody test and modified agglutination test for detecting Toxoplasma gondii immunoglobulin G antibodies in dog and cat. Parasitol Res 105: 35-40. Link: https://goo.gl/uEzhfq
- Dubey JP, Desmont G (1987) Serological responses of equids fed fed Toxoplasma gondii oocysts. Equine Vet J 19: 337-339. Link: https://goo.gl/Aokim8
- Wiengcharoen J, Thompson RC, Nakthong C, Rattanakorn P, Sukthana Y (2011) Transplacental transmission in cattle: is Toxoplasma gondii less potent than Neospora caninum? Parasitol Res 108: 1235-1241. Link: https://goo.gl/5bnxsX
- Chumpolbanchorn K, Anankeatikul P, Ratanasak W, Wiengcharoen J, Thompson A, et al. (2009) Prevalence of Toxoplasma gondii indirect fluorescent antibodies in naturally and experimentally infected chickens (Gallus domesticus) in Thailand. Acta Parasitologica 54: 194-196. Link: https://goo.gl/iJwDcr
- Mose JM, Kagira JM, Karanja SM, Ngotho M, Kamau DM, et al. (2016) Detection of Natural Toxoplasma gondii Infection in Chicken in Thika Region of Kenya Using Nested Polymerase Chain Reaction. Biomed Res Int. Link: https://goo.gl/kkRW4r
- Abraham-Sandi E, Vargas-Brenes O (2005) Serological prevalence of Toxoplasma gondii in free-range chickens from Costa Rica. Trop Anim Health Prod 37: 369-372. Link: https://goo.gl/XxV817
- Brandão GP, Ferreira AM, Melo MN, Vitor RW (2006) Characterization of Toxoplasma gondii from domestic animals from Minas Gerais, Brazil. Parasite 13: 143-149. Link: https://goo.gl/rTexcn
- Sukthana Y, Chintana T, Supathanapong W, Siripanth C, Lekkla A, et al. (2000) Prevalence of Toxoplasmosis in selected populations in Thailand. Trop Med Parasitol 23: 53-58.
- Sukthana Y, Kaewkungwal J, Jantanavivat C, Lekkla A, Chiabchalard R, et al. (2003) Toxoplasma Gondii Antibody In Thai Cats And Their Owners. Southeast Asian J Trop Med Public Health 34: 733-738. Link: https://goo.gl/qs5gxP
- Sukthana Y (2006) Toxoplasmosis: beyond animals to humans. Trends Parasitol 22: 137-142. Link: https://goo.gl/JF9dcf
- Sreekumar C, Graham DH, Dahl E, Lehmann T, Raman M, et al. (2003) Genotyping of Toxoplasma gondii isolates from chickens from India. Vet Parasitol 118: 187-194. Link: https://goo.gl/DMboh5
- Xu P, Song X, Wang W, Wang F, Cao L, et al. (2012) Seroprevalence of Toxoplasma gondii infection in chickens in Jinzhou, northeastern China. J Parasitol 98: 1300-1301. Link: https://goo.gl/isT6B6
- Dubey JP, Huong LT, Lawson BW, Subekti DT, Tassi P, et al. (2008) Seroprevalence and isolation of Toxoplasma gondii from free-range chicken in Ghana, Indonesia, Italy, Poland and Vietnam. J Parasitol 94: 68-71. Link: https://goo.gl/6619M5
- Gebremedhin E, Tesfamaryam G, Yunus H, Duguma R, Tilahun G, et al. (2015) Seroepidemiology of Toxoplasma gondii infection in free-range chickens (Gallus domesticus) of Central Ethiopia. Epidemiol Infect 143: 608-617. Link: https://goo.gl/X3znih
- Ayinmode AB, Olaosebikan RI (2014) Seroprevalence of Toxoplasma gondii infection in free ranged chicken from rural and urban settlements in Oyo State, Nigeria. Afr J Med Med Sci 43: 51-57. Link: https://goo.gl/eh8xfK
- Mercier A (2010) Approche écologique, épidémiologique et génétique de la biodiversité de Toxoplasma gondii en zone tropicale humide: exemples du Gabon et de la Guyane Française 1-289. Link: https://goo.gl/fXKsVh
- Sudan V, Jaiswal A, Shanker D (2013) Recent trends in the diagnosis of toxoplasmosis. Clin Rev Opinions 5: 11-17. Link: https://goo.gl/4mMcDx
