Open Journal of Trauma
Splenic trauma: Definition, classifications, clinical profiles and best treatments
Giulio Perrotta1*, Emanuele Guerrieri2 and Mario Guerrieri3
2Physician, “Medicina d‘Emergenza e Urgenza”, Università Politecnica delle Marche, Via Tronto n. 10/a, 60126, Torrette - Ancona, Italy
3Full Professor of surgery, “Clinica Chirurgica Generale e d’Urgenza”, Università Politecnica delle Marche, Via Tronto n. 10/a, 60126, Torrette - Ancona, Italy
Cite this as
Perrotta G, Guerrieri E, Guerrieri M (2021) Splenic trauma: Definition, classifications, clinical profiles and best treatments. Open J Trauma 5(1): 019-036. DOI: 10.17352/ojt.000038Copyright
© 2021 Perrotta G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The spleen is an organ commonly injured in abdominal trauma of the upper left quadrant and until just under two decades the first choice was always splenectomy; however, based on new research and clinical experience, there is a tendency to preserve the spleen as much as possible, precisely because of its immune function and risk of infection. On the basis of the trauma and of the patient’s anamnesis, after an objective examination, the primary ABCDE evaluation, the Eco-FAST, and if necessary also the CT scan (with contrast), it is possible to choose between surgical (OM) and non-surgical (NOM) management: in the first hypothesis are included total or partial splenectomy surgery, raffia, direct hemostasis through drugs or devices with hemostatic-adhesive action, and laparoscopy; in the second hypothesis are included treatments such as controlled nutrition, rest, anticoagulant drug therapy (and antibiotic, if necessary), and angioembolization (exclusive or accessory to a NOM). In particular, in the last few years, a dual interpretation has emerged on the findings necessary to favour splenectomy (total or partial) over angioembolization. From the best clinical practice emerges therefore the answer to the question at hand, namely that the patient is a candidate for angioembolization if 1) is hemodynamically stable (with systolic blood pressure > 90 mmHg, heart rate < 100 bpm, and transfusion of < 3 units of blood in 24 hours) or stabilizable (positive response to rapid infusion of 1000-2000 cc of crystalloids-Ringer Lactate-with restoration of blood pressure and heart rate values in the range of hemodynamic stability); 3) there is no open trauma to the abdomen or evidence of vasoconstriction (cold, sweaty skin, decreased capillary refill) or obvious intestinal lesions or perforative peritonitis or high-grade lesions to the spleen or peritoneal irritation or signs of exsanguination or contrast blush or effusion (exceeding 300ml) detected by Eco-FAST. This preference is optimal concerning both the risks of postoperative infection and immunological risks; finally, age and head trauma, compared to the past, seem to be no longer discriminating conditions to favour splenectomy regardless. Splenic immune function is thought to be preserved after embolization, with no guidelines for prophylactic vaccination against encapsulated bacteria. Other clinical signs finally, however, might argue for discontinuation of NOM treatment in favour of a surgical approach: 1) need to transfuse more than 3 units of blood or simply the need for transfusion in 24 hours to maintain a maximum systolic blood pressure greater than 90 mmHg, correct anaemia less than 9 g/100 ml, or a hematocrit less than 30%; 2) persistence of paralytic ileus or gastric distension beyond 48 hours (despite a nasogastric aspiration); 3) increased hemoperitoneum (on ultrasound or CT); 4) aggravation of the lesion evidenced by ultrasound and/or CT (so-called “expansive” lesions); and 5) subsequent appearance of signs of peritoneal irritation. A complete understanding of post-embolization immune changes remains an area in need of further investigation, as do the psychological and mental health profiles of the surgical patient.
Contents of the manuscript
Introduction. General profiles
The traumatic event is still one of the main causes of death in the population below the fourth decade and in itself represents a long-standing clinical dilemma that also has economic and social implications, as the direct social cost (resulting from treatment) and the indirect one (resulting from rehabilitation, and lack of productivity) affects the community as well as the individual. It is no coincidence that haemorrhage caused by trauma in the abdominal region is one of the leading causes of death with a percentage ranging between 40% and 80%, depending on the clinical history and subsequent events from the first treatment [1].
The spleen, in particular, is the organ most frequently affected by traumatic thoracoabdominal injuries, even before the liver and kidneys, with a percentage close to 50% [2] and a mortality rate around 7-10%, constant in the last 30 years, unlike the mortality rate for liver injuries that in the same period has been significantly reduced to around 4% [3,4].
The spleen is a richly vascularized organ, as it receives about 5% of cardiac output, and has an average parenchymal content of about 250 ml of blood with an arterial flow rate of about 250 ml/min, provided by the presence of vascular lacunae and terminal-type vessels (trabecular arteries), which are grafted into a relatively rigid stromal architecture that therefore responds poorly to self-hemostasis and retraction [5].
Splenic injuries are found in 25-30% of the cases of traumas closed by car accidents, while in the “occasional” civil accidents (sport, fall, etc...) they are found with a clear prevalence in the extreme age groups (adolescents and elderly) and a greater prevalence in the male sex (in a ratio that stands: M/F = 3:2). On the other hand, the extreme delicacy of this organ, in case of direct (compression, contusion, fracture) and indirect (recoil, avulsion) injuries, can be easily explained by the important blood content, in addition to the anatomical-functional structure [6]. In particular, iatrogenic splenic injuries during other abdominal surgical procedures account for approximately 20% of all splenectomies [7].
However, the management of splenic lesions has considerably changed during the last decades; if for a spleen lesion due to a penetrating trauma the surgical choice of splenectomy was compulsory, on the contrary, the treatment of closed contusive lesions of the spleen has undergone a considerable evolution, with the development of a protocol for spleen rescue and Non-Operative Management (N.O.M.) in patients specifically selected based on their clinical picture and medical history.
Historically, at least until the ‘60s of the last century, splenectomy was considered the only solution for any kind of splenic lesion, to prevent the often inevitable risk of protracted and unstoppable haemorrhages, also considering the theoretical assumption that did not consider the spleen an essential organ for life, but incapable of healing itself [8,9]. Later on, however, several studies allowed the scientific community to modify the theoretical assumption and the practical approach; in fact:
1) In primis: The finding of not always finding active bleeding at the time of a laparotomy performed for hemoperitoneum, although massive (although already T. Billroth had suggested this well over a century before) [10].
2) In secundis: P. Upadhyaya’s publication in 1968 noted that at the time of laparotomy, in most children’s spleen traumas, bleeding was already arrested [11].
3) In terzis: The observation of some surgeons, such as P. Upadhyaya in 1948 (who described the case of a child with intestinal occlusion and closed abdominal trauma) and G. Douglas (who described the self-healing capacity of the spleen) in 1971, who noted the circumstance according to which a splenic lesion could self-limit itself allowing a non-surgical approach (although always under strict clinical observation, to avoid the onset of infectious complications) [12].
4) In quartis: The recognition of the essential immune role of the spleen, especially in young people and the finding of a real “asplenia” pathology following splenectomy removal. Already in 1952 H. King and H. Shumacker described O.P.S.I. (overwhelming post-splenectomy infection) demonstrating the immunological consequences of splenectomy [13].
5) In quinquies: The advent of CT, with which it was possible to highlight that, despite the high frequency of splenic lesions, they do not necessarily lead to unstoppable bleeding. J. Mullix, in a 1980 study, on the impetus of this new technology, indicated how the introduction of spiral CT improved the recognition and classification of closed abdominal trauma [14].
Based on these preliminary remarks, it seems logical in modern times to no longer consider splenectomy as the absolute “gold standard” of treatment, thus being able to make use, in specific cases, of more conservative approaches, especially in patients with good hemodynamic compensation and younger than the fourth-fifth decade.
Anatomic-surgical references and pathophysiologic and dynamic profiles
Anatomic-surgical references: The spleen is an endoperitoneal organ that results from the differentiation of mesenchymal cells along the left side of the mesogastrium and is located in the left upper quadrant of the abdomen, in direct continuity with the ipsilateral hemidiaphragm. It is normally 13 x 8 x 3 cm in size, weighs 80 to 250 g, and is flattened ovoid (with possible variations on an individual basis: ovoid 44%; triangular 14%; tetrahedral 42%), with the major axis oriented obliquely from top to bottom, from back to front, and from inside to outside, parallel to the axis of the tenth rib. Accessory spleens are found in 10-30% of cases, most frequently near the splenic hilum (2/3 - 3/4 of cases), the pancreatic tail (20%), along the splenic artery, within the various fixation means and especially in the context of the large omentum; its intraoperative search plays an important role in the possible occurrence of a post-splenectomy syndrome [5,15].
The spleen [5] has two faces (one external diaphragmatic and one internal visceral), three margins, and two poles:
1. The diaphragmatic face, smooth and convex, with the interposition of the diaphragm relates the spleen to the pleura of the left lung and the costal wall;
2. The visceral face is divided by a longitudinal relief into gastric face, anteriorly concave and more extended, where the hilum of the organ is located, renal face and inferior colic face posteriorly;
3. The upper (or anterior) margin separates the gastric face from the diaphragmatic face and has some indentations;
4. The inferior (or posterior) margin, more rounded than the previous one, separates the diaphragmatic face from the renal face;
5. The inner margin separates the renal face from the gastric face;
6. The upper (or posterior) pole is rounded, located near the vertebral column at the level of the 10th thoracic vertebra;
7. The inferior (or anterior) pole is more acute, it is received in the lienal dimple that is located on the upper face of the left colic brake ligament.
Spleen ratios vary according to the volume of the viscera (stomach, colon) and respiratory activity. In any case, even under conditions of maximum inspiration, the lower pole does not surpass the costal arch and is palpable only in case of splenomegaly [5].
From a surgical perspective, the main anatomical features can be identified with [5,15].
1) Means of fixation: The spleen is lined by a fibroelastic capsule of dense connective tissue containing a meagre supply of smooth muscle tissue, 1-2 mm thick. Intraparenchymal septa originate from the capsule and contribute to the characteristic trabecular structure of the parenchyma. These septa do not divide the organ into lobes or lobules, because they stop at a short distance from the capsule itself contributing to determine the particular fragility. Only at the level of the hilum does the capsule deepen, representing the entry and exit point of blood vessels and nerves. The spleen is a quite mobile organ, using a series of suspensory ligaments and tissue connections with the aorta, diaphragm, pancreas, stomach, colon and kidney. This mobility determines a positive shock-absorbing effect for the normal variations of attitude (respiration and cough included) but represents at the same time a vulnerable element if a live force is focally applied right at the level of the capsule or of the pedicle, and it is just in these circumstances that the greater rigidity of the splenic capsule and the parenchymal shoots compared to the pulp can determine a laceration. The main suspensory ligaments are represented by the splenopancreatic, splenophrenic, gastrosplenic, splenorenal and splenocolic. In detail: A) the “splenopancreatic ligament” contains in its thickness the vascular-nervous pedicle of the spleen and the tail of the pancreas. It is stretched between the posterior lip of the hilum of the spleen and the posterior abdominal wall where the serosa covers the pancreas, the part of the anterior aspect of the left kidney that overlooks the transverse mesocolon, the anterior aspect of the adrenal gland and the diaphragm. B) The “splenophrenic ligament” is a fold formed by the upper part of the pancreatic-lienal ligament, leading from the upper pole of the spleen and the upper tract of the hilum to the diaphragm. C) The “gastrosplenic ligament” runs from the anterior lip of the hilum to the fundus of the stomach and appears as a vertical septum, directed from the back to the front and from the outside to the inside, contains the short gastric vessels, which anatomically connect fundus and great gastric curvature with the splenic pedicle. These vessels represent elements of surgical risk due to their brevity and proximity to the gastric fundus, both for possible lacerations during the splenic mobilization phase and for possible damage from clamping the gastric fundus during the resective phase. The remaining ligaments, on the other hand, in the absence of pathological circles, are relatively avascular and can usually be dissected with electrocautery. D-E) The “frenocolic” and “splenocolic” ligaments merge to form the “spleen hammock”, the main means of fixation of the lower pole; injury at this level due to excessive traction or misidentification of the cleavage plane, may result in further injury of the lower pole of the spleen.
