Open Journal of Pharmacology and Pharmacotherapeutics
Comparative evaluation of anti-depressant effects of Citalopram, Ketamine and their combination in animal models of depression: A potential anti-depressant?
Salim Sheikh1,2, Pankaj Sonone2, Veena Verma2, Chakra Dhar Tripathi1,2, Bushra Ahmed Karim3* and Girish Gulab Meshram1,2
Cite this as
Sheikh S, Sonone P, Verma V, Tripathi CD, Karim BA, et al. (2021) Comparative evaluation of anti-depressant effects of Citalopram, Ketamine and their combination in animal models of depression: A potential anti-depressant? Open J Pharmacol Pharmacother 6(1): 004-008. DOI: 10.17352/ojpp.000016Background: The prevalence of depression is highest amongst the mood disorders. Nearly one third of the patients suffer from major depression, characterized by increased rate of relapse, residual symptoms and impairment of functionality, along with increased tendency for suicide related behaviour. Therefore, it’s very important to acquire and explore in depth knowledge into the etiological aspect of the depression and complementing it with testing of potential newer anti-depression agents.
Methods: Evaluation for anti-depressant effect was done in through Tail Suspension Test and Forced Swim Test. Normal saline (control), citalopram, ketamine, and combination of ketamine with citalopram were tested in the 8 groups of mice. Each group comprised of 6 mice.
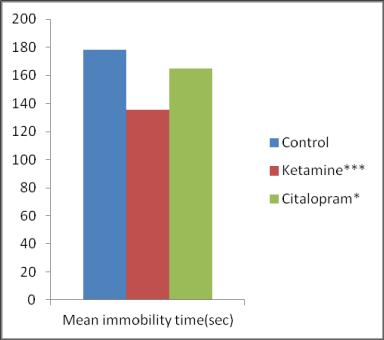
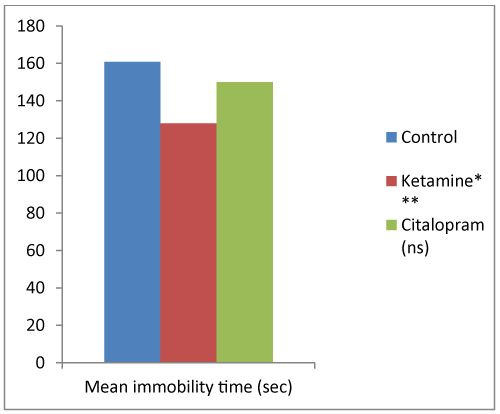
Results: In the TST, on comparison with the control group, the groups treated with citalopram, ketamine and their combination demonstrated a significant (p <0.05) reduction in the immobility time and increase in the time spent in struggle. In the FST, the group treated with citalopram alone showed a non-significant (P >0.05) decrease in the immobility time as compared to the control group.
Conclusion: Ketamine, an NMDA receptor antagonist has anti-depressant effect as evident in both TST & FST models. ketamine or its combination with citalopram was superior to citalopram alone. Not only it has antidepressant effect of its own, it also potentiates the antidepressant effect of citalopram.
Introduction
The prevalence of depression is highest amongst the mood disorders [1,2]. Nearly one third of the patients suffer from major depression [3], characterized by increased rate of relapse, residual symptoms, and impairment of functionality, along with increased tendency for suicide related behaviour [4]. Therefore, it’s very important to acquire and explore in depth knowledge into the etiological aspect of the depression and complementing it with testing of potential newer anti-depression agents.
The neurotransmitters serotonin (5-HT), norepinephrine and dopamine are the major targets for the currently available treatment [5]. Most preferable drugs are SNRI and SSRI drugs due to decreased incidence of side effects and better safety [6,7].
Treatment refractory depression still remains a major cause of concern in spite of a plethora of anti-depressant drugs because of the efficacy variations in the individuals [8]. Safety wise also there is increased incidence of early withdrawl from the treatment due to serious adverse effects [9]. In addition, there is higher rate of suicidal behavior during the initial phase of treatment owing to the therapeutic lag, which is around 3-4 weeks. The vulnerability of patient to suicide is highest during this period [10]. Therefore, testing of potential new agents with better efficacy and reduced toxicity which offers treatment option over the traditional therapy, need to be done.
The N-Methyl-D-Aspartate receptor family is a potential new target for treatment of depression. Several preclinical studies have shown drugs targeting NMDA receptor have enhanced antidepressant effect and decreased therapeutic lag [11-13]. Ketamine is a non-competitive NMDA antagonist and a derivative of phencyclidine (PCP) which was found to produce rapid, robust and persistent antidepressant effects in clinical studies [14]. Our study targeted the NMDA receptor to elaborate and demonstrate the potential activity of ketamine in animal models of depression.
