Open Journal of Plant Science
Distribution and Socio-economic Impacts of Invasive Alien Plant Species in Ethiopia: A Review
Belayneh Bufebo1* and Eyasu Elias2
2Associate Professor, Center for Environmental Science, Addis Ababa University P.O. Box 1167, Addis Ababa, Ethiopia
Cite this as
Bufebo B, Elias E (2018) Distribution and Socio-economic Impacts of Invasive Alien Plant Species in Ethiopia: A Review. Open J Plant Sci 3(1): 026-033. DOI: 10.17352/ojps.000012This paper is a review of various articles and documents on distribution and socioeconomic impacts of invasive alien plant species. It provides information on distribution and problems posed by these species. The spread of invasive alien species is neither easy to manage nor easy to reverse, threatening not only biodiversity but also economic development and human wellbeing. There are about 22 invasive alien species were identified in Ethiopia and prioritization of invasive alien species was done by considering facts such as the magnitude of invasiveness, threats to local biodiversity, socio-economic and human health impacts. Priority species are Parthenium weed (Parthenium hysterophorous), Mesquites (Prosopis juliflora), Water hyacinth (Eichhornia crassipes), Lantana (Lantana camara), and Acecia species (A.drepanolobium and A. mellifera). These invasive alien plant species pose the biggest threat to biodiversity after habitat destruction and also pose a serious threat to agriculture, livelihoods and human health in many parts of the country. Because of their unique characteristics they do not need special environmental requirement for seed germination, to have rapid seedling growth and produce seeds for longer period of time as long as environmental condition permit. These alien species outcompete, infect or transmit diseases, compete, hybridize with the native ones or attack them and these leads to sound effects on social instability and economic hardship, placing constraints on sustainable development, economic growth, poverty alleviation and food security. Therefore, the country should evaluate regularly the distribution and socioeconomic impact of these species to take proper protection measures and to prevent further introduction and spread of the invasive alien plant species in new areas that are not yet infested.
Introduction
Invasive alien species of plant are accidentally or intentionally introduced in to the country are subsequently escaping from their entry points and their spread is increasing at alarming rate from time to time. Even in the absence of precise figures, it is clear that the spread of invasive alien species in Ethiopia has increased in the last decade, both in terms of area coverage and plant density [1].
The spread of invasive alien species is neither easy to manage nor easy to reverse, threatening not only biodiversity but also economic development and human wellbeing [2]. Invasive Alien Species (IAS) refer to plants, animals or microorganisms that are not native to specific ecosystem and whose introduction threatens biodiversity, food security, health or economic development [3]. Invasive species are of concern because of their capability of spreading fast, their high competitiveness and ability to colonize new areas within short periods. The nature and severity of the impacts of these species on society, economic life, health and national heritage are of global concern [3].
Invasive species are recognized as one of the major threats to native species and ecosystems around the world [4]. Encroachment of rangelands by invasive species, reduction of crop yield, genetic erosion of biodiversity, disruption of water flow, poisoning of livestock, formation of impenetrable thickets, etc are some of the impacts of invasive species across a wide range of agro-ecologies. They are considered one of the key pressures on world’s biodiversity: altering ecosystem services and processes, reducing native species abundance and richness, and decreasing genetic diversity of ecosystems [4].
The spread of invasive plant species in Ethiopia is a growing concern in national parks, lakes, rivers, power dams, and urban green spaces - causing huge economic and ecological losses [5-7]. Among these invasive alien species, Priority species are Parthenium weed (Parthenium hysterophorus), Mesquites (Prosopis juliflora), Water hyacinth (Eichhornia crasspies), Lantana camara, and Acacia species (Acecia drepanolobium and Acecia mellifera) are causing major problems in the country. In general, invasive alien species is of a great concern posing serious problems to development, as well as big threat to biodiversity of the country and their spread is increasing at alarming rate from time to time [8]. Moreover, these species are causing severe damages to natural environment and habitats leading to the loss of many plant species of important to the natural heritage of a country in Ethiopia [9].
Therefore, this review work provides brief over view on the spread and socio economic impacts of Priority invasive alien plant species Parthenium weed (Parthenium hysterophorous), Mesquites (Prosopis juliflora), Water hyacinth (Eichhornia crassipes), Lantana (Lantana camara) Acecia species (Acecia drepanolobium and Acecia mellifera) based on different sources
Methodology
The methodology followed during the preparation of this manuscript was reviewing articles from known journals, reports and records related to the topic. Many published articles were reviewed in compiling this work. The available materials were systematically selected based on the relevance, content and time of their publication. Most of the journals and reports selected during review process were related with particular topic “Distribution and Socio Economic Impacts of Invasive Alien Plant Species” in Ethiopia. Therefore, these journals were taken as the base to review this manuscript.
