Open Journal of Tropical Medicine
Impact of Irrigation System on Malaria Transmission in Jazan Region, Saudi Arabia
Mohammed Hassan Alzahrani1, Philip McCall2, Amir Hassan2, Abdiasiis Ismael Omar1* and Abdelmohsin Mohammed Abdoon1
2Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Cite this as
Alzahrani MH, McCall P, Hassan A, Omar AI, Abdoon AM (2017) Impact of Irrigation System on Malaria Transmission in Jazan Region, Saudi Arabia. Open J Trop Med 1(1): 007-015. DOI: 10.17352/ojtm.000002Introduction: Jazan Region, in the Southwestern Saudi Arabia is known as the mostly affected region with malaria. Malaria occurs at hypo-to hyperendimic level where P. falciparum is the commonest parasite and An. arabiensis is the principal vector. Different types of crop irrigation systems were often blamed for aggravating the health risks of local communities. Reservoirs, irrigation canals, and dams are closely associated with vector-borne parasitic diseases. Thus, seasonal transmission period of a disease such as malaria may be extended as a result of these irrigation systems. The present study is meant to investigate vector breeding and transmission sites associated with the irrigation system in Jazan area for malaria through vector sampling and direct observation.
Methods: The study was conducted in three villages in irrigated and three villages in non-irrigated areas in Jazan Region. Entomological surveys were conducted over 12 months from January to December 2004. Larvae were sampled by standard dipping technique and adults were sampled by pyrethrum knockdown collection and CDC light traps. Anopheline mosquitoes were morphologically identified and further verified at the Natural History Museum, London, UK. Mosquito blood meals were tested by direct enzyme linked immune sorbent assay technique for source identification.
Results: Of the 3498 anophelines larvae collected, during this study, 53.2% were from the irrigated sites. The total collection of larvae revealed the prevalence of 7 Anopheles spp., of these An. d`thali and An. pretoriensis were the most abundant, and comprised about 50.77 % and 36.91%, of the total collection, respectively. Larvae of the known malaria vector, An. arabiensis comprised 9.43% (330), of which, 69.4% were harvested from habitats at the irrigated area. A total of 2938 adult anophelines were caught from both areas and 1539 (52.4%) were An. d`thali, An. multicolor and An. arabiensis from irrigated area. A total of 106 blood-meal specimens from An. d`thali and An. arabiensis females, were examined and 95.3% were of human origin.
Discussion and Conclusion: Of the seven anopheline species encountered, An. sergentii, An. multicolor and An. fluviatilis are first record in Jazan Region. The study showed no significant difference between irrigated and non-irrigated areas can provide mosquitoes with various breeding habitat types. The known malaria vector in the region, An. arabiensis were more abundant in irrigated areas as larvae (69.3%) and as adults (69.4%) and together with An. d`thali showed high tendency towards anthropophagic behaviour (95.33%).
Introduction
Environmental changes and ecological disturbances, due to both natural phenomena and human intervention, have exerted and continue to exert a marked influence on the emergence and proliferation of parasitic diseases [1,2]. Many diseases are associated with water either directly, such as the diarrhoeal diseases transmitted by ingestion of infected water, or indirectly, where a disease’s intermediate hosts or vectors depend on water for their development. In the latter case, the abundance and the consequent prevalence of the disease are often inadvertently increased when water resources are developed. Health Impact Assessment (HIA) is an approach that aims to identify, predict, safeguard and mitigate the impact of development projects on health; the assessment procedure involves identification of health hazards, interpretation of the health hazards as health risks attributable to the project and finally health risk management [3-6], conducted studies for predicting when vector-borne diseases might increase following water resource development and [7], Investigated changes in parasitic diseases in the same situations [8]. Mentioned that several case studies showed that dams may alter the composition and density of vectors and intermediate host species, increase the incidence of malaria schistosomiasis and possibly lymphatic filariasis. The water-based and water-related vector-borne diseases are most likely to be found in areas where irrigation has introduced large new water surface areas [9]. These include Malaria, Schistosomiasis and Lymphatic filariasis.
