Open Journal of Plant Science
Efficacy of Allopatric Tephrosia vogelii (Fabales: Fabaceae) against Pre-Emerginal Stages of Phlebotomus (Phlebotomus) Duboscqi (Diptera: Psychodidae)
Nathan M Aritho1, Paul K Ndungu1,3, Judith Inyangala4, Milkah Mwangi3, Michae1 M Gicheru1, Joseph JN Ngeranwa2 and Christopher O Anjili3*
2Department of Biochemistry and Biotechnology, Kenyatta University, PO Box 43844-00100, Nairobi, Kenya
3Centre for Biotechnology Research and Development, Kenya Medical Research Institute, PO Box 54840-00200, Nairobi, Kenya
4School of Biological Sciences, University of Eldoret, PO Box 1125-30100, Eldoret, Kenya
Cite this as
Aritho NM, Ndungu PK, Inyangala J, Mwangi M, Gicheru MM, et al. (2017) Efficacy of Allopatric Tephrosia vogelii (Fabales: Fabaceae) against Pre-Emerginal Stages of Phlebotomus (Phlebotomus) Duboscqi (Diptera: Psychodidae). Open J Plant Sci 2(1): 007-010. DOI: 10.17352/ojps.000006The efficacy of allopatric Tephrosia vogelii in killing eggs and larvae of the sand fly Phlebotomus duboscqi was tested in the laboratory. In this study effects of both water and crude powder extracs of Tephrosia vogelii were tested against against pre-emerginal stages of P. duboscqi in the laboratory. Three groups of eggs were wetted with T. vogelii extracts from Kilifi (TVK), Nairobi (TVN) and Vihiga Counties (TVV) and observed for morphological changes, motility, transformation and death. Six groups of 30 first instar larvae were fed on T. vogelii crude powder either alone or mixed with larvae food. All larvae given TVN powder become immortile, did not feed and died at the first instar stage, whereas those fed on TVV and TVK did not die. Combining all the three extracts also led the death of all first and second instar larvae, but gradual death and disintegration of third instar larvae was sequentially observed. The differences in activities of the allopatric plants can be attributed to allopatric introgression arising from geopgraphic separation leading to introgressive hybridization. The killing and anti-feedant activities of TVN could also mean that it is a chemotype 1 plant that contains rotenoid whereas TVK and TVV are chemotype 2 that lack rotenoids. Feeding deterrence has been shown to be caused by rutin and quercetin. Considering that only leaves were used, the toxicity of TVN can be attributed to single or combination actions of rotenone, elliptone, deguelin, rutin, rotenolone, tephrosin or quercetin that have been shown to be present in leaves. Results of this study suggest that T. vogelli has detrimental insecticidal effects on pre-emerginal stages of P. duboscqi and has potential for sand fly control. This control method can be achieved through direct introduction of crude dried T. vogelii powder into termite mound ventilation shafts and animal burrows where sand flies are known to breed and rest.
Introduction
Tephrosia vogelii has been shown to exhibit insecticidal activities. These properties have been attributed to the presence of isoflavonoid rotenoids in various parts of the plant [1]. Some of the active ingredients responsible for and entomocidal effects in T. vogelii are believed to be rotenoids, which are rotenone (C22H23O6) (Nagai, 1902; LaForge and Haller, 1932 [2,3]). deguelin (C23H22O6), munetone (C26H24O5) and tephrosin (C23H22O7) ([4] (McDavid and Lesseps, 1994) [5,6,7] rotenolone (C23H22O7) [8], toxicarol (C23H22O7) [9], sumatrol (C23H22O7) [10], malaccol (C20H16O7) [11], elliptone (C20H16O6) [12], rutin (C27H30O16) [13] and quercetin (C15H10O7) [14,15], flavonoids found mainly in leaves of T. vogelii, which has been shown to have deterrent effects on the feeding behavior, survival, and development of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) [16] and Rutin (quercitin 3-O-rutinoside), which has been shown to exhibit antibiotic and/or anti-feeding effect in several defoliating insects such as the sphinx moth Manduca sexta (Lepidoptera: Sphingidae) [17], the tobacco budworm Heliothis virescens (Lepideptera: Noctuidae) [18] and the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae) [19]. Rutin is well distributed in leaves of T. vogelii [14]. Its effects in slowing larval development of M. sexta is temperature dependent, being high at warm temperatures and slowed at low temperatures [20].