Ecological distribution and socio-economic impacts of parthenium hysterophorus
In Ethiopia, it is believed to have been introduced in 1976/77 with army vehicles from Somalia and has become a serious weed both in arable land and in grazing lands [10]. But in contrast to this [11], reported that it was introduced into Ethiopia around 1974. Others also believed that P. hysterophorus may have also been spread through the provision of humanitarian emergency food aid. For example, this weed was introduced to Africa through grain shipments for famine relief to Ethiopia [3]. The weed was first seen in 1980s near food-aid distribution centers in Ethiopia. However, currently, it is widely distributed in Ethiopia. In eastern Ethiopia, Tamado Tana and Milberg and Tamado Tana [12,13], reported that parthenium weed is the second most frequent weed (54%) after Digitaria abyssinica (63%). The presence of parthenium in Kenya and Somalia and the capacity of the seed to travel long distance through wind, water, and other means also suggested the possible entry into Ethiopia from these neighboring countries. The weed spread rapidly, and soon came to dominate pastures and crop fields because it has allelopathic properties, releasing chemicals that suppress the growth and germination of neighboring plants [14,15]. Its invasion of Ethiopia has not only had a devastating effect on crop production, but also results in grazing shortages, since the weed is unpalatable to livestock; if it is mixed with fodder, it taints the meat and milk [16].
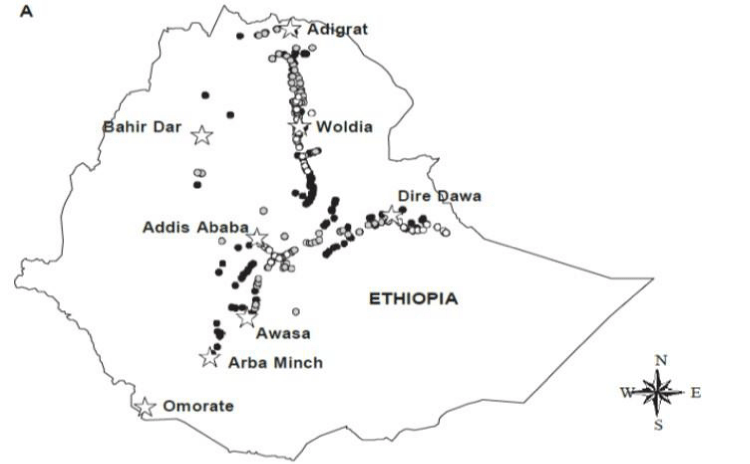
In the Amhara region, it is estimated that about 37,105 hectares of land is infested with parthenium [17]. It is abundantly found in Gojjam, in south and north Gonder with the potential to spread to agricultural districts of Metama and Setit Humera [17]. Furthermore, the weed is well established in many districts of south, north, and central Tigray. In one district alone, Alamata, about 10,000 hectares of the land has been infested with parthenium [17]. In much of the low lands of Wello, Parthenium has become the most dominant weed. In these areas, the weed has been reported in 42 districts. The weed is also a serious problem in the Regional State of Oromia although there is no actual survey data on the total area of land infested in the region. Currently, Parthenium is spreading at an alarming rate in Eastern Ethiopia; the central rift valley, and neighboring localities of Afar Region, East Shewa, Arsi, and Bale and in Southern Ethiopia. Taye Tessema [18], reported that the plant occurred in the towns, usually on roadsides, and vacant sites and grew only at irregular intervals. The introduction in these areas is very recent, probably since 1997 for there had been no parthenium weed observed in West Shewa region from 1995 – 1996 during which intensive qualitative and quantitative determination of weeds occurring in these areas took place. The distribution of Parthenium hysterop horus in Ethiopia can be shown as follows figure 1 [19].
The invasive nature of P. hysterophorus weed is evidenced from its ability to form huge mono cultural stands with no other plant in the vicinity. P. hysterophorus is known to cause a number of ecological and agricultural problems, such as the loss of crop productivity, fodder scarcity, biodiversity depletion and health problems for human beings and livestock. The impact of P. hysterophorus weed on livestock production is both direct and indirect, for it is known to affect grazing lands, animal health and on milk and meat products. According to Shashie [20], P. hysterophorus has negative impact on quality of meat that the meat could lose its quality having bitter taste and the soup prepared with the animal’s meat that was fed with P. hysterophorus is not favored as it impairs its normal flavor. Parthenium weed reduces the productivity and carrying capacity of rangeland pastures, invading crop and forage pastures, increasing agricultural production costs and causing environmental damage [21]. Range and pasture weeds are plants that reduce livestock production through poisoning, reducing stock growth (directly or by competition with preferred plants) or by inflicting mechanical damage. Pasture weed is defined as ‘a species whose presence results in a reduced economic output in a specific system. They may be poisonous or unpalatable to livestock or they may be edible, but provide less or poor quality forage when compared to other forage species. Various woody species are widely perceived to reduce the herbage supply through competition. They may inhibit the movements of livestock, reduce their access to water or forage, and make it more difficult to master livestock [22]. The presence of invasive plants in rangelands leads to large scale economic losses in the form of reduced level of animal productivity, increased herd mobility rates, more difficult stock handling and management and a considerably reduced property capital values. Weeds in pasture can endanger livestock and lower their products. For instance, they reduce the quality and quantity forage and make them unpalatable or even poisonous to livestock [23].