2) Relationships with surrounding structures: The major topographical connections of the spleen occur with the diaphragm, ipsilateral kidney-adrenal, left colon, and gastro-pancreatic complex. Those of greatest surgical importance are those with the pancreatic tail and gastric fundus. The pancreas is the organ that has the greatest relationship of continuity with the spleen, particularly at the level of the pancreatic tail, whose proximity to the splenic hilum can expose it to the risk of injury during splenectomy; in particular, if the spleen has a so-called “short” pedicle, that is, the terminal branches are particularly short and, therefore, closely connected to the pancreatic caudal parenchyma. Relations with the gastric fundus are mediated mainly by the short vessels and the gastrosplenic ligament; also, in this case, the presence of a very short ligament may contribute to lacerations or direct gastric injuries.
3) Normal vascular architecture and major anatomic variants: Arterial vascularization is provided by the splenic artery, which along with the hepatic artery and left gastric artery contributes to the celiac tripod. In 82% of cases, the splenic artery emerges directly from a short common trunk with the hepatic artery, although it may originate directly from the aorta or superior mesenteric artery. The splenic artery, with a length varying from 8 to 32 cm and a calibre varying from 0.5 to 1.2 cm, runs, with a sinuous path, from right to left, describing a slight arch with anterior concavity, over the homonymous vein, along the superior margin of the pancreas, at the level of the first lumbar vertebra, reaching the pancreatic tail on its anterior face. Along its course, it emits, as collateral branches, several pancreatic arteries and a posterior gastric artery. At a distance of 0.5 to 6 cm from the hilum of the spleen, before penetrating perpendicularly into the parenchyma, the artery divides into terminal segmental branches, usually, two, distinguished into superior and inferior, the latter dividing into one branch for the intermediate segment and one for the inferior segment. Each segmental artery then subdivides into trabecular arteries that cross the capsule to give the follicular arteries, usually distributed along a horizontal axis relative to the major splenic axis. The spleen has efficient collateral circles, particularly through the anastomotic arch of the great gastric curve (right and left gastroepiploic arteries: “arc of Josef Hyrtl”) so that a proximal ligation of the splenic artery does not alter the vascularization of the parenchyma. Frequently, accessory polar arteries may also be observed. The splenic venous circulation presents an extreme anatomic variability, is however based on a major venous trunk, with a parallel and sometimes spiral course concerning that of the artery, which is formed by the confluence, in two main trunks, of 6-8 large branches emerging from the parenchyma and located in the front or behind the arteries. The splenic vein, after receiving the left gastroepiploic vein at the anterior aspect of the tail of the pancreas, runs parallel to and below the artery; together with the inferior mesenteric vein, it forms the splenic-mesenteric trunk and then reaches the trunk of the portal vein. It must be pointed out that the so-called short vessels often drain in the direct context of the lienal parenchyma rather than that of the hilar pedicle, with obvious possibilities of laceration during surgical manoeuvres. Lymphatic vessels of the spleen can be distinguished into superficial and deep. The former is located in the thickness of the fibrous capsule, below the peritoneal envelope, and converge at the hilum. The deep lymphatics are usually satellites of the veins and also emerge from the hilum. These run in the thickness of the fibrous trabeculae that detach from the capsule. The red pulp and white pulp have no lymphatic vessels, which are exclusively associated with the coarser connective component of the organ. Superficial and deep lymphatic vessels afferent to lymph nodes located in the thickness of the pancreatic-lienal ligament, in the vicinity of the tail of the pancreas (pancreatic-lienal lymph nodes). The nerves of the spleen derive from the celiac plexus and reach the organ by following branches of the gastrolienal artery.
Physiological and functional profiles
Finally, from a functional point of view, the Spleen [13,15-21].
1) It is a lymphoid organ of the human body and its functions are closely related to its structure and its characteristic circulatory system. Of the arterial blood flowing along with the white pulp (lymphoid tissue) a part, crossing the capillary epithelium, passes directly into the venous circulation (theory of “closed” circulation). Most of the blood flow, on the other hand, enters the reticular system carpeted by macrophages and slowly returns to the venous circulation by passing through the venous sinuses (“open” circulation theory). The corpuscular component of the blood must pass through slits in the lining of the venous sinuses and, if unable to do so, is trapped in the spleen and ingested by splenic phagocytes.
2) It performs an important hematopoietic function until the 5th month of fetal development when the bone marrow then assumes a predominant role in hematopoiesis. In some pathological conditions, however, for example in myelodysplasia, the spleen can regain its hematopoietic function. Thanks to its particular circulatory system and lymphoid organization, the spleen continue throughout life to act as a fine filter, both by monitoring and managing the cellular component of the blood and by performing important immune functions.
3) It performs a mechanical filtration function that removes senescent erythrocytes and probably contributes to infection control by removing circulating intraerythrocytic pathogens, such as malaria parasites or bacteria, such as Bartonella species. Splenic filtration function is important in maintaining normal erythrocyte morphology and function. The spleen is an important site for processing immature erythrocytes and repairing or destroying deformed or old erythrocytes. Under conditions of asplenia, a variety of characteristic alterations in peripheral red cell morphology can be observed, with the presence of target cells (immature cells), Howell-Jolly bodies (core remnants), Heinz bodies (denatured haemoglobin), Pappenheimer bodies (iron granules), or irregular cells with the presence of incisure or spurs. Old red blood cells (120 days old) that have lost their enzymatic activity and membrane plasticity are trapped and destroyed in the spleen. This filtering function of the spleen also proves important in conditions of anaemia associated with abnormal erythrocyte morphologies. Splenectomy depriving the organism of these fundamental functions represents an event certainly serious, but not connected with lethal consequences in the short term from the intervention.
4) It plays a role in maintaining the normal immune response and host defence against certain types of infectious agents. Changes in the immune response due to splenectomy, and in particular OPSI (Overwhelming Post Splenectomy Infection), a rapidly evolving septic syndrome sustained predominantly by capsulated germs, were described in the 1950s by H. King and H. Shumacker. OPSI, which occurs with maximum peak incidence within the first two years after splenectomy, although cases are described even after decades, represents an infection characterized by non-specific symptoms, such as fever and general malaise, which can be initially mistaken for the trivial flu. But the course then evolves dramatically towards fatal sepsis, sustained by Streptococcus Pneumoniae, Haemophilus Influenzae and Neisseria Meningitidis. Other pathogens identified include Escherichia coli, Pseudomonas Aeuruginosa, Capnocytophaga canimorsus, type B Streptococci, and protozoa such as malarial. Splenectomized patients have a normal reimmunization response to antigens already encountered before splenectomy, but their response is not as optimal to new exposures to antigens, especially when administered venously. Larger amounts of antibodies are required for the removal of organisms such as capsulated bacteria. The spleen, because of its specialized circulatory system and the presence of numerous macrophages capable of phagocytizing organisms not well opsonized with antibodies, contributes greatly to their removal. It has been found that in asplenic subjects there are low levels of IgM and a suppressed immunoglobulin response by mononuclear cells in the peripheral blood. The spleen is the most important site of opsonin, properdin, and tuftsin production, and splenectomy results in decreased serum levels of these factors. Properdin may initiate the alternative pathway of complement activation to achieve the destruction of foreign and abnormal bacteria and cells. Tuftsin is a tetrapeptide capable of enhancing the phagocytic activity of both polymorphonuclear leukocytes and mononuclear phagocytes. The spleen is the main site of cleavage of tuftsin from IgG heavy chains, and the asplenia condition is accompanied by suppression of its circulating levels. Neutrophil function is reduced in asplenic patients, and this deficit appears to result from the absence of a circulating mediator. The true incidence of OPSI is not yet defined, being reported percentages varying from 1.0 to 2.1% of splenectomized subjects and with mortality, in this group, of more than 80%. The surgeon who performs a splenectomy, both in election and emergency, has the specific duty to implement the pharmacological measures necessary to avoid the onset of OPSI. Of course, these measures should not be performed in subjects undergoing conservative surgery, but the presence of accessory spleens is not a valid guarantee of the preservation of the immune status. Guidelines on the early administration of specific vaccines, antibiotic prophylaxis, adequate patient information, and timely treatment of possible infections have been developed by the British Committee for Standards in Hematology. The latest updated guidelines indicate to administer the vaccines (Pneumococcus, Haemophilus Influenzae and Neisseria Meningitidis) as soon as possible after an emergency splenectomy and at least 2 weeks before a scheduled splenectomy.
Splenic trauma: Definition and types
Splenic trauma is by definition the result of a traumatic event affecting the spleen, caused by an event capable of generating a lesion (single or multiple) with a negative impact on the organ, affecting both its structure and its functionality. The main symptom is represented by intense pain, mainly in the left hypochondrium, radiating to the ipsilateral shoulder and accentuated on palpation (Kehr’s sign). The abdomen may appear distended due to the accumulation of blood in the abdominal cavity and contracted muscle walls. Over time, bleeding will lead to a state of hemorrhagic shock, with the appearance of pallor, tachycardia, cold sweating, small and frequent pulse, and decreased level of consciousness. However, the clinical manifestations of splenic rupture are not always established early. Sometimes the objective examination may present lesions in the parenchymal organs, even if of minor degree. In other cases, the haemorrhage may not be immediate but occur later, with a latency of a few days from the trauma and late-onset of disorders, even after 6-7 days from the accident. In history investigate the dynamics of the accident, any congenital disorders of coagulation, vomiting, loss of consciousness. On physical examination, evaluate the general clinical conditions, look for any signs of hemodynamic instability, the presence of contusions of the abdominal wall, abrasions, lacerations or wounds penetrating the abdomen, abdominal distension, abdominal or shoulder pain, signs of peritoneal irritation [22].
The patient who has sustained an abdominal trauma usually comes to the clinician’s observation with a cursory description, without specific indications about the type and severity of the injuries. For these reasons, the following examinations should be performed [23-86].
a) Hematochemical examinations: blood count, blood group, azotemia, blood glucose, plasma electrolytes, transaminases (increased in hepatic trauma, AST > 400 and ALT > 250), lipase and amylase.
b) Ultrasound (FAST in hemodynamically unstable patients), looking for free endoperitoneal effusion (especially in the more declivous areas of the abdominal cavity).
c) Radiographs (RX) of the chest, looking for fractures, splenic trauma, pneumoperitoneum (due to traumatic perforation of the gastrointestinal tract).
d) CT scan, as the first choice in hemodynamically stable children or the absence of ultrasound instrument Figure 1.
Schematically, therefore, we can consider splenic traumas of two types [5,15].