Wadi Jazan Dam (1703’N, 4258’E; 15 km east of Abu Arish, Jazan Region) is probably the largest and most variable expanse of freshwater habitats in the southwestern (SW) provinces of Saudi Arabia. It was constructed to provide year-round water for irrigation purposes and for flood control. The reservoir is supplied by water from the major wadis and has a very large catchments area, extending south into Yemen. The depth of the reservoir has been reduced following sedimentation and, in flood periods, the reservoir can cover an area of 1,000 ha. Large quantities of silt have effectively cut off some pools and larger expanses of open water from the main reservoir; one can be regarded as an almost permanent lake of about 60 ha (MOA, 1995). There is a major possible change in land use in Jazan Dam area. Intensive cultivation continues to increase as the local human population expands [10]. The impact of water development schemes on health is unknown. The increase in the local human population poses the biggest threat of vector-borne diseases such as malaria and rift valley fever as more and more land is cleared for housing and agriculture. Methods of cultivation, field and water surface sizes and typical circular thatched dwelling huts give the Jazan Dam area, including nearby Wadis [10].
Following the extensive malaria control program in Saudi Arabia, malaria is now restricted to the southwestern part of the country (Tihama lowlands). About 5% of the national population of Saudi Arabia are at risk (1.04 million). Jazan region is in the SW of Saudi Arabia and bordering Yemen (the most malarious country in the region) accounting on average for 60-70% of all locally acquired malaria cases recorded during the period from 1983 to 2001 [11]. In wadis (valleys) and villages at the foothills of Sarawat Mountains in Jazan region where P. falciparum is common (over 90% of cases) and where rainfall is relatively abundant, malaria occurs as meso- to hyperendemic level [12,13].
Jazan region is divided into four zones with regard to malaria endemicity
Malaria free zone: malaria is absent in mountain areas at 1500 meter above sea level, e. g. Feifa and Al Hasa areas. It is also absent in the Farasan Islands.
Low endemicity zone: transmission in this zone is unstable and the area is subject to malaria epidemics. Periodic rains in the hills can lead to flooding in the wadis. This can leave water pockets suitable for breeding of mosquitoes on the coastal plain. In the past the spleen rate in this zone was found to be less than 10% [14,15].
Moderate endemicity zone: Malaria incidence in this zone is also unstable. Most the inhabitants in this zone are exposed to malaria transmission during periods of up to six months every year, depending on the pattern and amount of rain. This zone includes the interior plains and foothills. This is also where most of the population of Jazan reside, especially along the wadis. The spleen rate in this zone was 10- 50% [14,15].
High endemicity zone: Malaria in the Yemeni border areas is stable. Malaria cases reported from this zone constitute more than 50%of all cases reported in Jazan. Water suitable for mosquito breeding is available throughout the whole year. This persists in most areas despite repeated control measures. The malaria transmission season in Jazan extends from October to April with a prominent peak in January. The major factor that influences the extent and intensity of transmission season is rain [15].
The present study aims to investigate vector breeding and transmission sites associated with the irrigation system in Jazan region for malaria through vector sampling and direct observation.
Materials and Methods
Regional Profile: Jazan region lies in the southwest of the country, between 16” 12’ and 18” 25’ latitude north and altitude 420 00′ and 430 25′. It is bordered in the east by Sarawat Mountains, in the south by Arab Republic of Yemen and in the west by the Red sea. The total area of Jazan is about 22,000 square kilometers. The Jazan region runs along the Red Sea coast for almost 200 miles (300 km) and includes some 100 islands. In 2010 and according to the census, the population was 1.37 million, with 30% concentrated in six major cities, and the remainder living in over 1000 villages. Most of these villages of 50-500 people are scattered in remote areas, without ease of access. Jazan is one of the Kingdom’s richest agricultural regions. Topographically, it is divided into three zones: the mountains in the east (2,500 meters above sea level), the foothills (400-600 meters above sea level) and the extensive plains (less than 400 meters) occupying the area between the foothills and the Red Sea [15]. The region is drained by several permanent wadis, which play a crucial role in providing perennial habitats for mosquitoes and several intermittent wadis, which are wet except in the dry season and which are important in increasing the surface water providing many favourable breeding habitats and directly influence abundance of anopheline vectors. These wadis maintain a continuous steady flow into the foothill area [15,16]. During the period of heavy rains, the wadis may flood and water is carried along the entire wadis to the Red Sea. Thus water flow in these wadis is not stable and is subjected to various fluctuations depending on the amount of rainfall in the mountains. Water run off these wadis ranges from steady flow, excessive flow or sweeping flood. Under these situations the breeding places are not constant or stable and continuously depend on the water flow in the wadi. Twenty major wadis that drain rainwater from the mountains into the Red Sea traverse Jazan and have hundreds of tributaries in the foothill areas [15].