The rotenoids, especially rotenone has been shown to have low mammalian toxicity, and is extremely active as a contact and stomach poison against insect species in which it inhibits mitochondria electron transport [21]. In Kenya, where both cutaneous and visceral leishmaniases are andemic and are transmitted by bites of infected female Phlebotomus sand flies, the efficacy of plants containing rotenoids in the control of sand flies have not been tested. This study aimed at testing the effects of allopatric T. vogelii species in the control of pre-emerginal stages of P. duboscqi, the vector for Leishmania major (Kinetplastida: Trypanosomatidae) under laboratory conditions. The study was carried out using only the pre-emerginal stages because female adults feed on blood and both sexes suitable plant for carbohydrates [22]. It was envisaged that targeting the pre-emerginal stages can be achieved through introduction of the crude dried T. vogelii into the termite mound and animal burrow breeding and resting sites of the sand flies. Choice of pre-emrginal stages was because T. vogelii has been shown shown to deter adults from feeding [23].
Materials and Methods
Allopatric Tephrosia vogelii were collected from Kilifi County in Coast, Vihiga County in Western, and Karura in Nairobi provinces. Leaves were harvested and transported in paper bags to the laboratory where they were dried in a shade to a brittle state. The brittle leaves were ground to a fine powder using an electric mill and stored in glass bottles. The powder from the Nairobi plant was designated TVN, the Kilifi TVK and Vihiga TVV respectively.
Preparation of chloral hydrate mountant gum
Chloral hydrate gum which is used to clear sand flies was prepared according to the method of [24]. Briefly, 8 grams of gum arabic, 70 grams of chloral hydrate crystals were weighed. These were dissolved in a mixture of 10 ml distilled water, 5 ml glycerine and 3 ml glacial acetic acid. This mixture was stirred using an electric magnetic stirrer for uniformity and then used to mount both eggs and various larval stages of the sand fly.
Preparation of sand fly larvae food
Larvae food is usually prepared by mixing rabbit droppings with rabbit chow, which actually dried corn meal from Unga Feeds. Equal proportions of the two are ground together, spread on a plastic tray, sprinkled with water and covered for ten days for a fungus to grow. The fungus is then harvested, and then dried in an oven [25].
Toxicity assays
Equal proportions (200 grams) of TVK, TVN and TVV powders and larvae food were separately mixed and used as food for three groups of the thirty (30) larvae. Another group of larvae was fed on a mixture of all the three T. vogelli (TVK, TVN and TVV) powders mixed with larva food. Another set of 30 larvae was fed on normal food at the same time to serve controls. Each day after the larvae were given the mixtures, they were examined for ingestion, mortility, and death using a dissecting microscope. An additional 3 larvae per group were suffocated using CO2, mounted on slides using chloral hydrate mountant, left for 24 hours to clear and then examined using a light microscope for any internal changes. Live larvae were examined on a daily basis for any transformation to second, third, fourth in stars, pupae and adult emergence. Comparison was made between the time taken by the treated and the controls for the emergence of adult flies.