P. hysterophorus is a highly interfering in its nature and can cause substantial production losses up to 40% in India [24]. P. hysterophorus residues have been reported to exert a significant inhibitory effect on the growth and establishment of crops. A significant reduction in the seedling growth (measured in terms of seedling length and dry weight) was observed when test species were grown in soil amended with different amount of P. hysterophorus residues. The diverse problematic effect of P. hysterophorus do not end up only in its competitive ability and suppression by releasing the inhibiting chemical substances to the nearby crop or forage plants, but also it causes prolonged toxic effect to the soil environment. Kohli and Rani [25], stated that the soil underneath P. hysterophorus got polluted by the release of allelo chemicals from the weed leading to losses in crop yield.
P. hysterophorus can cause severe allergies in some people. It is known to cause human health problems, either through direct skin contact with the plant, or the pollen of the plant in the air, like asthma, bronchitis, dermatitis, and hay fever. In Poona (India), one in 60 men is said to be allergic, and so far, there have been 12 deaths attributed to the allergy [26]. A study, which was conducted in Bangalore (India), showed that 7.1 percent of the study population was suffering from allergic rhinitis resulting from exposure to P. hysterophorus pollen [27].
According to Shashie Ayele [20], hand weeding of parthenium is not advisable as the weed causes contact dermatitis, asthma and fever to human beings. In addition, hand weeding is laborious as it requires frequent work following the emergence pattern of the weed. Hoeing can also be used to get rid of parthenium, but repeated operation is needed as long as there is the seed of the weed in the soil. Manually removing parthenium is the most ideal method. However, it is effective as a method only in limited areas such as residential colonies and agricultural fields. The weed should be uprooted to prevent its regeneration from the remaining lateral shoots and that the uprooting should be done before its flowering period and when the soil is moist enough to facilitate easy removal. In Ethiopia, Presently, hand weeding of Parthenium is primarily conducted by men & women as well as school-age children as shown in figure 2.
Ecological distribution and socio-economic impacts of prosopis juliflora
The invasive woody plant, P. juliflora, is an evergreen tree native to North and South America. It is the most aggressive weed that cause great devastation to subtropical grasslands and was thought to have been introduced to Ethiopia during the establishment of irrigation water development project at Middle Awash as wind break, shade and shelter [28]. This species is now commonly found in Afar National Regional State and spreading to Oromia, Amhara, Somali, and Diredawa regions. Nowadays it is repeatedly reported to be one of the invasive and problematic trees in the Afar region as well as in the country. Many scholars reported that the species has been increasing in density as well as area coverage from year to year even from month to month (El-Keblawy and Al-Rawai, 2006). Currently, this noxious tree heavily infests most agricultural as well as potential rangelands in the Afar region [5]. The thorny nature of the plant, remarkable ability to withstand adverse conditions, non-browseable nature, and above all, the nomadic nature of the people have paved the way to invade most potential lands of the region. El-Keblawy [29], indicated that P. juliflora show a great depressive effect on the number, density, and frequency of native vegetations. The distribution of P. juliflora in Ethiopia is shown in the figure 3.
Prosopis dominates sites under sever biotic and edaphic conditions and the more the prosopis density, the less the non-prosopis woody vegetation density implying the advantage of prosopis over the other non-woody species due to its physiological properties to persist under harsh climatic conditions, herbivore attack, and probable synecological manifestations such as competition for resources. From indigenous tree species Acacia tortilis, Acacia senegal, Acacia saligna, Acacia nilotica, Salvadora persica and Balanitis aegiptica, has been lost due to prosopis invasion [7].
In the Ethiopian context, Prosopis juliflora was wrongly introduced in the 1970’s by Ministry of Agriculture to high quality pasturelands and irrigable areas, including the Awash River basin in the Afar National Regional State of Northeast Ethiopia. According to the result of both informal and formal surveys introduction of prosopis to Afar region has affected biodiversity of the area. Interviewed community leaders and individuals have the perception that several plant and animal species have disappeared from the area due to Prosopis. According to Senyit et al. [7], 94% of the individuals in the high infestation area and 97% in the medium infestation area believed that considerable number of plant species have disappeared from the area because of Prosopis. Disappearance of grasses from the area is the most disappointing to the local people as they heavily depend on grazing land for livestock production [7].
There is a general trend of increased rodent population that will in turn reduce the diversity and population of grass species (Kassahun et al., 2005). Rodents such as Merriam kangaroo rat (Dipodomys merriam) have been found to store mesquite seeds in shallow caches under the ground. Many of these seeds are never retrieved but instead, germinate and increase the infestation. As rodent population expands, they tend to move their caches farther into the grassland areas. Although rodents kill considerable number of mesquite seedlings by grazing, this is more than offset by their distribution activities (Kassahun et al., 2005). Dubale [30], reported that the population of predators such as Hyena, Fox, Lion, Snake, and Leopard, which kill livestock and warthog which damage crops, were increased because of invasion. On the other hand, it has also resulted in increased carnivore populations, which are coming nearer to human settlements and endanger man and his domestic animals [7].