1) Closed (or blunt) trauma: Where compression, concussion, and deceleration are the physical mechanisms underlying these injuries. The first two, compression and concussion, are produced by the impact of the body on a fixed obstacle, so site, impact force, and impact velocity must be evaluated. The injurious agents are often solid bodies (such as the steering wheel or the wall) on which is discharged the living force possessed by the patient or by direct blows in correspondence of the right hemiaddomen. In particular: the abdominal compression can determine the lesion of the hollow organs through a mechanism of laceration by burst increased endoluminal pressure or ischemic perforation by mesentery laceration; the concussion instead can determine intraparenchymal hematomas and is due to the transmission of the impact force inside them. Finally, deceleration occurs when there is a fall from above or an ejection, whereby the organ discharges its live force against the surrounding structures (in particular: rib cage), subjecting the fixed part of the viscera to traction on the ligaments, which may cause a tear injury. Characteristic deceleration injuries involve vascular structures such as the aorta at its fixation points, the hilar branches of the spleen and kidney, and the mesenteric branches at their origin. These conditions result, in decreasing incidence, injury to the spleen, liver, kidney and intestines and retroperitoneal haemorrhage. A further serious consequence of blunt trauma is represented by visceral injuries (laceration, crushing, tearing, bursting of viscera), which may be associated with hemoperitoneum and/or peritonitis or other previous existing medical conditions (e.g. taking anticoagulant or antiplatelet drugs, pregnancy, and some infectious diseases).
2) Penetrating trauma: Wounds of the abdominal wall can be distinguished into sharp-edged, lacerated-contused, with or without loss of substance, penetrating or piercing wounds, caused by compression, crushing or rolling by blunt or sharp injurious agents, or by blunt weapon, firearm or blast. The injuries are usually complex because, in addition to the spleen, they involve the surrounding anatomical structures (lung, rib cage, pancreas, stomach, kidney) and only the clinical examination can confirm or refute the initial hypothesis; however, especially in the case of gunshot wounds, the involvement of a parenchymal organ (eg, liver or spleen) or a viscera (eg, stomach or intestines) is not immediately diagnosable for the initially uncertain evolution of the symptoms. The haemorrhage that follows the trauma may be initially of modest entity, but prolonged over time and giving rise, in a few hours, to a conspicuous hemoperitoneum; by analogy, the perforation of a hollow viscera may be initially asymptomatic for the early tamponade of the lesion by the contiguous viscera. Whereas in blunt weapon wounds the type of weapon used and the shape of the wound can reasonably indicate which organs are likely to be injured, in gunshot wounds, this is not possible. In fact, after passing through the skin plane, the bullet may be deflected by bony structures so that its intra-abdominal path is not predictable based on external injuries; therefore, surgical exploration is necessary to exclude injuries even far from the entry and exit holes of the bullet, after performing a plain radiographic examination of the abdomen and a chest X-ray to search for signs of visceral perforation, associated thoracic-mediastinal injuries and retention of bullet fragments.
In both cases, the trauma patient is at high risk of venous thromboembolism and risks a hypercoagulative state within 48 hours of trauma, with a risk of deep vein thrombosis and pulmonary embolism that can increase mortality by up to 50% [30].
To stage the severity of the splenic injury, reference is made to the Organ Injury Scales for splenic injuries, developed by the Organ Injury Scaling Committee of the American Association for the Surgery of Trauma (AAST) [31]. This, along with classifications of injuries to other organs, provides a common nomenclature by which clinicians can describe injuries sustained and their severity Table 1.
Interventional profiles: The first approach, diagnosis, and treatment options
The first approach [15]: The complexity of the abdominal polytrauma phenomenon requires the following absolute and codified priorities, sequenced in the acronym “ABCDE”: A (Airway): patency and maintenance of the airway, protection of the cervical spine; B (Breathing): adequate ventilation; C (Circulation): adequate blood volume and cardiac output, control of haemorrhage; D (Disability): neurological assessment, establish the state of consciousness; E (Exposure): expose the patient’s body to identify any injuries by preventing hypothermia. Once these objectives have been achieved, the mechanisms responsible for the trauma can be investigated in detail.
However, history and physical examination remain of fundamental importance in the diagnosis of splenic trauma. In particular, it will be necessary to investigate on:
a) The existence or not of hemodynamic instability (after rapid volemic replenishment).
b) The existence or not of signs suggestive of a visceral perforation.
c) The existence or not of signs of focus (pain, skin lesions, seat belt marks).
On objective examination, signs of peritoneal irritation (pain, defensive contracture, rebound pain) may be evident; in other situations, the hemoperitoneum may have a mild irritating power on the peritoneum itself, allowing conspicuous blood effusions with relatively poor objectivity. In particular, for splenic trauma, there may be evidence of pain on percussion or obvious ecchymosis and signs of soft tissue contusion over the left posterior costal hemiarchate, which are signs generally present in splenic injury from direct trauma. Pain in the left upper abdominal quadrant or that referred to the left shoulder (Kehr’s sign) are often associated with splenic trauma. At least one-quarter of patients with fractures of the left last ribs also present with splenic trauma. However, these signs are unlikely to establish a direct correlation with splenic injury without further investigation. The onset of hemorrhagic shock at the arrival of the patient in the “Shock Room” is not very frequent, except in cases of complete or almost complete avulsion of the spleen, or its plurifragmentation. Faced with a polytraumatized patient, who presents alterations in hemodynamic conditions, with hypotension and tachycardia, it is always necessary to hypothesize the possibility of an intra-abdominal injury, especially splenic, remembering that even fractures of the long bones and pelvis can cause bleeding responsible for such hemodynamic instability, but the recognition of fractures should not exclude an intra-abdominal cause, and splenic injury is the most common. Since the classic J. West et al. studies of preventable deaths that directly contributed to the development of trauma care systems in the United States, mortality from missed or delayed diagnosis of splenic haemorrhages has remained at the top of the list of preventable causes of death. The presence of multiple injuries should not distract the examiner from correctly recognizing the source and severity of the haemorrhage; in particular, hemodynamic changes cannot be attributed, until proven otherwise, to the concurrent presence of neurologic injuries or substance abuse. Closed head trauma is associated in 30-40% of cases and compromises or even eliminates the reliability of the patient’s physical examination. Substance abuse has been documented in 40% of patients involved in motorcycle accidents.
Because of the unreliability of the objective examination in these conditions, newer, more objective diagnostic means have been developed. Before activating complex diagnostic procedures, however, it is important to ensure good venous access routes and, above all, to set up good monitoring, to highlight the premonitory signs of an abrupt transition (typical in the early phase in the elderly and a relatively late phase in the young) from a relative hemodynamic compensation to an overt shock.
Clinical examination [33,34]: The clinical examination is followed by the instrumental one, standard radiological examination (Rx thorax, Rx pelvis, usually already performed in the first phase of care) and an ultrasound FAST (Focused Assessment with Sonography in Trauma). Introduced during the ‘90s, ultrasound has become an important diagnostic aid in the evaluation of abdominal trauma. Its advantages include noninvasiveness, rapidity, and low cost. Compared with Diagnostic Peritoneal Lavage (DPL), for several years considered a diagnostic gold standard, ultrasound provides similar but more information. The presence of free intraperitoneal fluid can be detected and quantified, unlike diagnostic peritoneal lavage. These features make it the main diagnostic tool of the first level in any abdominal trauma, ensuring particularly high reliability in the detection of a free abdominal effusion (sensitivity 91.4%, specificity 97.8%, accuracy 95.6%) and sensitivity levels still satisfactory in the diagnosis of liver injury (75%) or splenic (72%). The FAST protocol has evolved into E-FAST and subsequently into FAST ABCDE: originally developed and codified to allow the recognition of hemoperitoneum and hemopericardium, it has been extended to a large number of other targeted clinical applications, always characterized by the rapidity of execution, with important diagnostic and therapeutic benefits; with E-FAST (Extended FAST) the goal is also the ultrasound recognition of pneumothorax. Recently, FAST ABCDE (Airways, Breathing, Circulation, Disability, Exposure), the latest evolution of the ultrasound methodological approach, makes the method help emergency physicians in resolving the many clinical questions that may arise during the evaluation in the scale of absolute priority that is followed in the approach to the traumatized patient. Further use is Contrast-Enhanced Ultrasound (CEUS); it is a real-life, noninvasive investigation done at the patient’s bedside, without radiation. Some studies suggest that CEUS is a good alternative to contrast-enhanced CT for the evaluation of traumatic lesions of abdominal solid organs, especially in patients with contraindications to CT due to allergies to contrast agents or in hemodynamically compromised patients. The exact role of CEUS in diagnosing patients with closed abdominal injuries will be further defined in the future. The subsequent diagnostic procedure is conditioned by the hemodynamic status of the patient: the presence of hemodynamic instability or insufficient response to volemic resuscitation will interrupt any other procedure, to proceed with urgent operative treatment. If instead, the patient has (or has achieved) a good hemodynamic compensation, the next investigation will be represented by a Computed Tomography (CT), the real diagnostic pillar for the implementation of any non-operative or conservative protocol. The current generation of CT with spiral technology is faster and has better resolution than in the past when 15 to 20 minutes were required for a complete examination, today only 1-2 minutes are needed. The resolution also allows a more precise definition of organ rupture and intraparenchymal vascular damage with very high sensitivity (99%). If, during CT with MDC, contrast overflow, arteriovenous fistulas and/or pseudoaneurysms are identified, in the patient who preserves a good hemodynamic compensation, the possibility of performing diagnostic-interventional Angiography with angioembolization can be considered, whose double diagnostic and therapeutic role is extensively used in many sites. The basic principle for any diagnostic and therapeutic choice remains dictated exclusively by the objective and hemodynamic evaluation: the presence of circulatory instability and visceral perforation represent absolute surgical indications. If the absence of these conditions is demonstrated, the treatment can be directed to more or less conservative protocols.
Non-Operative Management (NOM) in closed trauma [35-65,66]: The diagnosis and treatment of closed abdominal traumas with suspected splenic lesions have changed considerably in recent years: from an attitude almost constantly based on the studies of T. Kocher, who already in 1911 indicated splenectomy as the only treatment for splenic lesions, we have gradually moved to less aggressive and demolitive procedures, to the point of abstaining from surgery (Non-Operative Management, NOM).
The important therapeutic change was also a consequence of many clinical studies indicating that splenectomy increases the risk of susceptibility to infection with its most severe and fatal manifestation, OPSI, in 0.5% of all splenectomies for trauma and more than 20% of elective splenectomies for hematologic disorders. OPSI (risk of infection with capsulated germs) is more frequent in the first 2 years of asplenia, but there is a risk of permanent infection with a mortality of more than 80%. Based also on these studies, a conservative trend in therapy (nonoperative management, conservative surgery, and spleen autotransplantation) has been created; in fact, traumatic spleen injury is no longer an absolute indication for splenectomy.
Attempts at NOM have been described as far back as 1882 when S. Gross indicated bed rest and light diet for mild spleen lesions, lead acetate, ergot, and opium for severe lesions; surgery should be performed only if necessary. In 1968, P. Upadhyaya observed that children with splenic lesions often did not show signs of major blood loss. It was interesting to see that in most children with splenic lesions the bleeding had stopped by the time of laparotomy. This could be explained by different mechanisms: hypotension, clot formation, the buffering effect of the large omentum, perisplenic hematoma containing the bleeding, and intact splenic capsule. In 1971, G. Douglas and J. Simpson described 32 cases of children with clinical signs of a spleen injury, of whom, 25 did not require surgery. This study demonstrated that the spleen does indeed have the ability to heal itself with an excellent result in selected cases.
Early classifications for NOM criteria, developed in 1998, indicated:
1) Stable or readily stabilizable hemodynamics;
2) Lack of Blumberg’s sign and abdominal contracture;
3) Minor blood transfusions equal to 4 units;
4) Preserved state of consciousness;
5) age < 55 years;
6) Spleen injury documented on imaging.
Complex/severe splenic injury, preexisting spleen disease, number of units of blood transfused, brain injury, and age are no longer considered absolute contraindications to NOM.