Study area
The area for study is divided into two; irrigated and non-irrigated sites as follows:
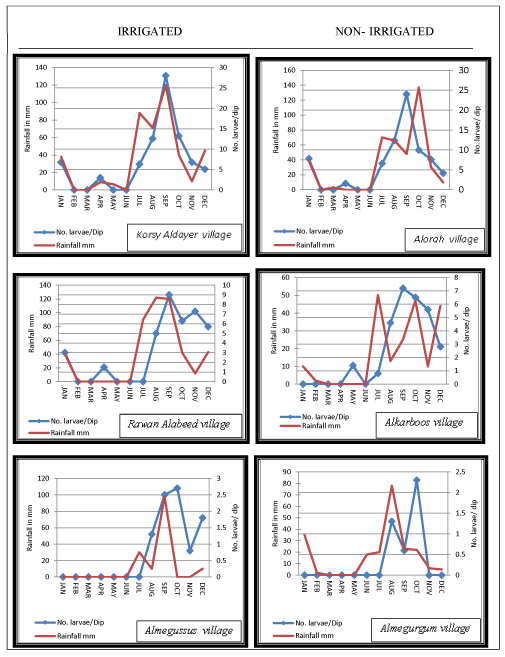
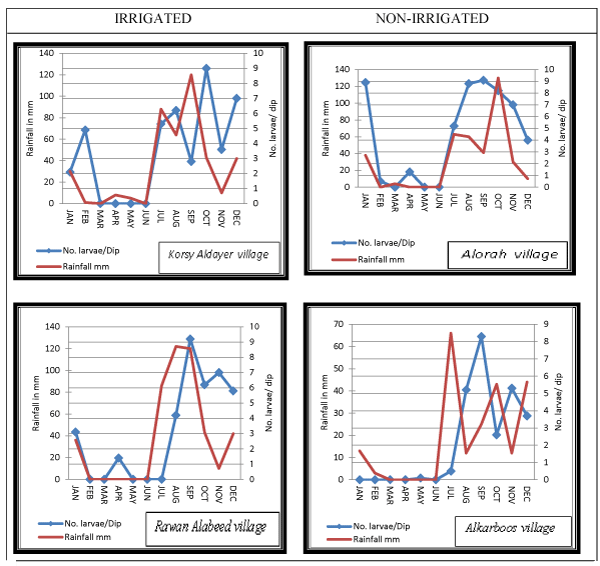
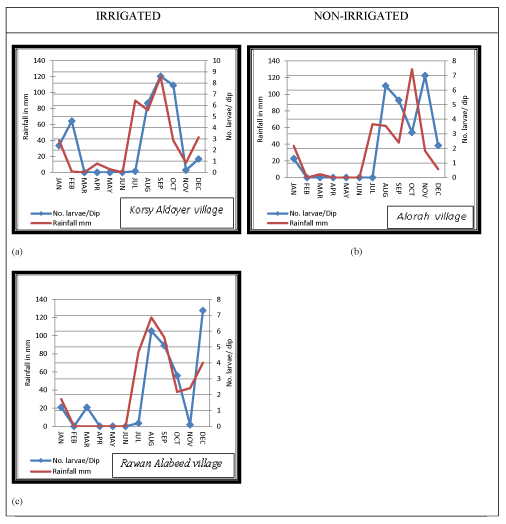
(1) Irrigated site at Jazan Dam and Irrigation Project at Wadi Jazan. Three villages in the irrigated site were randomly selected including; Korsy Aldayer village, Rawan Alabeed village and Almegussus village (Figure 1).
(2) Non-irrigated site in the control area (Khulab Valley), located 25 km from the irrigated site. Three villages were selected randomly, namely; Alorah village, Alkarboos village and Almegurgum village (Figure 1).
In the study area the population live mostly in villages, few of which have more than 500 inhabitants. Typically, the population ranges between 50 and 150 persons per village. Nearly, all the villages in the plain are situated along wadis and seldom lie further than 1km from the wadi. The natural vegetation along the wadis is dominated by Acacia spp. and Tamarix sp. The income is derived from sheep raising, commerce and the cultivation of sorghum. Each family maintains approximately 10 sheeps and goats and one cow, but wealthy herdsmen may own hundreds and hire shepherds. Most villages have electricity and a proportion of the population live in air-conditioned rooms. A few inhabitants have electric generators that they utilize at night. Inhabitants who have no electricity or air-conditioning sleep outside nearly all the year round, specially in the hot weather. Education is available for all levels until secondary school for boys and girls. Health services in the study area are represented by one primary health care center and two general hospitals. All health service facilities have laboratories and the first line treatment of malaria are available.