Materials and method
Ethical considerations
The approval for the experimentation of animals was obtained from the Institutional Animals Ethics Committee. The study was conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Testing of the animals was done for a single procedure only.
Study design and setting
The site for the study was the animal laboratory in the Pharmacology department of a medical college in New Delhi. The utilization of Swiss Albino male mice having weight of 22-25g was done for this study. The laboratory conditions for housing of the animals were light/dark cycle of 12 hours, temperature was maintained around 21±1°C and relative humidity was kept at 55±5% with 24 hour accessibility to food and water prior to the experiments. The allocation of housed animlas were done after acclimatization of 7 days into 8 random groups (Table 1) comprising of six mice per group. Concurrently, the control groups were also studied.
Experimental drugs
The procurement of Normal saline and Ketamine was from the hospital drug pharmacy. department of the hospital and Sigma Aldrich, India provided Citalopram. The drugs were given as per treatment schedule (Table 2). The test sessions were conducted 60 minutes after intraperitoneal injections. Normal saline was administered in the Control group. The doses of ketamine and citalopram were determined as per the previous studies and there was reduction of the drug doses in the combination group [15,16].
Animal models of depression
Experimental animals (1 mice for a single test only) were assessed with the Forced swim test (FST) and Tail suspension test (TST), both being valid animal models for depression [17-21]. Both models evaluate the behavior of the treated mice tests for antidepressant effects.
In FST, after placing the mice in an inescapable transparent water filled tank, its behavior in relation to mobility for escapism is observed. The dimensions of cylindrical tank of transparent Plexiglas is 30 cm height x 20 cm diameters. To ensure constant and consistent level of water, tank is marked 15 cm above the bottom. After each procedure, thorough cleaning of apparatus is done. Duel swimming sessions are conducted and videotaped: On first day, mice were trained for swimming for 15 min and on the test was performed the next day for 6min. Mice are properly cleaned, dried and kept warm before returning to their home cages, after each procedure. Injection of a single dose test drugs is given just before conduction of a test session. Videotaping of behavior and immobility time is recorded.
In TST, the test mice is acoustically and visually kept in isolation and suspension 50 cm above the floor by cello tape placed around 1 cm from the tail’s tip. Recording of immobility time is done for a 6 min period. Mice are considered immobile when they show no signs of motion. The parameter recorded is the immobility time.
Statistical analysis
Calculation of the mean+SEM of the immobility time was done and one way analysis of variance (ANOVA) was performed for the analysis of the results, supplemented by Tukey’s multiple range test, wherever required. Test were considered significant at the P values less than 0.05.
Results
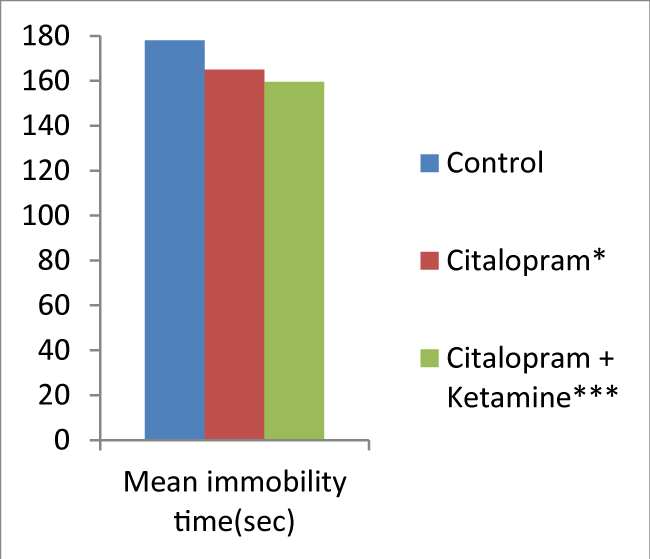
In the TST, on comparison with the control group, the groups treated with citalopram, ketamine and their combination demonstrated a significant (p <0.05) reduction in the immobility time and increase in the time spent in struggle (Figure 1). However, the decrease in immobility time was more pronounced with the groups treated with ketamine and its combination with citalopram than with citalopram itself (Figure 2).
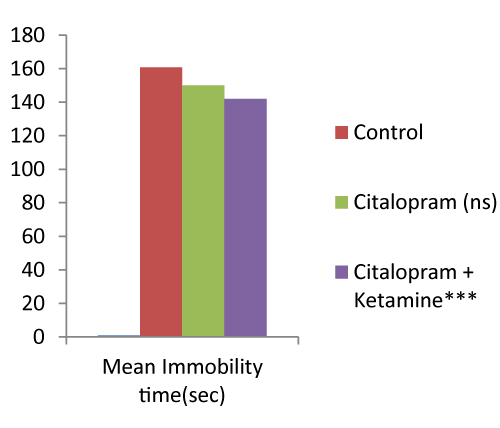
In the FST, the group treated with citalopram alone showed a non-significant (P >0.05) decrease in the immobility time as compared to the control group. Whereas, other groups treated with ketamine alone and its combination with citalopram showed a significant reduction of the immobility time and increase in the duration of struggle (Figure 3). However, when the combination was compared with citalopram group, not much change in immobility time was observed, and the effect was not significant (Figure 4).