NOM has been extensively demonstrated to have numerous advantages over OM: reduced complications reduced need for blood component transfusion, lower mortality and reduced costs, and preservation of immunologic function of the spleen and prevention of OPSI. Before the release of the new WSES guidelines, the only available guidelines on the topic were the 2012 Eastern Association for the Surgery of Trauma (EAST) guidelines and the 2016 Western Trauma Association (WTA) guidelines. Based on both guidelines, all peritoneal or hemodynamically unstable patients after closed splenic trauma should undergo urgent laparotomy. Conversely, surgery is not indicated in hemodynamically stable patients in the absence of peritonitis. The appropriateness of adopting NOM and the risk of FNOM are still debated in the scientific community, also because of some difficulties in interpreting the results of the various existing studies.
Currently, the age criterion is becoming less and less restrictive, many studies show little influence on the success of NOM; indeed, beyond 60 years of age, it seems that the capsule, which from youth is reduced in thickness, begins to acquire more consistency again. The only absolute indication for emergency laparotomy remains hemodynamic instability.
Exclusion criteria for NOM include
1) Signs of exsanguination;
2) Persistent hemodynamic instability (requiring more than 3 units of blood in 24 hours) and no response to initial resuscitation,
3) Open trauma of the abdomen or obvious bowel injury,
4) Associated intra- or extra-abdominal injuries requiring general anaesthesia.
5) The severity of head trauma, associated orthopaedic injuries, a high wound severity score or higher radiological classification of visceral injuries, or more solid organ trauma have not been considered as exclusion criteria in hemodynamically stable patients (although splenectomy was previously thought to be safer).
There are also predictive parameters for NOM (or FNOM) failure so that disproportionate risks can be avoided:
1) High-grade lesions (IV-V) or Injury Severity Score (ISS) of 25 or greater;
2) Positive FAST for free effusion;
3) Decrease in haemoglobin with the need for hemotransfusion (more than one unit) within the first 6 hours;
4) CT demonstration of contrast blush and effusion exceeding 300 mL.
Inclusion criteria for NOM are
1) Hemodynamic stability: systolic blood pressure > 90 mmHg, heart rate < 100 bpm, evidence of vasoconstriction (cold, sweaty skin, decreased capillary refill), altered level of consciousness, and/or dyspnea;
2) Positive response to rapid infusion of 1000-2000 cc of crystalloids (Ringer Lactate) with the restoration of blood pressure and heart rate in the range of hemodynamic stability;
3) The absence of objective and/or instrumental signs of perforative peritonitis.
To implement non-operative treatment, however, the presence of specific requirements is also required in the hospital where the patient is admitted:
1) The exclusive admission of patients with established hemodynamic stability;
2) The availability of an adequate hospitalization and monitoring environment (UTI, Sub-UTI);
3) The 24-hour availability of an operating department;
4) Accessible diagnostic imaging equipment and qualified personnel (CT, Echo, Angio).
If these principles are adopted, success can be expected in 98% of splenic lesions treated with NOM.
In addition, there are important parameters that dictate discontinuation of NOM treatment:
1) Need to transfuse more than 3 units of blood or simply the need for transfusion in 24 hours to maintain a maximum systolic blood pressure greater than 90 mmHg, correct anaemia less than 9 g/100 ml, or a hematocrit less than 30%;
2) Persistence of paralytic ileus or gastric distension beyond 48 hours (despite a nasogastric suction);
3) Increased hemoperitoneum (on ultrasound or CT scan);
4) Worsening of the lesion evidenced by ultrasound and/or CT (lesions called “expansive”);
5) The appearance of signs of peritoneal irritation.
Non-Operative Management (NOM) in the penetrating trauma [67-71]: Laparotomy has always been the gold standard in penetrating abdominal trauma. However, many studies have shown that the incidence of negative laparotomies ranges from 9 to 14%. Over the past 20 years, there has also been an increase in NOM in penetrating blunt and gunshot trauma. Carlin demonstrated that in a sample of 248 patients, mortality from penetrating trauma was not significantly higher than from closed trauma. OM is also associated with complications related to iatrogenic colic and pancreatic injuries, which on the one hand often require distal splenic-pancreatectomy, and on the other hand, lead to increased mortality and septic complications.
Specific treatment options [35-64,72,73]: The possible “therapeutic options” therefore involve treatments of two types:
1) Operative type (OT): They include total splenectomy, partial splenectomy, raffia, direct haemostasis using drugs or devices with haemostatic-adhesive action; these treatments, as already noted, should be instituted when there is haemodynamic instability or the need for an abdominal surgical approach for complex lesions (solid organs, visceral lesions).
2) Non-operative type (NOT): These treatments indicate procedures that do not involve access to the peritoneal cavity and include non-operative treatment proper (NOM) and angioembolization (exclusive or accessory to a NOM). Diagnostic-therapeutic laparoscopy, which, in well-selected subjects, represents a valid minimally invasive device, cannot be included among non-operative treatments.
Vediamoli nel dettaglio:
1)(OT) Urgent Splenectomy: Urgent surgical treatment is required in case of hemodynamic instability of the patient. In such circumstances, the spleen must be rapidly removed, according to the principles of damage control, especially in case of acidosis and coagulopathy, bleeding that cannot be otherwise controlled, severe lesions (OIS IV-V), comorbidity, the simultaneous presence of other hemorrhagic lesions, severe head trauma. The surgical technique to be practised during an emergency splenectomy differs substantially from that performed on an elective basis, both in terms of the access route and the general and specific surgical timing. Since the polytraumatized patient with hemodynamic instability could have a hemorrhagic lesion also of other organs, and not only of the spleen, we will proceed according to the principles of abdominal damage control: a) median, wide (xiphos-pubic) laparotomy; b) rapid removal of blood from the cavity using the aspirator but also and above all by laparotomic patches (to be counted and weighed). Autotrans is useful, if available, only if it is ascertained that the surgical field is not contaminated by any visceral perforations; c) inspection of the bleeding foci and control of this in the least invasive way possible. If it is evident that the splenic lesion is the one to be treated as a priority or exclusively, we will proceed to (a) mobilization and medialization of the spleen by a combined manoeuvre of grasping and section of the phrenosplenic ligament; (b) section of the frenocolic and splenocolic ligaments; (c) temporary packing in the splenic loggia thus freed and exteriorization/medialization by blunt route of the spleen and pancreatic tail; (d) decision of the next tactic after examination of the type of lesion, maintaining hemostasis with direct compression on the lesional focus and/or pedicle; (e) search for any accessory spleens. All these steps should be performed with caution to avoid further splenic injury.
2)(OT) Total Splenectomy: If the surgeon highlights the need for a total splenectomy, the following steps will be performed: a) careful inspection of the relationship between the splenic hilum and the pancreatic tail; b) section/tying of the gastro-splenic ligament, taking great care to avoid lacerations of the short vessels and to find a suitable space between the gastric fundus and the upper pole of the spleen (obligatory gastric decompression); c) ligation and section of artery and vein, keeping the spleen as vertical as possible and carefully avoiding caudal pancreatic lesions. Subsequent removal of the temporary packing previously left in the splenic lodge may reveal additional lesions that will need to be treated. At the end of the operation, if there are no other lesions to be treated, it is advisable to leave a drain in the splenic loggia, to be removed in the first postoperative days according to the clinical course of the patient. A recent study also suggests the application of Ligasure™ in blunt abdominal trauma for splenectomy as a replacement for silk sutures, as it can reduce the time of the operation, but can also decrease the volume of bleeding during the operation without any further increase in post-operative complications (recommended in traumatic splenic injuries requiring splenectomy to control bleeding).
3)(OT) Partial Splenectomy: Partial splenectomy, which consists of partial resection of the splenic parenchyma, usually extended to one of its poles, is indicated when there is a tear of one splenic pole with preservation of most of the parenchyma or when a segmental vascular lesion is observed. The initial surgical steps are essentially the same as those already described for total splenectomy. In most cases, after appropriate vascular ligation (selective clamping of the segmental branch destined to the lesion area), an anatomic resection of the splenic parenchyma is successful. In the end, the hemostasis in correspondence of the section slice is perfected with resorbable stitches, with detached stitches (Polyglycolic Acid), reinforced by resorbable pledgets. Other viable options include the use of a mechanical suture, electrocoagulation, direct suture (always with detached stitches). The use of topical hemostats (fibrin glue, fibrinogen and human thrombin sponge) is almost the rule because of the persistent bleeding that most often accompanies the partial spleen resection.
4)(OT) Splenorrhaphy. Splenorrhaphy: indicated in the case of linear lacerations with transversal major axis, is obtained by approaching the splenic capsule, applying mattress stitches in resorbable material, reinforced with pledgets, that allow obtaining a right tension avoiding further capsular lacerations. This is the simplest surgical solution but also the least frequently practicable due to the persistence of bleeding or the absence of any bleeding at the time of surgical inspection.
5)(OT) Direct hemostasis: Direct hemostasis and the application of topical hemostats and adhesives is the most frequently adopted form for minor bleeding injuries. The disadvantages of the use of these substances are the hypothetical risk of viral transmission especially for substances of human origin, transient hypotension and the possibility of anaphylactic reactions with fatal outcomes. The main indications for the use of biological substances and glues would seem to be grade 1 and 2 splenic lesions, either isolated or associated with lesions of other organs, more or less severe, to be specifically treated. In some cases, they may contribute to complete section hemostasis after partial splenectomy.
6)(OT) Spleen wrapping: Alternatively, hemostasis can be accomplished by wrapping, performed by wrapping the spleen in a resorbable mesh (usually Polyglycolic Acid) shaped to have an eyelet to be placed around the hilar vessels; the mesh is then sutured, achieving compression on the parenchyma that prevents further bleeding. Another technique involves covering the portion of damaged parenchyma (once hemostasis has been achieved) with a pedicled omental patch that is secured to the edges of the laceration with detached stitches, possibly reinforced by pledgets. This is a procedure that has very limited indications and is not accepted by all authors. Cases of recurrence of bleeding, splenic infarction from compression on the pedicle, and late intestinal occlusion from adhesions and dislocation of the wrap are also described.
7) (OT) Self-transplantation: Although the spleen possesses a remarkable capacity for regeneration and the technique is simple, splenic self-transplantation is neither a widespread procedure nor certainly effective in preventing post-splenectomy sepsis. Most authors suggest implantation in the great omentum both because of its rich vascularity and to ensure portal drainage. When the great omentum is not available, the retrocavity of the epiploon, mesocolon, or retroperitoneum can be used. Affixing metal clips around the fragments simplifies their later detection with scintigraphy. Revascularization of the implanted fragments is attested by scintigraphy, a normal platelet count or the absence of abnormalities in the peripheral circulation blood. The ability to protect against infection, on the contrary, is questioned by some cases of fatal fulminant septicemia, despite the presence of an ectopic or regenerated spleen, and by the insufficiency of experimental data.