The locations and altitudes of villages selected for the study in the irrigated and non-irrigated sites are shown in table 1. Jazan Irrigation Project is located in Almegussus area below the dam. Korsi Aldayer and Rawan Alabeed are a mountainous areas intersected by 4 main wadis and their tributaries, and with many hills and stony projections.Which reduce the available agricultural areas to patches of clay dependent on rain for cultivation of crops, mostly sorghum. Grazing grass is abundant during the rainy season.
Larval collections: Surveys were conducted over 12 months from January to December 2004 in the selected sites. Each site was sampled once per month. The wadis were sampled in stretches of approximately 1 km in length. Typical breeding places examined were static pools of water formed at the edges of the wadis and temporary pools resulting from overflow, seepage or receding water. Within nearby villages or farmland, all water bodies were examined for mosquitoes. All potential mosquito breeding places were visited and checked for the presence of water on each visit and those with water were examined for mosquitoes. Standard dippers of 350 ml volume were used to collect larvae. Collections were made following the protocols described by [17,18]. Hence, pools of one square meter or less in size were dipped five times while pools of larger size were sampled with more than 5 and number of dips were recorded for each sample to calculate the larval density.
Adult mosquito collection
a) Knockdown-sheet collections (K-SC): Sampling was carried out during the rainy season (from September to December 2004) once per month at each study site. On each visit, five houses were selected randomly in each site and K-SC carried out between 06:00 and 10:00 am. Selected rooms were evacuated from edible matters, white sheets were spread carefully on the ground all around the room and all openings of the room were closed leaving no way for mosquitoes to escape. Pyrethroid insecticide spray (RAID®) were used to knockdown mosquitoes and the room were left closed for at least 10 min after spray and before mosquito collection started. Mosquitoes of each room were gathered into a separate mosquito collection box with collection site, date and room number recorded.
b) CDC Light -trap collection: CDC light-traps baited with CO2 (Sudia & Chamberlain, 1962) operated from 6-V rechargeable gel-cell batteries were used to sample indoor and outdoor mosquitoes in each village houses and shelters. The body of the trap was made from a 15cm length of 8.25cm internal diameter. A slot on each side permitted the insertion of the motor support bracket for holding the motor. A 4-V bulb was mounted directly above the motor at the top of the trap body. Color-coded binding posts and ‘snap-on’ terminals permitted easy connection of leads from the battery and motor assembly. A detachable wire mesh screen was placed over the entrance of the trap to exclude large insects. A fine mesh cloth-collecting bag with a narrow neck was fitted to the end of the trap body. In bedrooms where normally people don’t use bed nets or shelters with ceilings, it was suspended about 15cm below the ceiling, opposite a window and close to sleeper or animal. Light traps were placed in randomly selected houses or animal shelters at each study village in both irrigated and non-irrigated sites and left to run from 18:00 to 06:00 hours period. Five traps were installed once per month in each of the six villages. These collections were conducted from January to December 2004.
Identification of mosquitoes: As larviciding operations by Jazan vector control centers occur weekly, larvae collection in the present study preceded these operations. Thus most of the larvae collected were at their early stages of development. Complete development in the laboratory of the early stages to 3rd and 4th stages larvae or further to adult stage was required for morphological identification. Mosquitoes (larvae and adults) were identified according to the keys of [19,20], All mosquito specimens were preserved in plastic bags containing silica gel and transferred to Liverpool School of Tropical Medicine for further analysis. Morphological identifications were further confirmed by Dr. Ralph Harbach at the Natural History Museum, London, United Kingdom.
Blood meal analysis: A Direct Enzyme Linked Immuno-Sorbent Assay (ELISA) [21], was used to identify the source of blood meals of mosquitoes collected by PKD. Each sample was tested for reactions to anti-sera for human, cattle, goat, sheep, dog, chicken, donkey and camel. Blood squash spots of mosquito were cut out and placed in labelled 1.5 ml eppendorf tubes to which 500 µI of phosphate buffered saline (PBS) were added and incubated at 4°C overnight to elute blood. A 96 well polyvinyl chloride (PVC) microtitre plate (Nuclon Surface) was first loaded with blood sample extract (50 gl/well). After an incubation of 3 hours at room temperature, the well contents were discarded and washed three times with PBS+0.05% Tween 20 (PBS-Tween 20). Peroxidase conjugated IgG antibody (diluted at a ratio of 1: 500 in blocking buffer) was added to wells (50 µl/ well) followed by another incubation period of 1 hour at room temperature. The blocking buffer contained sera of various possible potential hosts that were present in the collection area. The plates were then washed three times with PBS Tween 20. Finally, 100 µl of substrate solution of ABTS (2,2’ azino-bis 3-ethylbenzthiazoline-6-sulphonic acid) and hydrogen peroxide (Kirkegaard and Perry Laboratories, USA) mixed 1: 1 immediately before use, was added to each well and allowed to develop. Positive specimens turned green and all the results were read visually after 30 minutes.