Result and Discussion
When fed on larvae food mixed with T. vogelii, they were able to digest it without any detrimental effect. A summary of the activities of the allopatric T. vogelii on the pre-emerginal species of the sand fly are given in the table 1.
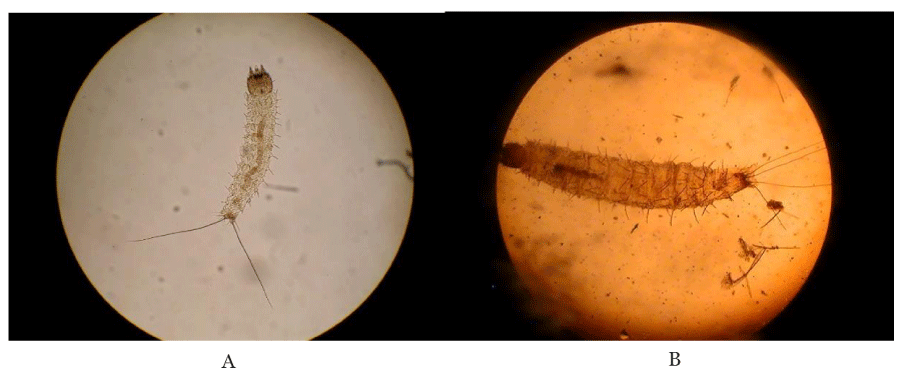
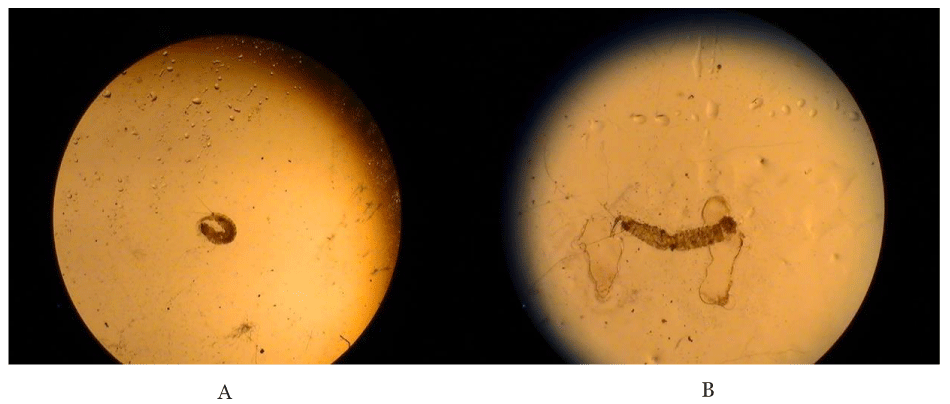
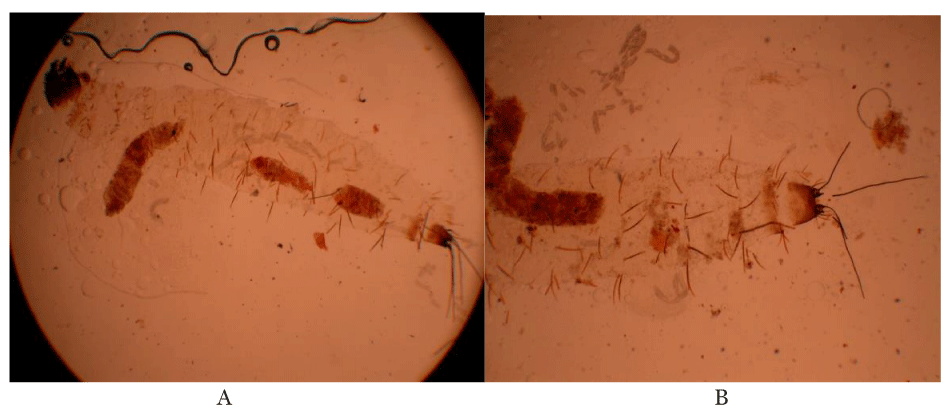
The first instar larvae with two caudal bristtles the third instar larvae with traces of T. vogelii in the fore gut, and with four caudal bristles are shown in Plate 1.
All larvae fed on extracts of Tephrosia vogelii from Nairobi County (TVN) exhibited 100% mortality. Results of this study showed that T. vogelii extracts from Kilifi (TVK) and Vihiga (TVV) are less potent as compared to the Nairobi plant. This could suggest that the Nairobi plant is a chemotype 1 that contains rotenoids whereas TVV and TVK are Chemotype 2 species that lack rotenoids [25]. The differences in activities of TVV, TVN and TVK could be as a result of allopatric introgression which is also known as has been reported in some plant species such as Juniperus virginiana (Pinales: Cupressaceae) [26]. Geographically separated plants of the same species usually exhibit introgressive hybridization, the movement of a gene (gene flow) from one species into the gene pool of another by repeated backcrossing of an interspecific hybrid with one of its parental species which changes chemical composition within the plant [26,27] (Plates 2,3).
Even though rotenone distribution varies in amount in most plants, leaves which were used usually contain the highest amount than in other plant parts as has been demonstrated by thin-layer densitometric analysis [27]. It has also been proven that the concentration of rotenone is high in leaves formed during the growing season and remains constant in a given leaf throughout its life on the plant [28]. Only leaves were used in the study, the killing and anti-feedant effects displayed by the sand fly can be attributed to single or combination actions of rotenone, elliptone, deguelin, rutin, tephrosin, rotenolone or quercetin that have been isolated from leaves [14,29].
The possibility of combination enhancement of toxicity can be because separately, the modified D and E rings deguelin and toxicarol are non-toxic whereas sumatrol and elliptone are less insecticidal compared to rotenone [30]. The anti-feeding effects caused T. vogelii extract which was observed in this study has also been reported for the spotted cereal stem borer, Chilo partellus (Lepidoptera: Crambidae) [31]. Feeding inhibition and lack of movement as was observed in 1st instar larvae fed on TVN can be useful in suppressing the spread of Leishmania major by P. duboscqi if T. vogelii is used. Quercetin and rutin have been shown to display properties of inhibiting the development, and increasing mortality of larvae of the moths Lymanria dispar (Lepidoptera: Erebidae) and Spodoptera litura (Lepidoptera: Noctuidae) [32,33]. It is possible that these compounds did the same to larvae of P. duboscqi when TVN was used.