8)(NOT) Non-operative Management (NOM): Angiography and Embolization. According to recent studies, NOM represents the gold standard for patients who have hemodynamic stability, especially if they are pediatric, and with positive indices for low predictivity of NOM failure. In addition to hemodynamic stability, the absence of other lesions requiring surgical correction is also necessary. No less important is also the availability of an adequately equipped department to perform close patient monitoring (vital parameters, laboratory tests, radiological follow-up). In selected patients, NOM has a success rate between 90-100% in OIS I-III lesions and 50-60% in OIS IV. Some authors advise against NOM in the elderly patient because of the high risk of failure: on the contrary, the pediatric patient is an optimal candidate, as he often presents an isolated splenic lesion and has a more resistant spleen (thanks to the thicker capsule), in which the tears, unlike in the adult, run more often parallel to the trabecular vessels with less bleeding from the parenchyma; in addition, as already pointed out, it is preferred to attempt a NOM in the pediatric patient because of the compromised cell-mediated immunity induced by splenectomy, which involves an increased risk of infection by capsulated germs (OPSI). Another setting in which the application of NOM is debated is the patient with mild-to-moderate splenic injury and severe head trauma, in whom any exsanguination is likely to worsen secondary brain injury; in these patients, a lower threshold must be set for deciding when to discontinue NOM and proceed to surgery. CT finding of contrast medium extravasation or vascular abnormalities is an unfavourable prognostic factor for successful NOM; in such cases, combining NOM with angioembolization, effective in 73-100% of cases, increases the chances of success. However, if during NOM the patient becomes hemodynamically unstable and an Echo-FAST confirms the abdominal origin of the bleeding, immediate surgery is required; in such cases, splenectomy is the primary option of choice. In case of rebleeding, if the hemodynamics remain stable, it may be useful to repeat a CT scan with an intravenous contrast medium, in the presence of parenchyma bleeding, angioembolization. If CT does not demonstrate parenchymal bleeding, laparoscopy may be performed, assuming optimization of surface hemostasis. Since 60-70% of NOM failures occur within 48-72 hours due to the appearance of re-bleeding, follow-up involves monitoring for three days, either on the ward (grades I-II) or in the ICU/Sub ICU (higher grades); the cornerstone remains the easy availability of diagnostic investigations (Echo, CT, Angio) and the presence of a 24-hour operatory department. The blood count should be repeated every six hours until two stable readings are obtained, then every 24 hours. In the spleen, there is a demonstrated percentage, up to 70%, of the late appearance of pseudoaneurysms, so it is appropriate to repeat, on the second day, an ultrasound in lesions I-II and/or CT with MDC (or Echo with MDC) in major lesions at the discretion of the surgeon. Appropriate radiologic follow-up with CT, in NOM trauma, at this time, has not been definitively established. If the injury is stable or regressing, after follow-up imaging, and the associated injuries are also in order, the patient can be started on outpatient follow-up with clinical examinations and follow-up ultrasound and resume normal physical activity after a few weeks. Generally, for isolated traumas of less than grade III, a seven-day stay (after the first CT scan) is considered acceptable in patients residing within a radius of 50 km Table 2, Figure 2.
Vaccines and antibiotic prophylaxis in splenectomized patients [74-83].
OPSI (infections with capsulated germs), as already indicated, is sepsis, meningitis, or fulminant pneumonia due in 50% of cases to infection with Streptococcus pneumonia or, in a smaller percentage of cases, with Haemophilus influenzae type B or Neisseria meningitidis. The risk of OPSI and associated mortality is highest in the first year after splenectomy, but remains high for more than 10 years and probably for life. The incidence of OPSI is 0.5-2% and mortality ranges from 30% to 70%. Most deaths occur within 24 hours of onset, and only prompt diagnosis and treatment can reduce mortality. Splenectomized children less than 5 years of age have a higher risk of OPSI and mortality than adults, and the risk is greater than 30% in infants. There is evidence showing that the immune function of the spleen after angioembolization is maintained, however for some authors it is reasonable to consider it less effective and therefore proceed to vaccination even in these patients. Annual influenza vaccine is recommended in all splenectomized patients over six months of age, as prevention of influenza allows avoidance of secondary bacterial infections by Pneumococci. Ideally, vaccinations against Streptococcus pneumonia, Haemophilus influenzae type B, and Neisseria meningitidis should be administered at least two weeks before splenectomy. Patients should be informed that vaccines can reduce the risk of OPSI, but not eliminate it (vaccines available to date cover only 23 of the 90 serotypes of S. pneumoniae and 5 of the 6 serotypes of N. meningitidis). In trauma patients, the correct timing of vaccines is no earlier than 14 days after splenectomy, because before 14 days the antibody response is suboptimal. However, in patients who are discharged before postoperative day 15, because the risk that they will not be vaccinated is high, vaccines should be administered before discharge. In any case, the primary care physicians of splenectomized patients should be notified to ensure that they receive the appropriate level of care. Splenectomized patients should receive antibiotic therapy in all cases of sudden or unexplained onset of fever or malaise. The recommended antibiotic regimens in adults are as follows: a) Amoxicillin: loading dose of 3g followed by 1g every 8 hours; b) Levofloxacin 500mg every 24 hours or Moxifloxacin 400mg every 24 hours (for patients allergic to beta-lactams). Antibiotic prophylaxis with Amoxicillin/Clavulanic Acid for 5 days is also recommended for dog or other animal bites. Treatment of OPSI should be intrahospital because clinical conditions can rapidly deteriorate even on adequate antibiotic therapy. The antibiotic regimen should then be adjusted based on culture examinations. In splenectomized patients, the risk of severe malaria is also increased, so patients travelling to an endemic area should receive appropriate prophylaxis and implement all necessary measures to avoid contracting the disease. Regarding the correlation between COVID-19 and spleen function, a very recent study showed that all patients who died had IgM memory B cell depletion, concluding that IgM memory B cells are commonly depleted in COVID-19 patients and this correlates with increased mortality and overlapping infections; therefore, administration of the anti-covid vaccine is suggested Table 3.
WSES guidelines for the management of adult splenic trauma [84].
The 2017 WSES guidelines on the management of splenic trauma in adult and pediatric patients were developed after a systematic review of the literature and following approval by a group of experts in the field using the Delphi method. The final version was discussed during the May 2017 WSES World Congress in Campinas, Brazil. Below is the final version of the splenic trauma guidelines for adult patients Tables 4 Figures 3,4:
Psychological and related profiles
The related issue of psychological profiles following abdominal trauma with splenic involvement is not debated in the medical literature, which could involve hospitalization because of the need to perform splenectomy for severe cases. This vulnus seems at least curious since the healthcare approach should always tend to the holistic preservation of the patient, even concerning profiles of disturbance of the serenity and mental well-being of the patient. Moreover, it is not new for ward professionals to encounter emotional [85] and physical phenomena of acute anxiety [86-89], phobia [90], obsession [91,92] and depression [93] or other kinds [94-96], concerning the near future that awaits the patient and his new quality of life; but such destabilization may hyperactivate certain personality traits [97-104] decompensating the patient already suffering from psychopathological disturbances [105-107] or prior neurologically based conditions or with traumatic brain comorbidity [108-120] or prone to easily altered perception (as in psychotic states or psychotic tendencies) [121,122], especially if associated with the traumatic condition there is a neoplastic origin or comorbidity [123], and therefore having to resort to psychotherapeutic or psychiatric intervention [124,125] to restore the lost conditions of well-being [126-129]. Another profile of extreme clinical interest, also because of the numerous correlations with psychological disorders, is intestinal dysbiosis [130-132] caused by prescribed drug therapy, which in turn can negatively affect the balance of intestinal flora and all its functions. These profiles, therefore, deserve greater attention and dignity, especially in a holistic key, also to avoid serious psychological imbalances that can promote episodes or suicidal thoughts [133].
Conclusions
Therefore, it is possible to conclude the following:
1) patients presenting with hemodynamic instability and/or peritonitis should be referred for urgent surgical intervention;
2) non-operative treatment of splenic lesions is now the treatment modality of choice in hemodynamically stable patients, regardless of the grade of the lesion;
3) the method of Non-Operative Management of splenic trauma is confirmed as a method characterized by lower morbidity, mortality and hospitalization compared to the surgical method;
4) low morbidity and overall mortality is achieved when the NOM procedure is applied to an appropriate patient population;
5) nonoperative management of splenic lesions should only be considered in a setting that offers the possibility of serious clinical monitoring and evaluations and that has an operating room available for 24-hour urgent laparotomies;
6) CT with intravenous contrast medium is the diagnostic modality of choice for evaluation of closed splenic lesions;
7) adjunctive therapies such as angiography with embolization remain important adjuncts to the bloodless treatment of splenic lesions;
8) Most patients with splenic-dominant blunt trauma are managed with NOM.
Thus, over time, the use of embolization has increased while open surgery has decreased, and mortality has improved for all treatment methods. Compared with splenectomy, embolization is associated with a shorter hospital stay, but it is still used relatively infrequently [86]; However, a complete understanding of post-embolization immune changes remains an area in need of further investigation, as do the psychological and mental health profiles of the surgical patient.
- Van der Vlies CH, Olthof DC, Gaakeer M, Ponsen KJ, van Delden OM, et al. (2011) Changing patterns in diagnostic strategies and the treatment of blunt injury to solid abdominal organs. Int J Emerg Med 4: 47. Link: https://pubmed.ncbi.nlm.nih.gov/21794108/
- Costa G, Tierno SM, Tomassini F, Venturini L, Frezza B, et al. (2010) The epidemiology and clinical evaluation of abdominal trauma. An analysis of a multidisciplinary trauma registry. Ann Ital Chir 81: 95-102. Link : https://bit.ly/3pkcB4l
- Richardson JD (2005) Changes in the management of injuries to the liver and spleen. J Am Coll Surg 200: 648-669. Link: https://bit.ly/3AYANLU
- Forsythe RM, Harbrecht BG, Peitzman AB (2006) Blunt splenic trauma. Scand J Surg 95: 146-51. Link: https://bit.ly/2Zg9hfU
- Dionigi R (2017) Chirurgia. Basi teoriche e chirurgia generale. VI ed Edra Ed. Link: https://bit.ly/3DXXgdX
- Beauchamp RD, et al. (2002) Milza. Trattato di chirurgia. Le basi biologiche della moderna pratica chirurgica. Sabiston.
- Seccia M (2008) I traumi splenici. Splenic Trauma in Emergency Surgery Manual. Edizioni Alpes.
- Jeremitsky E, Smith RS, Ong AW (2013) Starting the clock: defining non-operative management of blunt splenic injury by time. Am J Surg 205: 298-301. Link: https://bit.ly/3aVhdpg
- Sherman R (1980) Perspectives in management of trauma to the spleen: 1979 presidential address, American Association for the Surgery of Trauma. J Trauma 20: 1-13. Link: https://bit.ly/3DZqtFe
- Lucas CE (1991) Splenic Trauma-Choice of Management. Ann. Surg 213: 98-112. Link: https://bit.ly/2XzCIZO
- McClusky III DA, Skandalakis LJ, Colborn GL, Skandalakis JE (1999) Tribute to a Triad: History of Splenic Anatomy, Physiology, and Surgery-Part 2. Word J Surg 23: 311-25. Link: https://bit.ly/3pjo6ZT
- Douglas GJ, Simpson JS (1971) The conservative management of splenic trauma. J Pediatr Surg v6, i5, 565-570. Link: https://bit.ly/2Zc1S1a
- King H, Shumacker HB jr (1952) Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg 136: 239-242. Link: https://bit.ly/3E1Qwfa
- Mullinix AJ, Foley WD (2004) Multidetector computed tomography and blunt thoracoabdominal trauma. J Comput Assist Tomogr., 28 Suppl., 1: S20-7. Link: https://bit.ly/2ZiKLuC
- Gozzoli M (2013) Le lesioni traumatiche della milza: recenti acquisizioni e prospettive. Tesi specialistica. Università degli Studi di Pisa, Facoltà di Medicina e Chirurgia, a.a. 2012/2013. Link: https://bit.ly/3jmsq6G
- Groom AC (1987) The Microcirculatory Society Eugene M. Landis award lecture. Microcirculation of the spleen: new concepts, new challenges. Microvasc Res 34: 269-289. Link: https://bit.ly/2XA4SDY
- Drew PA, Kiroff G K, Ferrante A, Cohen R C (1984) Alterations in immunoglobulin synthesis by peripheral blood mononuclear cells from splenectomized patients with and without splenic regrowth. J Immunol 132: 191-196. Link: https://bit.ly/3aTvwuB
- Bohnsack JF, Brown EJ (1986) The role of the spleen in resistance to infection. Annu Rev Med 37: 49-59. Link: https://bit.ly/3jk7mha
- Foster PN, Bolton RP, Cotter KL, Losowsky MS (1985) Defective activation of neutrophils after splenectomy. J Clin Pathol 38: 1175-1178. Link : https://bit.ly/3B1RrtS
- Newland A (2005) Preventing severe infection after splenectomy. BMJ 331: 417-418. Link: https://bit.ly/3C2gb6O
- Beuran M, Gheju I, Venter MD, Marian RC, Smarandache R (2012) Non-operative management of splenic trauma. J Med Life 5: 47-58. Link: https://bit.ly/3vwAHKm
- Pizzilli G (2018) Trattamento multimodale delle lesioni traumatiche di fegato e milza: proposta e verifica di un protocollo operativo. EAI ed.