Discussion
Delay in the therapy responsiveness and unsatisfactory control along with not so good patient compliance owing to adverse effects are the major challenges in modern day anti-depressant therapy [8]. This necessitate the need of potential new drugs with faster onset of action and sustained prolonged effects. Ketamine has fast acting and sustainable antidepressant effect, making it a valuable next generation of rapidly acting antidepressants [13,14,22]. In our study, we explored the possible role of citalopram (SSRI) and ketamine (NMDA receptor antagonist) in animal models of depression (TST & FST). Good predictive validity along with rapid and economical detection of potential antidepressant like activity are the hallmarks of these tests [23].
Anti-depressant effect of Citalopram was evident in our study as it significant decrease in the immobility time and increase in the struggle period in TST model [24]. SSRIs acting on SERT causes increased levels of serotonin at post synaptic receptor due to reuptake inhibition. However, it caused non-significant anti-depressant effect in FST model. This effect is similar to previous studies where single dose of citalopram did not decreased the immobility time significantly [25,26]. Interestingly, when citalopram and fluoxetine are administered chronically they cause significant reduction in the immobility time [25-27], probably explaining the therapeutic lag seen in SSRIs.
In our study, mice treated with Ketamine reduction of immobility time both in FST & TST models, which was significant. This result was consistent with previous studies demonstrating reduction of immobility time in a dose dependent by MK-801, ketamine & imipramine, but not by fluoxamine [28]. Ketamine acting as a NMDA receptor antagonist, explains this effect. In addition, the increased Brain Derived Neurotropic Factor levels and activated mammalian target of rapamycin (mTOR), may explain this reduction due to more synaptic signaling protein in the pre frontal cortex [29-31].
In present study, ketamine with citalopram combination reduced the immobility time and increased the struggle time significantly in both the models as compared to control group. This is attributed to synergistic activity of citalopram with ketamine as seen in earlier studies [28,32,33]. Additionally, ketamine affect mood due to its interaction with monoamine and opiate systems [34-36].
Limitation of the study is the assessment of an acute effect of the drugs. Acute administration might be sufficient for ketamine’ mechanism of action but not for citalopram’s effect onset.
Conclusion
Ketamine, an NMDA receptor antagonist has anti-depressant effect as evident in both TST & FST models. Not only it has antidepressant effect of its own, it also potentiates the antidepressant effect of citalopram, suggesting possible involvement of NMDA receptors and its interaction with the monoaminergic system in depression.
Author contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, et al. (2007) Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370: 851-858. Link: https://bit.ly/2QXzOL7
- Kessler RC, Berglund P, Demler O, Jin R, koretz D, et al. (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication(NCS-R). JAMA 289: 3095-3105. Link: https://bit.ly/2Q3jXdh
- Thase ME (2000) Treatment of severe depression. J Clin Psychiatry 61: 17-25.