Results
A total of 3498 anopheline larvae were collected and identified during the present study. The collection revealed the presence of eight species of anopheline larvae, namely, An. d`thali, An. pretoriensis, An. arabiensis, An. sergentii Theobald*, An. multicolor Cambouliou*, An. rhodesiensis rupicolus, An. turkhudi Liston* and An. fluviatalis. As shown by table 2, An. d`thali was the most frequent species in the study area, comprised more than 50 % of the total larvae collected, followed by An. pretoriensis larvae comprising about 37% of the total collection. An. arabiensis, the principal malaria vector in Jazan Region, comprised less than 10%. An. sergentii, An. multicolor, and An. turkhudi were very scanty in their presence and were considered as first record in Jazan Region.
In both, irrigated and non-irrigated sites of the study area, An. d`thali and An. pretoriensis larvae constituted the most abundant among other prevalent Anopheles species. An. arabiensis was encountered in two villages of the irrigated site, while only one village of the three investigated showed its existence (Table 3). However, it showed low collection rate compared to An. d`thali and An. pretoriensis and the difference is highly significant (p <0.05). The majority of anopheline larvae were collected from villages located at the upper part of wadis in both irrigated and non-irrigated sites, 77.4% of the total catch at the irrigated sites was collected from Korsi Aldayer village and 95.3% of the total catch of the non-irrigated site was collected from Alorah village. Conversely, very few were collected from villages at the lower wadis, less than 1% of the total catch at the irrigated site was collected from Almegussus village and less than 1% of total catch of the non-irrigated site was collected from Almegergum village. In the middle reaches of wadis in irrigated and non-irrigated sites, 21.5% of anopheline larvae were obtained from Rawan Alabeed village (upper Jazan dam in the irrigated sites) compared with very few (4.1%) collected from Alkarboos village in the non-irrigated site (Table 3). In the irrigated site, about 12.7% of An. arabiensis larvae were collected from 2 villages located above the Jazan dam, while nothing was obtained from Almegussus village located below the dam.
Interestingly, in Rawan Alabeed village which located above Jazan dam and very close to the lake, the abundance of An. arabiensis fluctuated over the months of the study. A significance t test shows that there is no significant difference between irrigated and non-irrigated site in anopheline larvae collection rate. In the non-irrigated site, a total of 101 larvae of An. arabiensis were collected from Alorah in the upper part of the wadi and there were no An. arabiensis larvae recorded in the other two villages (Alkarboos in the middle part of the wadi and Almegurgurn in the lower part of the wadi (Table 4).
A total of 2938 anopheline adults were collected during the study period (Table 5). In adult collections, 53% were An. d`thali Patton, 31% were An. pretoriensis Theobald, and 7% were An. arabiensis. Of all anopheline mosquitoes collected in this study, two species, An. arabiensis and An. d`thali are known vectors of malaria elsewhere. An. fluviatalis was collected as adult but not as larvae. This was the first record of this species in Jazan Region.
In all study villages the larval density of Anopheles spp. was closely associated with rainfall pattern. Peaks of rainfall amount was mostly followed by increased larval density (Figures 1-3). An. d`thali was found in all study villages and was observed to occur between June and February, with the highest peak in September. This period coincides with peaks of rainfall recorded (Figure 1). The range of density of this species in the study area lied between 0 – 30 larvae/ dip. However, the larval density between irrigated and non-irrigated site was not statistically significant (p >0.05). An. pretoriensis was found in 2 villages of the irrigated site and 2 villages of the non-irrigated site. The range of density of this species in the study area lied between 0 – 10 larvae/ dip. Its larval density appeared in June and continued up to February with the highest peak recorded in September (Figure 2). Larvae of An. arabiensis was collectable from 2 villages (Korsy aldayer and Rawan Alabeed) at irrigated site and from only one village (Alorah) at non-irrigated site. The density range of this species was 0 -9 larvae/ dip. In both, irrigated and non-irrigated sites larval density of An. arabiensis started to appear in August and continued till March. In the irrigated site the peak of density was observed in September at Korsy Aldayer village and in December at Rawan Alabeed village. In Alorah village at the non-irrigated site the peak of An. arabiensis larval density was found to occur in November.