- Aroussi A, Vignoles P, Dalmay F, Wimel L, Dardé ML, et al. (2015) Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite 22: 14. Link: https://goo.gl/AMgXoP
- Sedla K, Literak I, Vitula F, Benak J (2000) High susceptibility of partridges (Perdix perdix) to toxoplasmosis compared with other gallinaceous birds. Avian Pathol 29: 563-569. Link: https://goo.gl/QoEiaw
- Dubey JP, Ruff MD, Camargo ME, Shen SK, Wilkins GL, et al. (1993) Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am J Vet Res 54: 1668-1672. Link: https://goo.gl/EuD3Fb
- Sunanta C, Inpankaew T, Pinyopanuwat N, Chimnoi W, Kengradomkij C, et al. (2009) Comparison of diagnostic techniques for detection of Toxoplasma gondii infection in dairy cows in Thailand. Kasetsart J (Nat. Sci.) 43: 48-52. Link: https://goo.gl/EtyLnB
- Casartelli-Alves L, Boechat VC, Macedo-Couto R, Ferreira LC, Nicolou JL, et al. (2014) Sensitivity and specificity of serological tests, histopathology and immunohistochemistry for detection of Toxoplasma gondii infection in domestic chickens. Vet Parasitol 204: 346-351. Link: https://goo.gl/zkmksL
- Kotresha D, Noordin R (2010) Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 118: 529-542. Link: https://goo.gl/UufXSW
- Holec-Gasior L (2013) Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin Vaccine Immunol 20: 1343-1351. Link: https://goo.gl/UYDV13
- Gatkowska J, Dziadek B, Dziadek J, Dzitko K, Dlugonska H (2015) Recombinant MAG1 Protein of Toxoplasma gondii as a Diagnostic Antigen. Pol J Microbiol 64: 55-59. Link: https://goo.gl/JmYCgj
- Singh H, Tewari A, Mishra A, Maharana B, Sudan V, et al. (2015) Detection of antibodies to Toxoplasma gondii in domesticated ruminants by recombinant truncated SAG2 enzyme-linked immunosorbent assay. Trop Anim Health Prod 47: 171-178. Link: https://goo.gl/2sW1Yp
- Zhang D, Wang Z, Fang R, Nie H, Feng H, et al. (2010) Use of Protein AG in an Enzyme-Linked Immunosorbent Assay for Serodiagnosis of Toxoplasma gondii Infection in Four Species of Animals. Clin Vaccine Immunol 17: 485-486. Link: https://goo.gl/qu3nTR
- Wang Z, Ge W, Huang S, Li J, Zhu X, et al. (2014) Evaluation of recombinant granule antigens GRA1 and GRA7 for serodiagnosis of Toxoplasma gondii infection in dogs. BMC Vet Res 10: 158. Link: https://goo.gl/92h1wu
- Wang Z, Ge W, Li J, Song M, Sun H, et al. (2014) Production and evaluation of recombinant granule antigen protein GRA7 for serodiagnosis of Toxoplasma gondii infection in cattle. Foodborne Pathog Dis 11: 734-739. Link: https://goo.gl/CdgXNp
- Sun X, Wang Z, Li J, Wei F, Liu Q (2015) Evaluation of an indirect ELISA using recombinant granule antigen GRA1, GRA7 and soluble antigens for serodiagnosis of Toxoplasma gondii infection in chickens. Res Vet Sci 100: 161-164. Link: https://goo.gl/iHwSJH
- Hamidinejat H, Nabavi L, Mayahi M, Ghourbanpoor M, Pourmehdi Borojeni M, et al. (2014) Comparison of three diagnostic methods for the detection of Toxoplasma gondii in free-range chickens. Trop Biomed 31: 507-513. Link: https://goo.gl/BFvoXZ
- Millar P, Alves F, Teixeira V, Vicente R, Menezes E, et al. (2012) Occurrence of infection with Toxoplasma gondii and factors associated with transmission in broiler chickens and laying hens in different raising systems. Pesq Vet Bras 32: 231-236. Link: https://goo.gl/HuaSeP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