- McFadyen JG, Ramaiah R, Bhananker S (2012) Initial assessment and management of pediatric trauma patients. Int J Crit IIIn Inj Sci 2: 121-127. Link: https://bit.ly/3GepEum
- Basile G, Mari P, Chiarenza S, Magrì A, Primus A, et al. (2005) Traumi chirurgici addominali in età pediatrica, Ann Iul Chir LXXVI: 1. Link: https://bit.ly/3jjvOzz
- Barbuscia M, Praticò C, Pergolizzi FP, Lizio R, Ilaqua A, et al. (2007) Closed trauma of the spleen. Indications to surgical treatment. G Chir 28: 217-221. Link: https://bit.ly/3jeU3if
- Capasso L, Cuomo UM, D'Ambrosio R, Buonincontro S, Iarrobino G, et al. (2008) Treatment of splenic trauma in paediatric age. Ann Ital Chir 79: 129-134. Link: https://bit.ly/3pkP03q
- Fingerhut A, Etienne JC (1995) Chirurgia conservativa della milza. EMC - Tecniche Chirurgiche - Addominale.
- Bradburn EH, Frankel HL (2010) Diagnosis and Management of Splenic Trauma. The Journal of Lancaster General Hospital v5 Link: https://bit.ly/2Zbv5c8
- Trauma, American College of Surgeon’s Commitee on. Advanced Trauma Life Support® (ATLS®) Student manual. 9th, 2016. Link: https://bit.ly/3E27vxW
- Rostas JW, Manley J, Gonzalez RP, Brevard SB, Ahmed N, et al. (2015) The safety of low molecular-weight heparin after blunt liver and spleen injuries. Am J Surg 210: 31-34. Link : https://bit.ly/3vHeauB
- Moore EE, Shackford SR, Pachter HL, McAninch JW, Browner BD, et al. (1989) Organ injury scaling: spleen, liver and kidney. J Trauma 29: 1664-1666. Link: https://bit.ly/3pBNCtJ
- Bouillon B, Kanz KG, Lackner CK, Mutschler W, Sturm J (2004) The importance of Advanced Trauma Life Support (ATLS) in the emergency room. Unfallchirurg 107: 844-850. Link: https://bit.ly/3G4zI8Z
- West JG, Trunkey DD, Lim RC (1979) Systems of trauma care. A study of two counties. Arch Surg 114: 445-460. Link: https://bit.ly/3aWR35G
- Richards JR, McGahan PJ, Jewell MG, Fukushima LC, McGahan JP (2004) Sonographic patterns of intraperitoneal hemorrhage associated with blunt splenic injury. J Ultrasound Med 23: 387-394. Link: https://bit.ly/3m0Lk4R
- Catalano O, Aiani L, Barozzi L , Bokor D , De Marchi A , et al. (2009) CEUS in abdominal trauma: multi-center study. Abdom Imaging 34: 225-234. Link: https://bit.ly/3pmOiD5
- Cocanour CS, Moore FA, Ware DN, Marvin RG, Clark JM, et al. (1998) Delayed complications of nonoperative management of blunt adult splenic trauma. Arch Surg 133: 619-624. Link: https://bit.ly/2XwBQFf
- Bhullar IS, Frykberg ER, Siragusa D, Chesire D, Paul J, et al. (2012) Age Does Not Affect Outcomes of Nonoperative Management of Blunt Splenic Trauma. J Am Coll Surg 214: 958-964. Link: https://bit.ly/3E0drr1
- Raza M, Abbas Y, Devi V, Prasad KV, Rizk KN, et al. (2013) Non operative management of abdominal trauma – a 10 years review. World J Emerg Surg 8: 14. Link: https://bit.ly/2Z7ImCG
- Bhullar IS, Frykberg ER, Siragusa D, Chesire D, Paul J, et al. (2012) Selective angiographic embolization of blunt splenic traumatic injuries in adults decreases failure rate of nonoperative management. J Trauma Acute Care Surg 72: 1127-1134. Link: https://bit.ly/3m1zmrM
- Berguer R, Staerkel RL, Moore EE, Moore FA, Galloway WB, et al. (1991) Warring: Fatal reaction to the use of fibrin glue in deep hepatic wounds. Case reports. J Trauma 31: 408-411. Link: https://bit.ly/3aWJ8Ff
- Reddy CG, Chalasani V, Pathma-Nathan N (2004) Splenic preservation: an additional haemostatic measure during mesh splenorrhaphy. ANZ J Surg 74: 596-597. Link: https://bit.ly/3pBTiE3
- Pisters P, Pachter L (1994) Autologous splenic transplantation forsplenictrauma. AnnSurg. 219: 225-235. Link: https://bit.ly/3joNfP3
- Holdsworth RJ (1991) Regeneration of the spleen and splenic transplantation. Br J Surg 78: 270-278. Link: https://bit.ly/3E44a15
- Cimbanassi S, Chiara O (2012) Trauma dell’addome. Trauma care. La cura definitiva del trauma maggiore. Elsevier. Link: https://bit.ly/3GaEke2
- Smith Jr JS, Cooney RN, Mucha Jr P (1996) Nonoperative management of the ruptured spleen: A revalidation of criteria. Surgery 120: 745-750. Link: https://bit.ly/2XwGVgN
- Upadhyaya P, Simpson JS (1968) Splenic trauma in children. J Am Coll Surg 126: 781-790. Link: https://bit.ly/3nbbwta
- Haan JM, Bochicchio GV, Kramer N, Scalea TM (2005) Management of blunt splenic injuries: A 5-year experience. J Trauma. 58: 492-498. Link: https://bit.ly/3CdUsIY
- Schurr MJ, Fabian TC, Gavant M, Croce MA, Kudsk KA, et al. (1995) Management of blunt splenic trauma: computed tomographic contrast blush predicts failure of nonoperative management. J Trauma 39: 507-512. Link: https://bit.ly/2Xv6f6I
- Sabe AA, Claridge JA, Rosenblum DI, Lie K, Malangoni MA (2009) The Effects of Splenic Artery Embolization on Nonoperative Management of Blunt Splenic Injury: A 16-Year Experience. J Trauma 67: 565-572. Link: https://bit.ly/3vwqsWe
- Peitzman AB, Heil B, Rivera L, Federle MB, Harbrecht BG, et al. (2000) Blunt splenic injury in adults: multi-institutional study of the eastern association for the surgery of trauma. J Trauma 49: 177-187. Link: https://bit.ly/3vwQB7l
- Weinberg J, Magnotti LJ, Croce MA, Edwards NM, Fabian TC (2007) The utility of serial computed tomography imaging of blunt splenic injury: still worth a second look? J Trauma. 62: 1143-1147. Link: https://bit.ly/3B11Ndy
- Mutschler M, Nienaber U, Brockamp T, Wafaisade A, Fabian T, et al. (2013) Renaissance of base deficit for the initial assessment of trauma patients: a base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the Trauma Register DGU®. Crit Care 17: R42. Link: https://bit.ly/2ZbvXgU
- Mutschler M, Nienaber U, Münzberg M, Wölfl C, Schoechl H, et al. (2013) The Shock Index revisited - a fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Crit Care 17: R172. Link: https://bit.ly/3vv7H5N
- Carr JA, Roiter C, Alzuhaili A (2012) Correlation of operative and pathological injury grade with computed tomographic grade in the failed nonoperative management of blunt splenic trauma 38: 433–438. Link: https://bit.ly/3C1XEaN
- Doody O, Lyburn D, Geoghegan T, Govender P, Munk PL, et al. (2005) Blunt trauma to the spleen: Ultrasonographic findings. Clin Radiol, 60: 968-976. Link: https://bit.ly/3lY2vEk
- El-Matbouly M, Jabbour G, El-Menyar A, Peralta R, Abdelrahman H, et al. (2016) Blunt splenic trauma: Assessment, management and outcomes; Surg 14: 52–58. Link: https://bit.ly/3E0Lxvb
- Bee TK, Miller PR,Croce MA, Pritchard FE, Fabian TC (2001) Failures of splenic nonoperative management: is the glass half empty or half full?; J Trauma 50: 230–236. Link: https://bit.ly/3C5mmqA
- Clark R, Hird K, Misur P , Ramsay D , Mendelson R (2011) CT grading scales for splenic injury: Why can’t we agree? J Med Imaging Radiat Oncol 55: 163-169. Link: https://bit.ly/3vAoxQz
- Anderson SW, Varghese JC, Lucey BC, Burke PA, Hirsch EF, et al. (2007) Blunt splenic trauma: delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology. 243: 88-95. Link: https://bit.ly/3nhkJ2O
- Marmery H, Shanmuganathan K, Mirvis SE, Richard H 3rd, Sliker C, et al. (2008) Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg 206: 685-693. Link: https://bit.ly/3jjskNu
- Hafiz S, Desale S, Sava J (2014) The impact of solid organ injury management on the US health care system. J Trauma Acute Care Surg 77: 310-314. Link: https://bit.ly/3G4NacZ
- Stassen NA, Bhullar I, Cheng JD, Crandall ML, Friese RS, et al. (2012) Eastern Association for the Surgery of Trauma. Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg.; 73 (5 Suppl 4): S294-300. Link: https://bit.ly/3vwT4yO
- Gaspar B, NEGOI I, PAUN S, HOSTIUC S, GANESCU R, et al. (2014) Selective nonoperative management of abdominal injuries in polytrauma patients: a protocol only for experienced trauma centers. Medic - J Clin Med 9: 168-172. Link: https://bit.ly/3m2rt5C
- Moore FA, Davis JW, Moore EE Jr, Cocanour CS, West MA, et al. (2008) Western Trauma Association (WTA) critical decisions in trauma: management of adult blunt splenic trauma. J Trauma 65: 1007-1011. Link: https://bit.ly/2ZiuUw9
- Rowell SE, Biffl WL, Brasel K, Moore EE, Albrecht RA, et al. (2016) Western Trauma Association Critical Decisions in Trauma: Management of adult blunt splenic trauma. J Trauma Acute Care Surg 82: 787-793. Link: https://bit.ly/3E1UuUZ
- Fugazzola P (2016) Il trauma splenico chiuso: studio multicentrico su due TCI e validazione della nuova classificazione WSES. Tesi di Specializzazione in Chirurgia Generale, Università degli Studi di Milano.
- Demetriades D, Rabinowitz B (1987) Indications for operation in abdominal stab wounds. A prospective study of 651 patients. Ann Surg 205: 129-132.