Blood-meal analysis; A total of 106 anopheline (An. d`thali and An. arabiensis) blood meal specimens were stored from PKD collections from both irrigated (78) and non-irrigated (28) areas and assayed by ELISA. In both irrigated and non-irrigated sites human was the most common blood meal source in all species (Table 6). In the irrigated site, more than 90% of the blood meal specimens of An. d`thali were of human origin, while only 64% of An. arabiensis fed on human. In non-irrigated site both species relied entirely on human as a source of blood (94% - 100% human feeding rate). The human blood feeding rates were approximately the same for anopheline species 82% (n-64) in irrigated site with 96.4 % (n-27) in non-irrigated site.
Discussion
This study was conducted following the extensive use of pesticides during the 2000 epidemic of RVF in Saudi Arabia that consequently led to rapid decline in malaria cases reports the following years as was noticed during this study. The entomological surveys of the present study indicated that Anopheles spp., including vectors of malaria, breed readily in both irrigated and non-irrigated sites, and the significance t test shows that there is no significant difference between irrigated and non-irrigated sites. However, irrigated sites were found to provide more water surface area, and hence many more suitable breeding places. Most of the anopheline larvae were obtained from the irrigated site, but the difference is not significant compared to those from non-irrigated site. In the middle reaches of Wadis at irrigated and non-irrigated sites, 389 anopheline larvae were collected from Rawan Alabeed (upper Jazan dam in the irrigated area) compared to the few collected from Alkarboos in the non-irrigated site (Table 3). This can most likely be attributed to the number of breeding sites available at each site: at Rawan Alabeed there was Jazan Dam Lake and Jazan Wadi providing perennial sources for breeding but at Alkarboos, the non-irrigated site, there was only one perennial wadi (Khulab Wadi) which remained dry until the seasonal rains. Seasonal rain usually increases the numbers of mosquito breeding places, while higher relative humidity and growth of vegetation cover provided a cool shaded environment for the development of aquatic stages and aided the survival of young adults [22-24]. Most of the sources of breeding places in the area of study were wadis, although, they were dry during much of study period due to the low rainfall. The total amount of rainfall recorded in the study period (Jan. to Dec. 2004) was 182.2mm and during 3 months (May, June, July) there was no rainfall. Interestingly, in the irrigated site the abundance of larval population of An. arabiensis and other anophelines was not correlated with rainfall while in the non-irrigated site the abundance of larvae of the two species was correlated with rainfall pattern. In Rawan Aalabeed, located at the upper Jazan dam area and very close to the Dam Lake, the abundance of An. arabiensis was 1.3 larvae/dip in March 2004 although no rain fell from February to May 2004 (Figure 3c). This was most likely because the dam lake and the irrigation project created additional suitable breeding places for this species. A study in Jazan Region to determine the vectors of RVF found that more breeding sites were found in the Jazan dam area providing good mosquito larval habitats (Jupp et al., 2002).
In addition to the widespread use of insecticides, the shortage in rain during the study period (as compared with previous four years) was one of the limitations of adult vector density in both irrigated and non-irrigated sites. Clearly, the amount of rain in both sites and the creation of additional breeding places by irrigation in irrigated site can influence the quantity and quality of breeding sites available for breeding of An. arabiensis. In the dry savanna of Africa, vector species can have seasonal fluctuations in abundance, declining to low levels in dry seasons [25-28]. ELISA test conducted in the present study showed that An. d`thali and An. arabiensis rely predominantly on human blood. The other blood meals were taken from cattle, mainly by An. arabiensis in the irrigated site. The domestic animals found in both areas were mainly goats, sheep, cattle, camel and donkey. Although cows were available in both areas, there were more cows in irrigated site (estimated at 250 compared to 30 cows or less in the non-irrigated site). The presence of domestic animals has been associated with a decrease in malaria transmission rates due to zoophilic deviation [29]. In some parts of Africa, zooprophylaxis is used and cattle are intentionally kept near or inside houses to divert mosquitoes from humans to cattle [30]. It was suggested that cattle could play a role in reducing transmission of malaria by An. arabiensis by distracting the prints create more breeding sites. In East Africa, An. arabiensis shows a preference for feeding on cattle, sheep, goats and donkeys [31,32]. All these hosts were common in our study in the irrigated area. In Kenya An. arabiensis has been reported to move into houses after feeding outside on cattle [1,33,34]. Also showed that a high proportion of An. arabiensis resting inside human habitations in northern Tanzania had fed on cattle. For control measures, preventing mosquito feeding on animals by application of insecticide on domestic animals might also be useful [35,36].