- Pattanayak RD, Sagar R (2014) Depressive Disorders in Indian Context : A Review and Clinical Update for Physicians. J Assoc Physicians India 62: 827-832. Link: https://bit.ly/3tsUqrG
- Mendlewicz J (2001) Optimising antidepressant use in clinical practice: towards criteria for antidepressant selection. Br J Psychiatry 42: S1- S3. Link: https://bit.ly/3o06rUu
- Ellis P (2004) Australian and New Zealand clinical practice guidelines for the treatment of depression. Aust N Z J Psychiatry 38: 389-407. Link: https://bit.ly/3bbe9pu
- Roose SP (2003) Compliance: the impact of adverse events and tolerability on the physician’s treatment decisions. Eur Neuropsychopharmacol 13: S85- S92. Link: https://bit.ly/3vTha5J
- Al-Harbi KS (2012) Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6: 369-388. Link: https://bit.ly/33rLmZH
- Cartwright C, Gibson K, Read J, Cowan O, Dehar T (2016) Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence 10: 1401-1407. Link: https://bit.ly/3fcfEFk
- Selvaraj V, Veeravalli S, Ramaswamy S, Balon R, Yeragani VK (2010) Depression, suicidality and antidepressants: A coincidence? Indian J Psychiatry 52: 17-20. Link: https://bit.ly/3o0Aqf1
- Lipson R, Beyer S, Hughes CE, Khawaja ZA, Rajarao X, et al. (2007) Differentiating antidepressants of the future: Efficacy and safety. Pharmacol Ther 113: 134-153. Link: https://bit.ly/3hjcKkz
- Szewczyk B, Pałucha-Poniewiera A, Poleszak E, Pilc A, Nowak G (2012) Investigational NMDA receptor modulators for depression. Expert Opin Investig Drugs 21: 91-102. Link: https://bit.ly/3y2uKWf
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R (2006) A randomized trial of an N- methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856-864. Link: https://bit.ly/2Q2nnwU
- Skolnick P, Popik P, Trullas R (2009) Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30: 563-569. Link: https://bit.ly/2Q6uzIw
- Sheikh S, Sonone P, Tripathi CD, Verma V, Karim BA (2020) An experimental study targeting N-methyl-D-aspartate receptor in depression; beyond ketamine. Ann Psychiatry Treatm 4: 057-061. Link: https://bit.ly/2RHHC3j
- Baumann P (1996) Pharmacology and pharmacokinetics of citalopram and other SSRIs. Int Clin Psychopharmacol 11: 5-11. Link: https://bit.ly/3bheAP5
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730-732. Link: https://go.nature.com/3o4LiZ3
- Borsini F, Meli A (1988) Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology 94: 147-160. Link: https://bit.ly/3vWrVo6
- Lucki I (1997) The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8: 523-553. Link: https://bit.ly/3o1gsk6
- Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85: 367-370. Link: https://bit.ly/2RFMm9E
- Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29: 571-625. Link: https://bit.ly/3ezW0Ug
- Sheikh S, Sonone P, Verma V, Tripathi CD, Karim BA, et al. (2021) Ketamine; A better anti-depressant? An animal study evaluating the efficacy of citalopram,ketamine and their combination in animal models of depression. J Neurol Neurol Sci Disord 7: 019-023. Link: https://bit.ly/3uBcukG
- Castagne V, Moser P, Roux S, Porsolt RD (2011) Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 8: 10. Link: https://bit.ly/3bi0blE
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, et al. (2015) Comparison of ketamine, 7, 8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 232: 4325-4335. Link: https://bit.ly/3o60Ege
- Mombereau C, Gur TL, Onksen J, Blendy JA (2010) Differential effect of acute and repeated citalopram in mouse model of anxiety and depression. Int J Neuropsychopharmacol 13: 321-334. Link: https://bit.ly/3beW1eh
- Dulawa SC, Holick KA, Gundersen B, Hen R (2004) Effects of Chronic Fluoxetine in Animal Models of Anxiety and Depression. Neuropsychopharmacology 29: 1321–1330. Link: https://bit.ly/3w02DW9
- Cryan JF, Page ME, Lucki I (2005) Diffrential behavioral effect of the antidepressant reboxtine, fluoxetine and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology 182: 335-344. Link: https://bit.ly/2RFMJRA
- Chaturvedi HK, Bapna JS, Chandra D (2001) Effect of fluvoxamine and N-methyl-D-aspartate receptor antagonists on shock-induced depression in mice. Indian J Physiol Pharmacol 45: 199-207. Link: https://bit.ly/3vVB6oI
- Reus GZ, Stringari RB, Kirsch TR, Fries GR, Kapczinski F, et al. (2010) Neurochemical and behavioural effects of acute and chronic memantine administration in rats: Further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res Bull 81: 585-589. Link: https://bit.ly/3bhftXV
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91-95. Link: https://go.nature.com/3ttYPul
- Mathew JS, Shah A, Lapidus K, Clark C, Jarun N, et al. (2012) Ketamine for Treatment-Resistant Unipolar Depression. CNS Drugs 26: 189-204. Link: https://bit.ly/3ewexks
- Rogoz Z, Skuza G, Maj J, Danysz W (2002) Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology 42: 1024-1030. Link: https://bit.ly/2SFwxR2
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351-354. Link: https://bit.ly/3y1N8hW
- Lindefors N, Barati S, Connor WTO (1997) Differential effect of single and repeated ketamine administration on dopamine, serotonin, and GABA transmission in rat medial prefrontal cortex. Brain Res 759: 205-212. Link: https://bit.ly/3vWsDlg
- Elliot K, Kest B, Man A, Kao B, Inturrisi CE (1995) N-Methyl-D-Aspartate(NMDA) receptors, µ and κ opiod tolerance, and perspective on new analgesic drug development. Neuropsycopharmacol 13: 347-356. Link: https://bit.ly/3tCVXLQ
- Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS (1996) Effect of NMDA receptor antagonist on inhibition of morphine tolerance in rats: Binding at µ-opiod receptors. Eur J Pharmacol 297: 27-33. Link: https://bit.ly/3hgjeAK
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