- Velmahos GC, et al. (2001) Selective nonoperative management in 1,856 patients with abdominal gunshot wounds: should routine laparotomy still be the standard of care? Ann Surg 234: 395-403.
- Renz BM, Feliciano DV (1994) Gunshot wounds to the right thoracoabdomen: a prospective study of nonoperative management. J Trauma 37: 737-744.
- Carlin AM, Tyburski JG, Wilson RF, Steffes C (2002) Factors affecting the outcome of patients with splenic trauma. Am Surg 68: 232-239. Link : https://bit.ly/3C0EwtK
- Inaba K, Barmparas G, Foster A, Talving P, David JS, et al. (2010) Selective nonoperative management of torso gunshot wounds: when is it safe to discharge? J Trauma 68: 1301-1304. Link: https://bit.ly/3lZggCC
- Musetti S (2015) L’impatto dell’embolizzazione dell’arteria splenica nel trattamento dei traumi della milza. Tesi di laurea in Medicina e Chirurgia, Università degli Studi di Pisa.
- Malihe K, Amirkazem VS (2017) Randomized clinical trial of Ligasure versus conventional splenectomy for injured spleen in blunt abdominal trauma. Int J Surg 38: 48-51. Link: https://bit.ly/3B2yd7w
- Leone G, Pizzigallo E (2015) Bacterial infections following splenectomy for malignant and nonmalignant hematologic diseases. Mediterr J Hematol Infect Dis 7: e2015057. https://bit.ly/3E77UPL
- Skattum J, Naess PA, Gaarder C (2012) Non-operative management and immune function after splenic injury. Br J Surg 99: 59-65. Link: https://bit.ly/3lY84CI
- Lynn KN, Werder GM, Callaghan RM, Sullivan AN, Jafri ZH, et al. (2009) Pediatric blunt splenic trauma: A comprehensive review. Pediatr Radiol 39: 904-916. Link: https://bit.ly/2ZbDAUA
- Spelman D, Buttery J, Daley A, Isaacs D, Jennens I, et al. (2008) Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern Med J 38: 349–356. Link: https://bit.ly/30xMWuD
- Salvadori MI, Price VE (2014) Preventing and treating infections in children with asplenia or hyposplenia. Paediatr Child Heal 19: 271-278. Link: https://bit.ly/3vu4ByG
- Gross J, Woll NL, Hanson CA, Pohl C, Scorpio RJ, et al. (2013) Embolization for pediatric blunt splenic injury is an alternative to splenectomy when observation fails. J Trauma Acute Care Surg 75: 421-425. Link: https://bit.ly/3jg7qyr
- Skattum J, Loekke RJ, Titze TL, Bechensteen AG, Aaberge IS, et al. (2014) Preserved function after angioembolisation of splenic injury in children and adolescents: A case control study. Injury 45: 156-159. Link: https://bit.ly/3plaFsC
- Schimmer JAG, van der Steeg AF, Zuidema WP (2016) Splenic function after angioembolization for splenic trauma in children and adults: A systematic review. Injury 47: 525-530. Link: https://bit.ly/2Z9AIYM
- Shatz DV (2002) Vaccination practices among North American trauma surgeons in splenectomy for trauma. J Trauma 53: 950-956. Link: https://bit.ly/3C30j3O
- Lenti MV, Aronico N, Pellegrino I, Boveri E, Giuffrida P, et al. (2020) Depletion of circulating IgM memory B cells predicts unfavourable outcome in COVID-19. Scientific Reports 10: 20836. Link: https://go.nature.com/30D5gT7
- Coccolini F, Montori G, Catena F, Kluger Y, Biffl W, et al. (2017) Spleen Trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg 12: 40. Link: https://bit.ly/3B2ydEy
- Perrotta G (2021) The "Human Emotions" and the "Perrotta Human Emotions Model" (PHEM): The new theoretical model. Historical, neurobiological and clinical profiles. Arch Depress Anxiety 7: 020-027. Link: https://bit.ly/2XkVTWY
- Perrotta G (2019) Anxiety disorders: definitions, contexts, neural correlates and strategic therapy. J Neur Neurosci 6: 046. Link: https://bit.ly/2WSmiaT
- Perrotta G (2021) Maladaptive stress: Theoretical, neurobiological and clinical profiles. Arch Depress Anxiety 7: 001-007. Link: https://bit.ly/3sDs39Y
- Perrotta G (2019) Panic disorder: definitions, contexts, neural correlates and clinical strategies. Current Trends in Clinical & Medical Sciences 1. Link: https://bit.ly/38IG6D5
- Perrotta G (2019) Obsessive-Compulsive Disorder: definition, contexts, neural correlates and clinical strategies. Cientific Journal of Neurology 1: 08-16. Link: https://bit.ly/3pxNbNu
- Perrotta G (2019) Tic disorder: definition, clinical contexts, differential diagnosis, neural correlates and therapeutic approaches. J Neurosci Rehab 2019: 1-6. Link: https://bit.ly/36UJme5
- Perrotta G (2019) Depressive disorders: Definitions, contexts, differential diagnosis, neural correlates and clinical strategies. Arch Depress Anxiety 5: 009-033. Link: https://bit.ly/2KADvDm
- Perrotta G (2019) Neural correlates in eating disorders: Definition, contexts and clinical strategies. J Pub Health Catalog 2: 137-148. Link: https://bit.ly/3mWmf8s
- Perrotta G (2019) Post-traumatic stress disorder: Definition, contexts, neural correlations and cognitive-behavioral therapy. J Pub Health Catalog 2: 40-47. Link: https://bit.ly/3rvaCc6
- Perrotta G (2019) Sleep-wake disorders: Definition, contexts and neural correlations. J Neurol Psychol 7: 09. Link: https://bit.ly/3hoBiGO
- Perrotta G (2020) Perrotta Integrative Clinical Interview, LK ed.
- Perrotta G (2020) The structural and functional concepts of personality: The new Integrative Psychodynamic Model (IPM), the new Psychodiagnostic Investigation Model (PIM) and the two clinical interviews for the analysis of personality disorders (Perrotta Integrative Clinical Interview or PICI) for adults and teenagers (1TA version) and children (1C version), Psychiatry Peertechz, E-book. Link: https://bit.ly/2SqQevV
- Perrotta G (2020) First revision of the Psychodiagnostic Investigation Model (PIM-1R) and elaboration proposal of a clinical interview for the analysis of personality disorders (Perrotta Integrative Clinical Interview or PICI-1) for adults, teenagers and children, Psychiatry Peertechz. Link: https://bit.ly/2MQe3dY
- Perrotta G (2020) "Perrotta Integrative Clinical Interview (PICI-1)": Psychodiagnostic evidence and clinical profiles in relation to the MMPI-II, Ann Psychiatry Treatm 4: 062-069. Link: https://bit.ly/3q0bYLP
- Perrotta G (2021) "Perrotta Integrative Clinical Interview" (PICI) for adults and teenagers (1TA version) and children (1C version): new theoretical models and practical integrations between the clinical and psychodynamic approach. Ann Psychiatry Treatm 5: 001-014. Link: https://bit.ly/3546iGM
- Perrotta G (2021) Perrotta Integrative Clinical Interviews (PICI-2), LK ed, III ed.
- Perrotta G (2021) Perrotta Integrative Clinical Interview (PICI-1): a new revision proposal for PICI-1TA. Two single cases. Glob J Medical Clin Case Rep 8: 041-049. Link: https://bit.ly/3rtXLaq
- Perrotta G (2021) Perrotta Integrative Clinical Interviews (PICI-2): innovations to the first model, the study on the new modality of personological investigation, trait diagnosis and state diagnosis, and the analysis of functional and dysfunctional personality traits. An integrated study of the dynamic, behavioural, cognitive and constructivist models in psychopathological diagnosis. Ann Psychiatry Treatm 5: 067-083. Link: https://bit.ly/3DTK2yC
- Perrotta G (2020) Borderline Personality Disorder: definition, differential diagnosis, clinical contexts and therapeutic approaches. Ann Psychiatry Treatm 4: 043-056. Link: https://bit.ly/3hx2B1N
- Perrotta G (2020) Psychotic spectrum disorders: definitions, classifications, neural correlates and clinical profiles. Ann Psychiatry Treatm 4: 070-084. Link: https://bit.ly/2QI9kNc
- Perrotta G (2021) Avoidant personality disorder: Definition, clinical and neurobiological profiles, differential diagnosis and therapeutic framework. J Neuro Neurol Sci Disord 7: 001-005. Link: https://bit.ly/3DPRF9A
- Perrotta G (2019) Bipolar disorder: definition, differential diagnosis, clinical contexts and therapeutic approaches. J Neuroscience and Neurological Surgery 5. Link: https://bit.ly/34SoC67
- Perrotta G (2019) Autism Spectrum Disorder: Definition, contexts, neural correlates and clinical strategies. J Neurol Neurother 4: 136. Link: https://bit.ly/36UNF9b
- Perrotta G (2019) Alzheimer's disease: definition, contexts, neural correlates, strategies and clinical approaches. J Aging Stud Ther 1. Link: https://bit.ly/35icsDY
- Perrotta G (2019) Parkinson's disorder: definition, contexts, neural correlates, strategies and clinical approaches. J Neurosci Neurol Surg 4. Link: https://bit.ly/2LoIiaS
- Perrotta G (2020) General overview of “human dementia diseases”: definitions, classifications, neurobiological profiles and clinical treatments. Journal of Gerontology & Geriatrics Studies 6: GGS.000626. Link: https://bit.ly/3hRNbp4
- Perrotta G (2020) Amnesia: definition, main models, classifications, neurobiological profiles and clinical treatments. Arch Depress Anxiety 6: 037-044. Link: https://bit.ly/2JuNeKM
- Perrotta G (2020) Apraxia: definition, clinical contexts, neurobiological profiles and clinical treatments. Global J Medical Clin Case Rep 7: 059-061. Link: https://bit.ly/3mW3MJo .
- Perrotta G (2020) Agnosia: definition, clinical contexts, neurobiological profiles and clinical treatments. Arch Gerontol Geriatr Res 5: 031-035. Link: https://bit.ly/3hpis2p
- Perrotta G (2020) Dysarthria: definition, clinical contexts, neurobiological profiles and clinical treatments. Arch Community Med Public Health 6: 142-145. Link: https://bit.ly/3n539NL
- Perrotta G (2020) Agraphia: definition, clinical contexts, neurobiological profiles and clinical treatments. J Neuroscience and Neurological Surgery 6: 4. Link: https://bit.ly/2KGYggo
- Perrotta G (2020) Epilepsy: from pediatric to adulthood. Definition, classifications, neurobiological profiles and clinical treatments. J Neurol Neurol Sci Disord 6: 014-029. Link: https://bit.ly/2L0GC7d
- Perrotta G (2020) The pharmacological treatment of epileptic seizures in children and adults: introduction, clinical contexts, psychopharmacological profiles and prospects in the neurogenetic field. Journal of Neuroscience and Neurological Surgery 6: 8. Link: https://bit.ly/34QPtzv
- Perrotta G (2020) Aphasia: definition, clinical contexts, neurobiological profiles and clinical treatments. Ann Alz Dement Care 4: 6. Link: https://bit.ly/3rEti9l
- Perrotta G (2019) Delusions, paranoia and hallucinations: definitions, differences, clinical contexts and therapeutic approaches. Cientific Journal of Neurology (CJNE) 1: 22-28.