The authors are indebted to the General Health Affairs Directorate of Jazan Region for providing field and laboratory facilities to carry out this work. We are also thankful to all our colleagues, the technical staff of the Vector-borne Diseases Control Administration for their co-operation and encouragement during this work.
- Patz JA, Graczyk TK, Geller N, Vittor AY (2000) Effects of environmental change on emerging parasitic diseases. Int J Parasitol 30: 1395-1405. Link: https://goo.gl/YMDwZr
- Brunner FS, Eizaguirre C (2016) Can environmental change affected host/parasite-mediated speciation? Zoology (Jena) 119: 384-394. Link: https://goo.gl/i9s7bj
- Birley MR, Peralta GL (1995) Health impact assessment of development projects, pp. 153-170 in Vanclay, F., and Bronstein, D. A. (eds.) Environmental and Social Impact AssessmentJ, ohn Wiley and SonsL td., Chichester.
- Birley MH (1991) Guidelines for forecasting the vector-borne disease implications of water resources development. Geneva, World Health Organization. Link: https://goo.gl/Dfs3Ht
- Birley MH (1995) The health impact assessmenot f developmentp rojects. London, HMSO Link: https://goo.gl/AFnc97
- Birley MH, R Bos CE, Engel, P Furu (1996). "A multi-sectoral task-based course: Health opportunities in water resources development". Education for Health: Change in Training and Practice 91: 71-83.
- Hunter JM, L Rey, KY Chu, EO Adekolu-John, KE Mott (1993) Parasitic Diseases in Water Resources Development. The Need for Intersectoral Negotiation. World Health Organization, Geneva. Link: https://goo.gl/ve59rC
- Yewhalaw D, S Hamels, Y Getachew, PR Torgerson, M Anagnostou, et al. (2014) Water resource developments in Ethiopia: potential benefits and negative impacts on the environment, vector-borne diseases, and food security. Environmental Reviews 22: 364-371. Link: https://goo.gl/hqGKrd
- Tiffen M (1991) Guidelines for the incorporation of health safeguards into irrigation projects through intersectorial cooperation. PEEM Guidelines series 1 World Health Organization, Geneva. Link: https://goo.gl/xgiU7s
- Rahmah T (1994) Study of Jizan dam, Saudi Arabia. M Sc. Thesis, King Saud University, Riyadh, Saudi Arabia.
- AI-Sheikh A (2004) Studies on the ecology, vectorial role and population structure of Anopheles arabiansis in the Tihama region of Saudi Arabia and Yemen. PhD Thesis, Liverpool University, Liverpool. Link: https://goo.gl/90xs5z
- Al-Seghayer SM (1983) Control of malaria in the Kingdom of Saudi Arabia. Postgraduate Diploma Thesis. University of Leeds, UK. Link:
- Al-Seghayer SM, Kenawy MA, Ali OT (1999) Malaria in the Kingdom of Saudi Arabia epidemiology and control. Scientific J of King Faisal University 6-20. Link: https://goo.gl/X3e8nP
- MOH (1984) Malaria Control Programme in the Kingdom, 1983 .Malaria Control Service, Saudi Arabia.
- Ageel A (1990) Changing Epidemiological Pattern of Malaria during the period from 1981-1989 in Gizan Province-Saudi Arabia with Particular Reference to Control Measures. M.Sc. Thesis, Military Medical Academy, Cairo.
- Al-Sharif A (1983) "Geography of Kingdom of Saudi Arabia. The second part: Southwestern Region of Saudi Arabia, "Mareekh Riyadh.
- WHO (1975) Manual on Practical Entomology. Part II: Methods and Techniques. Geneva. World Health Organization.
- WHO (1992) Entomological field techniques for malaria control. Part 1: Geneva. World Health Organization.