Prosopis has an effect on human health. The most important effect of prosopis on human health is that its thorns cause itching and bring tetanus. Its thorns can even cause blindness [7]. There are some distinct environmental impacts of Prosopis served as a windbreak by preventing movement of sand-drift and typhoon. There is an indication of reducing air temperature and creating a mild weather. It has affected the flora by suppressing grass species and the natural regeneration of native tree species [31].
In an investigation to study the allelopathic effects of prosopis foliage on seed germination and seedling growth of bermuda grass (Cynodon dactylon) [31,32], showed that germination was significantly reduced by aqueous leaf extracts in comparison with distilled water control. The reduction increased with increasing extract concentration until germination was totally inhibited. They indicated that mesquite foliage contains water soluble allelochemicals which could inhibit seed germination and significantly retard the rate of germination and seedling growth of Bermuda grass. Because prosopis tends to establish itself so well, including in arid lands where other tress fail to survive [32]. Indigenous tree and dry season grazing pasture were lost due to invasion of prosopis. This is because of its high competitiveness and production of allelochemicals, which hinder the growth, and development of other species growing in association [31].
Prosopis provides more firewood than other species due to its high biomass production on marginal land [6]. The wood is probably the most important product of woody invasive plant species use either as a fuel wood or structural material [33]. The wood is heavy and hard with excellent fire generating quality. The wood can be commercialized for industrial fuel wood production, brick furnaces and bakery oven and grill charcoal for household consumption. However, due to bushy character and small size more labor is required to gather and transport it than other species. The resulting fire wood is also crooked and irregular in size [6], Figure 4 [34].
Observation in Ethiopia showed that Prosopis trees stumped 10cm below the ground had not coppiced over six months after cutting. This was thought to be a good option to control Prosopis invasion if it is specially linked with schemes that facilitates utilization of the harvested wood for fuelwood or charcoal production [5]. Geesing et al. [35], indicated that depth of cutting to prevent coppicing depends on the size and age of the tree and the density of the vegetation stands. They reported that matured trees where there is thick coverage should be cut 30cm below the ground to remove the bud zone of the root system to prevent reshooting. Csurhes [36], also commented that for effective control through coppicing below ground level, emerging seedlings from the seed in the ground should be uprooted or killed by spraying chemicals or fire. Felling trees and uprooting seedlings from pasturelands using hand tools and machineries was the attempt made to control Prosopis invasion in Ethiopia. Moreover, Clearing of P. juliflora is carried out by the pastoralist communities for crop cultivation (Figure 5).
Ecological distribution of water hyacinth
In Ethiopia, this weed was first reported in 1956 in Koka Lake and the Awash River. At that time, although the infestation was small, the concerned authorities were notified of its potential negative effects on these water bodies and the community at large. However, no subsequent action was taken. Senait et al. [7], also indicated appearance of water hyacinth in Dugda Bora destrict that was reported to be between 1949 and 1958. The weed is now present in Gambella (Baro, Gillo and Pibor rivers), at Koka Dam, Wonji/Shoa Sugar Estate Water reservoirs, Awash River, Lake Akaki Beseka, Lake Aba Samuel, Gora River and in the Rift Valley lakes, except Lake Ellen (Eight km North of Alemtena town). Other Lakes: Ziway, Langano, Abiyata, Shala and Awassa are proved to be free from water hyacinth, but the potential risk for their infestation is still there [7,37] (Figure 6).
In Ethiopia, water hyacinth poses serious socioeconomic and environmental problems on human population in areas where people depend on lakes and rivers for transportation, irrigation, electric generation, fishing, drinking, and meeting other needs, and is therefore an added constraint on development [38]. Water hyacinth was the most abundant aquatic weed on the water bodies and perceived as one of the most important noxious weeds [39]. As reported by different scholars, impact of water hyacinth gets higher whenever there were mats. The most noticeable impacts that were reported by most researchers include: restricting proper water flow, water loss through excessive evapotranspiration, interference with fishing, grazing and crop production activities (accessibility to land water hindered), effect on power generation, increase siltation, flooding, increase cost of production and effect on native plants [39]. Though vital epidemiological data pertaining to incidence of human diseases were not obtained during this review, there is a general increase in disease incidences as a result of provision of vector breeding grounds. Some of the human diseases reported include skin rash, malaria, and bilharzias. These results showed that the impact of water hyacinth might be categorized into social, economical and environmental impacts. Fishers, riparian communities, Institute of Biodiversity, National Agricultural Research Institute, sugar corporation/sugar factories, Ministry of Energy and Water Resources, Ministry of Agriculture and Environmental Protection Authority were identified as the organizations and communities affected by this noxious weed [40]. Similarly, other studies have indicated that the weed poses a great threat to agriculture, fisheries, transportation, hydroelectric power generation, health and the environment [40]. The negative impacts of water hyacinth are due to its dense, impenetrable mats, which restrict access to water. These mats affect fisheries and related commercial activities, functioning of irrigation canals, navigation/transport, hydroelectric programmes and tourism [41]. Ecologically, benthic and littoral diversity is reduced [38], while population of vectors of human and animal diseases such as bilharzias and malaria are increased with water hyacinth infestation as these plants interfere with pesticide application [42].