The ability of P. duboscqi larvae to feed on T. vogelii from Western and Coast and without dying can be taken to suggest these are C2 plants lacking rotenoids, and that there is need to screen allopatric species from different regions before use. The development of natural resistance or innate immunity to rotenoid pesticides have rarely been reported despite their widespread and continued use since 1948 in Central America [34]. This is thought to be because insects are not known to detoxify rotenoids [35]. Being easy to grow, coupled with being biodegradable, T. vogelli can be used to control the leishmaniases in Kenya.
In this study, the proven efficacy of crude T. vogelii extracts against sand fly larvae is a good indication that the plant can be readily used by the population at risk without necessarily purifying the active ingredients. Similar observations were made when crude M. sericea crude extracts from Coastal and Western Kenya regions were used against Culex quinquefasciatus (Diptera: Culicidae) eggs and larvae in the laboratory, semi-field and field conditions in Kibera slums of Nairobi County, Kenya. Unlike T. vogelii, Mundulea sericea extracts known to contain rotenoids from two allopatric regions were toxic to both eggs and larvae but at different concentrations, with the coast species being more toxic than the western one [36]. The study recommended the adoption of M. sericea crude extract as a biological larvicide and ovicide to control Cx. quinquefasciatus. For the control of sand flies in the field, application of crude dried T. vogelii powder into termite mounds and animal burrows can be a cost-effective method of sand fly control.
Conclusion
Semi-field and field studies need to be done in order to determine how to use T. vogelii toxic chemotype in the control of sand fly vectors of the leishmaniases in Kenya.
- Belmain SR, Amoah BA, Nyirenda SP, Kamanula JF, Stevenson PC (2012) Highly Variable Insect Control Efficacy of Tephrosia vogelii Chemotypes. J Agric Food Chem 60: 10055-10063. Link: https://goo.gl/gDlw8T
- Nagai K (1902) Reseraches on the poisonous principals of Rhoten I J Tokyo Chem Soc 23: 744-777.
- LaForge FB, Haller HL (1932) the chemical structure of rotenone. J Am Chem Soc 54: 810
- Hanriot M (1907) Sur less substances actives du Tephrosia vogelii. Comptes Rendus Chimie, 144: 150-152.
- McDavid A, Lesseps RSJ (1994) Effects of Tephrosia vogelii leaf extracts on several crop pests. In: Natural Products as Pesticides. Proceedings from the First National Symposium in Zambia held in Lusaka 2-5. Link: https://goo.gl/kewKBt
- Delfel NE, Tallent WH, Carlson DG, Wolff I (1970) Distribution of rotenone and deguelin in Tephrosia vogelii and separation of rotenoid-rich fractions J Agric Food Chem 18: 385-390. Link: https://goo.gl/wzdBVj
- Lee SK, Luyengi L, Gerhauser C, Mar W, Lee K, et al. (1999) Inhibitory effect of munetone, an isoflavonoid, on 12-O-tetradecanoylphorbol 13-acetate-induced ornithine decarboxylase activity Cancer Lett 136: 59-65. Link: https://goo.gl/74ANa9
- Lambert N, Trouslot MF, Nefcampa C, Chrestin H (1993) Production of rotenoids by heterotrophic and photomixotrophic cell cultures of Tephrosia vogelii. Pytochemistry, 34: 1515-1520. Link: https://goo.gl/41qJ3W
- Clark EP (1930) Toxicarol. A constituent of the South American fish poison Cracca (Tephrosia) Toxicara. J Am Chem Assoc 52: 2461-2464. Link: https://goo.gl/QbtRoh
- Meyer TM, Koolhas DR (1938) new constituents of derris root. II J Roy Chem Soc 58: 875-884. Link: https://goo.gl/lhvrlC
- Buckley TA (1936) The toxic constituents of derris root J Soc Chem Ind London, 55: 285-291.
- Rangaswami S, Rao GR (1955) Occurrence of rutin in the leaves of Tephrosia lanceolata. IJP 17: 136-137.
- Marston A, Msonthi JD, Hostetmann K (1984) on the reported molluscicidal activity from Tephrosia vogelii leaves. Phytochemistry 23: 1824-1825. Link: https://goo.gl/2rNAQC
- Sharma, Gupta H (2016) Quercetin - A Flavanoid. Chron Young Sci, 1:10-5.