- Mattingly PF, Knight KL (1956) The mosquitoes of Arabia. Bull. Brit. Mus. Nat. Hist. (Ent) 4: 91-141. Link: https://goo.gl/QwYJdT
- Du Bose W, Curtin T (1965) Identification Keys to the Adult and Larval Mosquitoes of the Mediterranean Area. Journal of Medical Entomology, 1: 349-355. Link: https://goo.gl/iDyy2A
- Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, et al. (1988) Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol 25: 9-16. Link: https://goo.gl/8bxXey
- Evans A (1938) "Mosquitoes of The Ethiopian Region II: Anophelini, "Oxford University Press, London". Link: https://goo.gl/9GZmIR
- Igbinosa IB (1989) Investigations on the breeding site preferences of mosquitoes in kpoma, Nigeria. Journal ofApplied Entomology 107: 325-330. Link: https://goo.gl/eLds44
- Okogun G, Nwoke B, Okere A, Anosike J, Esekhegbe A (2003) Epidemiological implications of preferences of breeding sites of mosquito species in Midwestern Nigeria. Annals of Agricultural and Environmental Medicine 10: 217-222. Link: https://goo.gl/UZa83A
- Taylor C, Toure Y, Coluzzi M, Petrarca V (1993) Effective population size and persistence of Anopheles arabiensis during the dry season in west Africa. Medical and Veterinary Entomology, 7: 351-357. Link: https://goo.gl/Uese6r
- Charlwood J, Alecrim W, Fe N, Mangabeira J, Martins V (1995) A field trail with Lambda-cyhalothrin (ICON) for the intradomiciliary control of malaria transmitted by Anopheles darlingi Root in Rondonia, Brazil. Acta Tropica 60: 3-13. Link: https://goo.gl/XwjJxJ
- Lemasson J, Fontenille D, Lochouarn L, Dia I, Simard F, et al. (1997) Comparison of behaviour and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera: Culicidae) in Barkedji, a Sahelian area of Senegal Journal Medical Entomology 34: 396-403. Link: https://goo.gl/PysVqo
- Simard F, Lehmann T, Lemasson J, Diatta M, Fontenille D (2000) Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirectet approach using microsatellite loci. Insect Molecular Biology 9: 467-479. Link: https://goo.gl/ROVcDB
- Bruce-Chwatt LJ, Garrett B, Weitz B (1966) Ten years study (1955-64) of host selection by anopheline mosquitos. Bull. Wd HIM Org 35: 405-439. Link: https://goo.gl/YEBsGN
- Burkot TR, Graves PM, Paru R, Lagog M (1988) Mixed blood feeding by the malaria vectors in the Anopheles punctulatus complex (Diptera: Culicidae). J Med Ento-mol 25: 205-213. Link: https://goo.gl/i35lgV
- White GB (1974) Anopheles gambiae complex and disease transmission in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 68: 278-301. Link: https://goo.gl/stQ5CZ
- Garrett-Jones C, Boreham PFL, Pant CP (1980) Feeding habits of anophelines (Diptera: Culicidae) in 1971-78, with reference to the human blood index: a review. Bulletin of Entomological Research 70: 165-185. Link: https://goo.gl/2y6OOU
- Githeko A, Brandling-Bennett A, Beier M, Atieli F, Owaga M, et al. (1992) The reservoir of Plasmodiumfalciparum malaria in a holo-endemic area of western Kenya. Transactions ofthe Royal Society of Tropical Medicine and Hygiene 86: 355-358. Link: https://goo.gl/BLrGNQ
- McCall P, Mosha F, Njunwa K, Sherlock K (2001) Evidence for memorized site-fidelity in Anopheles arabiensis. Transactions ofthe Royal Society of Tropical Medicine and Hygiene 95: 587-590. Link: https://goo.gl/5dptAj
- Hewitt S, Rowland M (1999) Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Tropical Medicine and International Health 4: 481-486. Link: https://goo.gl/zlwdIF
- Rowland M, Durrani N, Kenward M, Mohammed N, Urahman H, et al. (2001) Control of malaria in Pakistan by applying deltarnethrin insecticide to cattle: a community-randomisedtr ial. Lancet 357: 1837-1841. Link: https://goo.gl/Vyfdfk
- Ministry of Agriculture and Water. (1995). Dams in The Kingdom of Saudi Arabia. Projects Execution Department.
- Service MW (1993) The Anopheles vector. In Essential malariology, eds., H. M. Gilles, and D. A. Warrell. London: Edward Arno.
- Spiers AA (2003) Seasonality and life history traits of the Anopheles gambiae complex in MalawL PhD Thesis University of Liverpool.
- Service M, Townson H (2002) The Anopheles Vectors. In "Essential Malariology" (D. Warrell, & H. Gilles, Eds.), Arnold, London.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