This review indicated that water hyacinth has become a major invasive alien weed in the water regions of the country having successfully established and invaded the different water bodies. In all water bodies, there was a high degree of variability in water hyacinth infestation. Most of the lakes of Ethiopia were predominated with high water hyacinth infestation and a few spots had low to medium infestation. The infestation of water hyacinth in Lake Tana, is shown as follows in figures 7,8 [43].
The cost involved in the management of water hyacinth is high in terms of economy and also environment. Hence, environmentally safe and economically sustainable controls are necessary to provide long term solutions to weed infestation [37]. The reasons for the inefficiency and limited success in responding to infestations in Ethiopia are lack of an appropriate integrated strategy, lack of awareness to problem in the infested water bodies, lack of coordination or communication and efforts to control and manage water hyacinth have been poorly funded [38]. The strategy developed to manage water hyacinth dealt with the related social and technical factors and specific control measures [38].
Socio-economic impacts of lantana camara
Lantana camara was introduced to Ethiopia as an ornamental plant due to its beautiful aromatic flowers [44]. However, because of prolific seed production and easy dispersal, it escaped cultivation and become a pest in the social, ecological and economic concerns. Presently, it has spread almost all over the country, but still it is not much perceived as a chronic environmental problem, except in few parts of Ethiopia, such as Oromia and Somali regions [44]. Currently, there is little information available on spatial distribution of Lantana camara invasion and its potential geographic spread. Lantana is relatively unpalatable, poisonous to stock, causing loss of appetite, frequent urination, dehydration, and yellowing of inner mouth and eyes. Hairs are lost from the skin, the mouth and eyes smell and ulcerate, and animals may die with one or four weeks. The fruits are also poisonous to children. In some areas, lantana thickets provide a breeding ground to tsetse flies, which transmit the parasitic trypanosomes that cause an animal form of sleeping sickness. According to Belayneh Bufebo et al. [9], some of the problems of Lantana camara invasion, include encroaching bush lands, quickly takes over valuable grazing lands and its dense growth suppressed grasses and other useful forages under its canopy.
Socio-economic impacts of accacia spp
Native species of Acacia such as A. drepanolobium and A. mellifera are encroaching on the rangelands of the Borena zone, which is known for its endemic cattle breeds in the country and the problem is threatening the biodiversity in rangeland ecosystems [45]. According to Tamene (1990), most invading woody species are thorny plants, particularly the Genus Acacia. Acacia drepanolobium, commonly known as a whisthing thorny plant (family Fabaceae) is one of the indigenous species widely distributed in the Borana rangelands. The species for several reasons is notoriously invading the Borana rangelands and emerging as an indigenous invasive woody plant in the southern region of Ethiopia [46,47].
Encroachment is excessive and undesirable increase in woody plant abundance resulting in the suppression of palatable grasses and herbs by encroaching woody species often unpalatable to domestic livestock. Progressive growth in the density of woody plants in dry savanna beyond a critical density suppresses herbaceous plant production and decline range productivity, mainly due to severe competition for available soil and water [48]. It is the major form of rangeland degradation in Borana, characterized by invasion of undesirable woody species and unpalatable forbs, losses of grass layer and increased soil erosion [49].
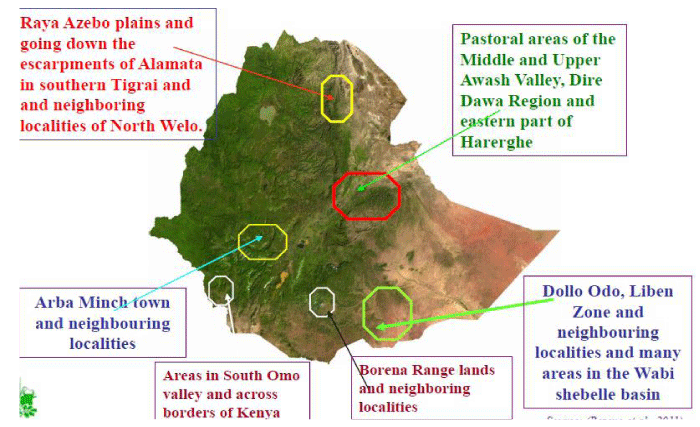
The extent of woody species encroachment in Borana was estimated to be 40% in 1980 and within a decade the cover doubled, and currently it is becoming a great challenge to livestock production and livelihood of pastoralists and/or agropastoralists [50]. The major encroaching species were Acacia drepanolobium, A. mellifera, A. bussei, A. bresvispica, A. senegal, A. oerfota and Commiphora species figure 7 [51].
According to Berhanu Lemma et al. [52], A. mellifera formed impenetrable thicket that prohibit the movement of cattle to the available forages and destroy grassland biodiversity. The spread pattern of A. mellifera was observed from the lowland border districts to the nearest districts in the high lands. Similarly, its abundance decreased as one goes from lowlands to highlands of the Borena zone.