- Perrotta G (2021) The state of consciousness: from perceptual alterations to dissociative forms. Defining, neurobiological and clinical profiles. J Neuro Neurol Sci Disord 7: 006-018. Link: https://bit.ly/3pk3rop
- Perrotta G (2019) The acceptance in the elaboration of mourning in oncological diseases: definition, theoretical models, and practical applications. Needs analysis and subjective oncological reality. Biomed J Sci Tech Res 21.
- Perrotta G (2020) The strategic clinical model in psychotherapy: theoretical and practical profiles. J Addi Adol Behav 3: 5. Link: https://bit.ly/3aPMx9X
- Perrotta G (2020) Accepting "change" in psychotherapy: from consciousness to awareness. Journal of Addiction Research and Adolescent Behaviour 3.
- Perrotta G (2021) Strategic psychotherapy and the "decagonal model" in clinical practice. Ann Psychiatry Treatm 5: 028-035. https://bit.ly/3pgS14Y
- Perrotta G (2019) The reality plan and the subjective construction of one's perception: the strategic theoretical model among sensations, perceptions, defence mechanisms, needs, personal constructs, beliefs system, social influences and systematic errors. J Clinical Research and Reports 1. Link: https://bit.ly/3b34baH
- Perrotta G (2020) Psychological trauma: definition, clinical contexts, neural correlations and therapeutic approaches. Curr Res Psychiatry Brain Disord: CRPBD-100006. Link: https://bit.ly/37UD3bz
- Perrotta G (2020) Human mechanisms of psychological defence: definition, historical and psychodynamic contexts, classifications and clinical profiles. Int J Neurorehabilitation Eng 7: 1. Link: https://bit.ly/2L0I5dJ
- Perrotta G (2020) Oxytocin and the role of “regulator of emotions”: definition, neurobiochemical and clinical contexts, practical applications and contraindications. Arch Depress Anxiety 6: 001-005. Link: https://bit.ly/2JqvAYr
- Perrotta G (2021) The intestinal microbiota: towards a multifactorial integrative model. Eubiosis and dysbiosis in morbid physical and psychological conditions. Arch Clin Gastroenterol 7: 024-035. Link: https://bit.ly/3m0E1dH
- Perrotta G (2021) Intestinal dysbiosis: definition, clinical implications, and proposed treatment protocol (Perrotta Protocol for Clinical Management of Intestinal Dysbiosis, PID) for the management and resolution of persistent or chronic dysbiosis. Arch Clin Gastroenterol 7: 056-063. Link: https://bit.ly/3naUmvz
- Perrotta G (2020) The clinical and psychopathological implications in the forms of hyperhistaminosis. Online J Neuro Br Disord 4: 12. Link: https://bit.ly/3rBIh40
- Perrotta G (2020) Suicidal risk: definition, contexts, differential diagnosis, neural correlates and clinical strategies. J Neuroscience Neurological Surgery 6: 114. Link: https://bit.ly/3aMqcu5
- Chahine AH, Gilyard S, Hanna TN, Fan S, Risk B, et al. (2020) Management of Splenic Trauma in contemporary clinical practice: a National Trauma Data Bank Study. Acad Radiol S1076-6332(20)30645-0. Link: https://bit.ly/3jjCzkQ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
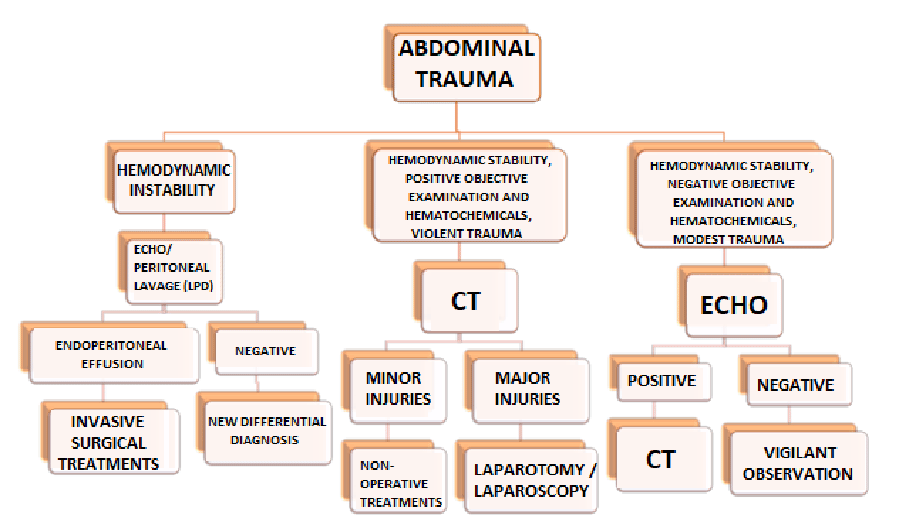
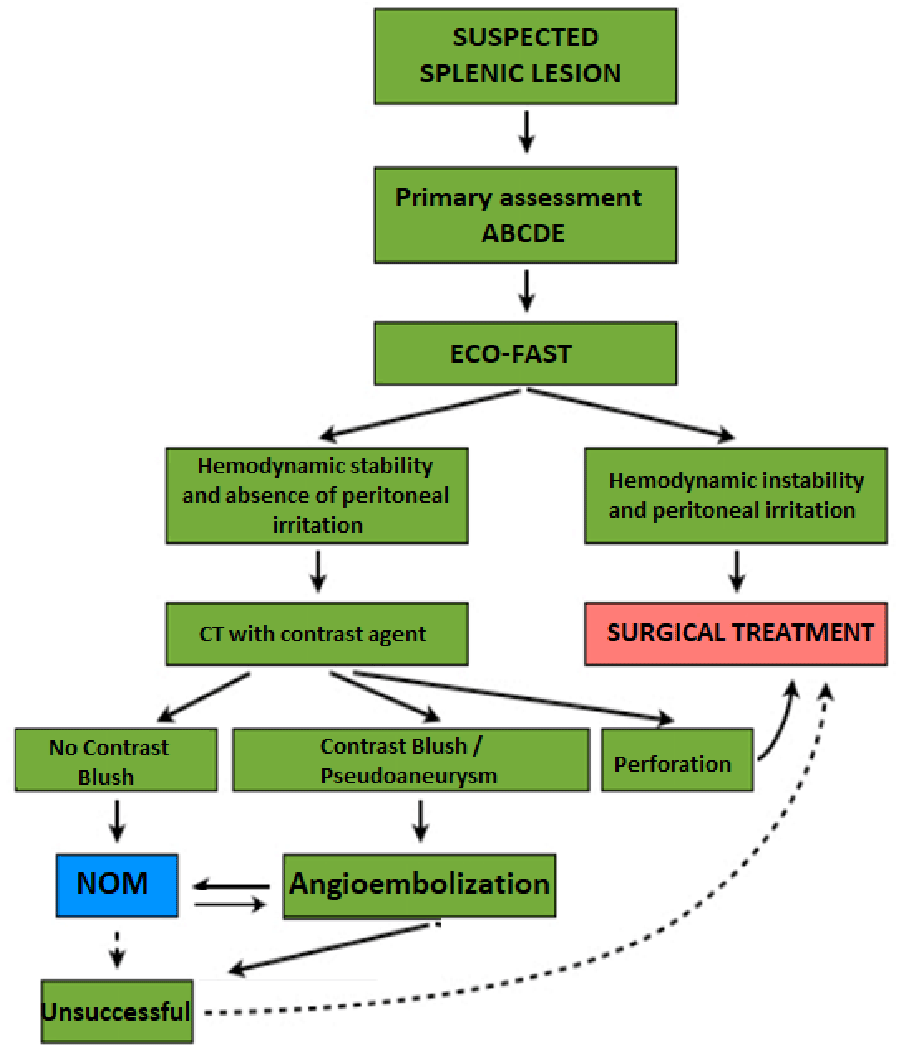
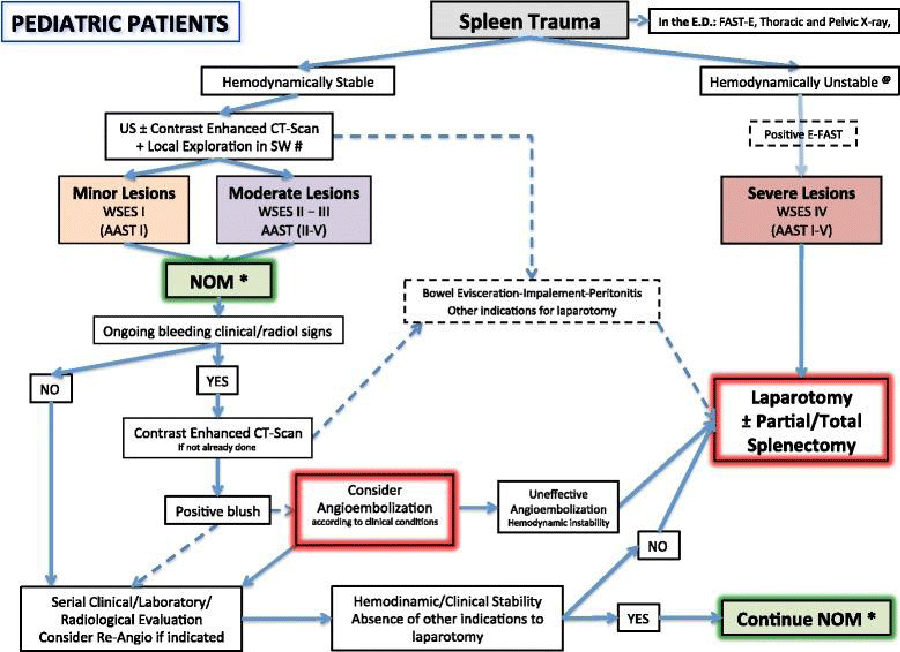
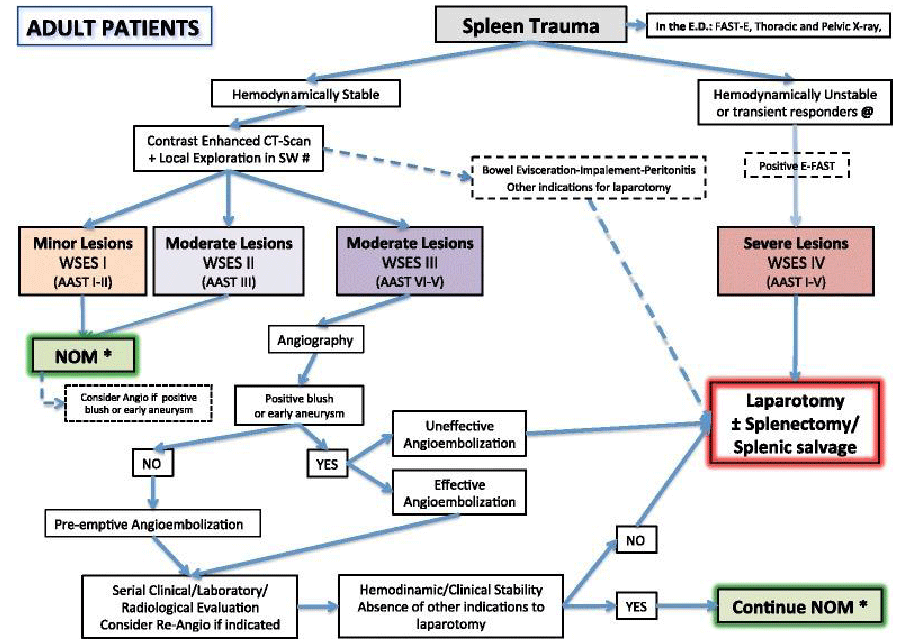

 Save to Mendeley
Save to Mendeley