- Diaz-Napal GN, Palais SM (2015) Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J Pest Sci 88: 629-635. Link: https://goo.gl/Ug2PWF
- Stamp NE, Skrobola CM (1993) Failure to avoid rutin diets results in altered food utilization and reduced growth rate of Manduca sexta larvae. Entomol Exp Appl 68: 127-142. Link: https://goo.gl/R6VZzi
- Hoffmann-Campo CB (1995) Role of the flavonoids in the natural resistance of soybean to Helioths virescens (F.) and Trichoplusia ni (Hübner). The University of Reading, Reading. Link: https://goo.gl/nMB2Xu
- Hoffman-Campo CB, Harborne JB, McCaffery AR (2001) Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomol Exp Appl 98: 181-194. Link: https://goo.gl/NDjloF
- Stamp NE (1990) Growth versus molting time of caterpillars as a function of temperature, nutrient concentration and the phenolic rutin. Oecologia, 82: 107-113. Link: https://goo.gl/v8vjrh
- Fakami J, Mitsui T, Fukanga K, Shishido T (1970) The selective toxicity of rotenone between mammal, fish and insect. Biochemical toxicity of insecticides. Academic Press, 159-178.
- Beach RF, Kiilu G, Hendricks LD, Oster C, Leeuwenburg J (1984) Cutaneous leishmaniasis in Kenya: transmission of Leishmania major to man by the bite of a naturally infected Phlebotomus duboscqi. Tran Roy Soc Trop Med Hyg 78: 747-751. Link: https://goo.gl/TVBtdU
- Alao FO, Adebayo TA (2015) Comparative efficacy of Tephrosia vogelii and Moringa oleifera against insect pests of the watermelon (Citrillus lanatus Thumb). ILN 35: 71-78. Link: https://goo.gl/TbNxwx
- Schlein Y, Jacobson RL, Muller GC (2001) Sand fly feeding on noxious plants: a potential method for the control of leishmaniasis. Am J Trop Med Hyg 65: 300-303. Link: https://goo.gl/LaF57D
- Minter DM (1963) Thre new sandflies (Diptera: Psychodidae) from East Africa with notes on other species. Bull Ent Res 54: 484-495. Link: https://goo.gl/PRztDN
- Stevenson PC, Kite GC, Lewis GP, Forest F, Nyirenda SP, et al. (2012) Distinct chemotypes of Tephrosia vogelii and implications for their use in pest control and soil enrichment. Phytochemistry 78: 135-146. Link: https://goo.gl/E9fXUQ
- Flake AH, Urbbatsch L, Turner BL (1978) Chemical documentation of allopatric introgression in Juniperus. Syst Botany 3: 129-144. Link: https://goo.gl/I1xGVp
- Anderson E (1949) Introgressive hybridization. New York: Wiley & Sons. Link: https://goo.gl/jPjyr4
- Hagemann JW, Pearl MB, Higgins JJ, Delfel NE, Earle FA (1972) Rotenone and deguelin in Tephrosia vogelii at several stages of maturity. J Agr Food Chem 20: 906-908. Link: https://goo.gl/Khqb05
- Dzenda T, Ayo JO, Adelaiye AB, Adaudi AO (2007) Ethnomedical and veterinary uses of Tephrosia vogelii Hook F (Fabaceae): a review Nig Vet J 28: 24-39. Link: https://goo.gl/kmSQu5
- Martin JT (1942) The problem of the evaluation of rotenone containing plants. VI. The toxicity of L-elliptone and poisons applied jointly, with further observations on the rotenone equivalent method of assessing the toxicity of Derris root. Ann Appl Biol 29: 69-81. Link: https://goo.gl/7axkQq
- Machocho AK (1992) Flavonoids from the roots of tephrosia emoroids and their antifeeding effects on the larvae of the spotted stalk borere. Chilio partellus. P120. Thesis (MSc) Kenyatta University. Link: https://goo.gl/arBNGh
- Gould Lister KSC (2006) Flavonoid functions in Plants. In Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press LLC: Boca Raton, FL. 397-443. Link: https://goo.gl/WBE5eO
- Mallikarjuna N, Kranthi KR, Jadhav DR, Kranthi S, Chandra S (2004) Influence of foliar chemical compounds on the development of Spodoptera litura in interspecific derivatives of groundnut J Appl Entomol 128: 321-328. Link: https://goo.gl/P0wLtT
- De Jesus NF (2016) Reviving the Derris production industry. Link: https://goo.gl/jKxYoj
- National Research Council (U.S.). (1969). Toxicity and fate of Insecticides. In: Insect Pest Management Control. 385.
- Mwanjulu MA (2014) Evaluation of larvicidal and ovicidal effect of Mundulea service in the control of Culex quinquefasciatus. MSc thesis. Link: https://goo.gl/g9VJjf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