Acacia drepanolobium, commonly known as a whisthing thorny plant (family Fabaceae) is one of the indigenous species widely distributed in the Borana rangelands. Another peculiar feature of A. drepanolobium is its association with populations of symbiotic ants [53], with several impacts on the ecology of grazing [49]. The Borana rangeland also has stretches of Acacia-Commiphora small-leaved deciduous woodlands, with a mixture of the genera Acacia, Boswellia and Commiphora.
Generally, it is argued that a ban on the use of traditional range fire is probably one of the reasons for the expansion of bush encroachment at the expense of important rangeland species and human livelihood [46-48]. Most threatened plant species have significant values as human food, for medicinal purposes, feed for animal, construction materials, gum-resin sources and/or are useful for other cultural purposes [47]. According to Gemedo et al. [54], Gum-resin bearing species are among the predominant components of rangeland resources in Borana. However, the influence of Acacia drepanolobium on rangeland resources and useful woody species has been rarely investigated. Due to its potential impact on the resilience of rangeland resources, conservation of biodiversity and local livelihood security, understanding these multiple impacts of Acacia drepanolobium is a priority.
Conclusion
Invasive plant species can directly or indirectly affect the food security of local residents and increase vulnerability to hazards and risks. In areas where they spread, invasive species can destroy natural pasture, displace native trees, and reduce grazing potential of rangelands. Invasive species of Plants like water hyacinth also block water ways for irrigation, navigation, electricity generation, and livestock watering. Ecologically, benthic and littoral diversity is reduced because of dense and impenetrable mats of water hyacinth .Some species of invasive plants pose health risks to livestock and humans in the invaded areas by impairing mobility or causing injuries. These species can affect crop production, animal husbandry, human health and biodiversity. Moreover, invasive alien species have adversely impacted numerous industries, such as fisheries, tourism, and water production. Apart from their threat to biodiversity and altering ecosystem services, invasive alien plant species have significant socio-economic impacts. Attempts to combat the threat of invasive plant species in Ethiopia have followed the usual piecemeal approach. Therefore, there is a need to take a concerted look at the likely effects of invasive alien plant species on socio-economic and biodiversity impact and devise appropriate measures to mitigate the effects of these invasive plant species.
We are grateful to all the authors of the scientific papers used in this article. We also thank our colleagues of center for environmental science of Addis Ababa University who gave comments during the period of development of this paper.
- Demissew Sertse (2005) Controlling the spread of P. juliflora in Ethiopia by its utilization. Link: https://goo.gl/F2y1FK
- UNEP (2012) Fifth Global Environment Outlook (GEO5): Environment for the future we want. United Nations Environment Programme, Nairobi. Link: https://goo.gl/WzkqzT
- McNeeley JA, Mooney HA, Neville LE, Schei P, Waage JK (2001) Global Strategy on Invasive Alien Species. UCN – the World Conservation Union, Gland. Link: https://goo.gl/VPr4QX
- Kathiresan RM, Gnanavel I, Anbhazhagan R, Padmaria SP, Vijayalakshmi NK (2005) Ecology and control of Parthenium invasion in command area. In: Proceedings of Second International Conference on Parthenium Management 5-7 Dec 2005. Bangalore, India. pp 77-80. Link: https://goo.gl/AkGc8w
- Shiferaw H, Teketay D, Nemomissa S, Assefa F (2004) Some biological characteristics foster the invasion of Prosopis juliflora (Sw.) DC at Middle Awash Rift Valley Area, Northeastern Ethiopia. Journal of Arid Environments 58: 135–154. Link: https://goo.gl/PBwo25
- Kassahun Z, Yohannes L, Olani N (2004) Prosopis juliflora: Potentials and Problems. Arem 6: 1-10. Link: https://goo.gl/rA9FrA
- Senayit R, Agajie T, Taye T, Adefires W, Getu E (2004) Invasive Alien Plant Control and Prevention in Ethiopia. Pilot Surveys and Control Baseline Conditions. Link: https://goo.gl/x5VUyT
- Ababu A, Fasil R, Getachew T, Asefa A, Yigzaw A (2004) Review of IAPS related policies and strategies in Ethiopia. EIAR, Addis Ababa, Ethiopia, Pp60.
- Belayneh B, Taye T, Rezene F (2016) Spatial Distribution and Abundance of Invasive Alien Plant Species in Gamo Gofa Zone, Ethiopia. International journal of innovative research and development ISSN 2278 – 0211 5: 23-33. Link: https://tinyurl.com/ycd2pkdu
- Tamado T, Schütz W, Milberg P (2002) Germination ecology of the weed Parthenium hysterophorus L. in eastern Ethiopia. Ann Appl Biol 140: 263-270. Link: https://goo.gl/H2ntZD
- Hedberg I, Friis I, Edwardes S, Mesfin Tadesse (Eds.) (2004) Flora of Ethiopia and Eritrea. V. 4: P.2. Addis Ababa, Ethiopia. Uppsala, Sweden. Link: https://goo.gl/BfbcUY
- Tamado T, Milberg P (2000) Weed flora in arable fields of Eastern Ethiopia with emphasis on the occurrence of Parthenium hysterophorus L J Weed Research 40: 507- 521. Link: https://goo.gl/xNdfri
- Tamado T (2001) Biology and management of parthenium (Parthenium hysterophorus L.) in Ethiopia. PhD thesis. Swedish University of Agricultural Sciences, Uppsala. Link: https://goo.gl/vG71LM
- Tadelle T (2002) Allelopathic Effects of Parthenium hysterophorus Extracts on Seed Germination and Seedling Growth of Eragrostis tef. J Agronomy & Crop Science 188: 306-310. Link: https://goo.gl/wMteqN
- Singh PH, Batish RD, Pandher KJ, Kohli KR (2005) Phytotoxic effects of Parthenium hysterophorus residues on three Brassica species. Weed Biology and Management 5: 105–109. Link: https://goo.gl/u2DLt9
- GISP (2004) Africa Invaded: The Growing Danger of Invasive Alien Species. Global invasive Species Programme, CapeTown. Link: https://goo.gl/S63QBL
- Bezabih F, Araya T (2002) Spread and status of partheinium in Tigray Regional State at Woreda level. Tigray Bureau of Agriculture and Natural Resources.
- Taye T (2002) Investigation of Pathogens for Biological control of Parthenium (Parthenium hysterophorus L.) in Ethiopia. PhD thesis, Humboldt– Universitat zu Berlin, Landwirtschaftlich-Gartnerischen Fakultat, Berlin. 152 pp. Link: https://goo.gl/CYj6Gt
- McConnachie AJ, Strathie LW, Mersie W, Gebrehiwot L, Zewdie K, et al. (2011) Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (Asteraceae) in eastern and southern Africa. Weed Research 51: 71–84. Link: https://goo.gl/a4XWVe
- Shashie A (2007) Impact of parthenium (Parthenium hysterophorus L.) on the range ecosystem dynamics of the Jijiga rangeland. An MSc Thesis Presented to the School of Graduate Studies of Haramaya University, Ethiopia. Link: https://goo.gl/NXqNGg
- Navie SC, McFadyen RE, Panetta FD, Adkins SW, (1996) The biology of Australian Weeds 27: Parthenium hysterophorus L. Plant Protection Quarterly 11: 76- 87. Link: https://goo.gl/g9i1H5
- Auld BA, Menz KM, Medd RW (1979) Bioeconomic methods of weed in pastures. Journal of Agro- Economics 5: 69-84. Link: https://tinyurl.com/y9sm4gbp
- Klingman GC, Ashton FM (1995) Weed Science: Principles and Practices. John Wiley and Sons Inc., New York. 449p. Link: https://goo.gl/To6ays
- Khosla SN, Sobti SN (1981) Effective control of Parthenium hysterophorus L. Pesticides.
- Kohli RK, Daiz R (1992) Identification and bio efficacy of soil chemicals of Parthenium hysterophorus. Extended abstract Indian Society of Allelopathy. Link:
- Kololgi PD, Kololgi SD, Kololgi NP (1997) Dermatological hazards of parthenium in human beings. In: Mahadevappa M, Patil VC (eds) Proceedings of the First International Conference on Parthenium Management, 6 - 8 October 1997, University of Agricultural Sciences, Dahrwad, India, 18-19. Link: https://goo.gl/Cv5i5a
- Sriramarao P, Nagpal S, Rao BS, Prakash O, Rao PV (1991) Immediate hypersensitivity to Parthenium hysterophorus. II. Clinical studies on the prevalence of parthenium rhinitis. Clin Exp Allergy 21: 55-62. Link: https://goo.gl/buPo4J
- Abiyot B, Getachew T (2006) The P. juliflora Dilemma, Impact on Dry land Biodiversity and Some Controlling Methods. Journal of the Dry Lands 1: 158-164. Link: https://tinyurl.com/y87s98k3
- El-Keblawy A (2002) Causes and Consequences of the Invasion of the Exotic P. juliflora to the Environment of the UAE In: Third Annual Conference for Research Funded by UAE University.
- Dubale A (2006) Impacts of Prosopis juliflora invasion and control using charcoal production in Afar National Regional State, Ethiopia. MSc thesis (distinction), University of Wales, Bangor, UK.
- Al-Humaid, A.I. and Warrage, MOA, 1998. Allelopathic effects of Prosopis juliflora foliage on seed. Link: https://tinyurl.com/y8mz8g47
- Germination and seedling growth of Bermuda –grass (Cyanodon dactylon). Journal of Arid Environment 38: 237-243. Link:
- Mwangi E, Swallow B (2005) Invasion of Prosopis juliflora and local livelihoods: Case study from the lake Baringo area of Kenya. ICRAF Working Paper – no. 3, Nairobi, World Agroforestry Centre. Link: https://goo.gl/ASrmKw
- Shetie G (2008) The Ecological Distribution and Socio-Economic impacts of Prosopis juliflora (Sw.) DC. In the Amibara Woreda, Afar National Regional State. An M.sc Thesis presented to School of graduate studies Addis Ababa University. Link: https://goo.gl/zUzLqg
- Geesing M, Khawlani, Abba ML (2004) Management of introduced Prosopis species: Can economic exploitation control an invasive species? Unasylva 55: 36-44. Link: https://goo.gl/kVmS7E
- Csurhes S (1996) Mesquite (Prosopis spp.) in Queensland: Pest status review series Land Protection Branch. Department of Natural resources, Queensland, Australia. 19p. Link: https://goo.gl/z9TYMy
- Rezene F (2005) b/ Water hyacinth (Eichhornia crassipes): A review of its weed status in Ethiopia. Arem/ 6: 24-30.
- Taye T (2007) The prospects of Biological control of weeds in Ethiopia. Ethiopian Journal of Weed Management 1: 69-70. Link: https://goo.gl/vwtpqH
- Midgley JM, Hill MP, Villet MH (2006) The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr J Aqua Sci 31: 25-30. Link: https://goo.gl/gprgVp
- Mailu AM (2001) Preliminary assessment of the social, economic and environmental impacts of water hyacinth in the Lake Victoria basin and the status of control. In: Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. ACIAR Proc. No. 102. Link: https://goo.gl/wQhS4T
- Navarro L, Phiri G (2000) Water hyacinth in Africa and the Middle East. A survey of problems and solutions. International Development Research Centre, Ottawa (CA). Link: https://goo.gl/UfZmKA
- Harley KL, Julien MH, Wright AD (1996) Water hyacinth: a tropical worldwide problem and methods for its control. In: Brown H, Cussans GW, Devine MD, Duke SO, Fernandez-Quintanilla C, A Helweg, RE Labrada, M Lanedes, P Kudsk and JC Streibig (eds.). Proceeding of the 2nd International Weed Control Congress, Copenhagen, Denmark. 639 – 644. Link: https://goo.gl/ZERnST
- Adisu Abebe (2009) Bush Encroachment and its Impacts on Plant Biodiversity in the Borana Rangelands: MSc thesis Addis Ababa
- Binggeli P, Desalegn D (2002) Lantana camara: The Invasive Shrub That Threatens to Drive People out of Their Land. Wildlife and Natural History Society 4-6. Link: https://goo.gl/UrZx15
- EARO (2003) Removing Barriers in Invasive Plant Management in Africa. Global Environmental Facility (GEF) Proposal for PDF B Block Grant. EARO, Addis Ababa. Link: https://goo.gl/7iqaSa
- Tamene Y (1990) Population Dynamics of the Problem Shrubs, Acacia drepanolobium and Acacia brevispica in the Southern Rangelands of Ethiopia. MSc thesis. University of New South Wales, Australia. Link: https://goo.gl/sh3Asr
- Coppock DL (1994) The Borana Plateau of Southern Ethiopia Synthesis of Pastoral Research. Development and Change 1980-1991. Link: https://goo.gl/84N4Yd
- Oba G, Post E, Syvertsen PO, Stenseth NC (2000) Bush Cover and Range Condition Assessment in Relation to Landscape and Grazing in Southern Ethiopia. Landscape Ecology 15: 534-546. Link: https://goo.gl/F1tDeT
- Oba G (1998) Assessment of indigenous range management knowledge of the Borana pastoralists of southern Ethiopia.Commissioned by GTZ-Borana Lowland Pastoral Development Program in collaboration with the Oromiya Regional Bureau for Agricultural Development, Negelle/Borana Ethiopia.
- Anteneh Tesfaye, Alemayehu Negassa (2005) Systematic Approach to the Problem of Bush Encroachment in the Borana Low Land. Forest Research center. Addis Ababa, Ethiopia.
- Terefe B, Limenih M, Gure A, Angassa A (2009) Impact of Acacia drepanolobium (An Invasive Woody Species) on Gum-Resin Resources and Local Livelihood in Borana, Southern Ethiopia. Link: https://goo.gl/zVYVA1
- Berhanu L, Taye T, Rezene F (2015) Distribution, abundance and socio-economic impacts of invasive plant species (IPS) in Borana and Guji Zones of Oromia National Regional State, Ethiopia. Link: https://tinyurl.com/ybsl5ko6
- Young TP, Stubblefield CH, Isbell LA (1997) Coexistence among obligate acacia ants. Oecologia 109: 98-107
- Gemedo D, Brigittie LM, Johannes I (2005) Plant Biodiversity and Ethnobotany of Borana Pastoralists in southern Oromia, Ethiopia. Economic Botany 59: 43-65. Link: https://goo.gl/egYjRL
- Wassie A (2014) Water Hyacinth Coverage Survey Report on Lake Tana. Technical Report Series 1. Link: https://goo.gl/Lvqtsf
- Kefelegn H (2015) Invasive Alien Weed Species Impacts on Biodiversity and Socio-Economic Aspect in Ethiopia: A Review International Journal of Science and Research (IJSR) 4: 2319-7064. Link: https://goo.gl/GoNafo
- Pasiecznik NM, Felker P, Harris PJ, Harsh LN, Cruz G, et al. (2001) The Prosopis juliflora – Prosopis pallida Complex: A Monograph. HDRA, Coventry, UK. pp 162. Link: https://goo.gl/t3uGsF
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.







 Save to Mendeley
Save to Mendeley
