Open Journal of Pharmacology and Pharmacotherapeutics
Possibility of Drug-Drug Interaction through Prescription Analysis at the National Institute of Cardiovascular Disease (NICVD), Bangladesh
Mohammad Borhan Uddin1, NiluferYeasmin Nipa1, Shamim Ahmed1, Bushra Haider1, Shakura Binte Hasan1 and Abu Yousuf2*
2Faculty of Engineering Technology, University Malaysia Pahang, Kuantan-26300, Malaysia
Cite this as
Uddin MB, Nipa N, Ahmed S, Haider B, Hasan SB, et al. (2016) Possibility of Drug-Drug Interaction through Prescription Analysis at the National Institute of Cardiovascular Disease (NICVD), Bangladesh. Open J Pharmacol Pharmacother 2(1): 007-010. DOI: 10.17352/ojpp.000004Introduction: Medicaments are the ultimate choice of treatment when lifestyle and diet changes are unable to serve the preventive strategy for cardiovascular diseases. Contradictorily, detrimental Drug–Drug Interactions (DDI) between cardiovascular drugs with the non-cardiovascular drugs may lead to alterations in the therapeutic responses, and pose a grave health concern leading to early morbidity and mortality.
Purpose: The main objective of this study was to find out drug-drug interactions of cardiovascular drugs with non-cardiovascular drugs and this study also took into account the pattern of prescriptions written by physicians especially cardiologists.
Methods: It was carried out on indoor cardiac patients of the National Institute of Cardiovascular Diseases (NICVD), Bangladesh. These prescriptions were collected over a period of three months and analyzed using Microsoft® Office and Microsoft® Excel 2007 software.
Results: Rigorous analysis revealed that the incidence of potential DDI with at least one interacting drug combination (56%) was the most frequent. A total of 14 potentially harmful drug interactions were identified. Clopidogrel-Omeprazole (33.47%), Clopidogrel-Esomeprazole (27.75%), Frusemide-Cephalosporin (10.62%), Atorvastatin-Vitamin B (7.76%) were the most frequent interacting pairs.
Conclusions: This study concludes that concomitant use of a proton pump inhibitor (omeprazole, esomeprazole) and clopidogrel increases the risk of myocardial infarction (MI). Combination treatment with gliclazide and aspirin has not proven efficient in controlling blood glucose level. Co-administration of frusemide and cephalosporin might increase the risk of nephrotoxicity. It also focuses on the point that proper therapeutic planning might reduce the possible interaction risk.
Introduction
The complexity of drug therapy had increased with the increasing incidence of diverse disease states in patients. Owing to this, the risks and chances of Drug Drug Interaction (DDI) increases [1]. Negative drug reactions, drug-drug interactions, idiosyncratic reactions, and hypersensitivity reactions are the drug associated concerns that remained a major challenge in clinical practice [2]. Drug-Drug Interactions (DDIs) may affect pharmacokinetically or pharmacodynamically and overall lead to variations in the therapeutic response [3]. DDIs are estimated to be responsible of about 6-30% of all adverse drug reactions (ADRs), among which 2.8% ADR results in hospital admissions every year [4,5].
Worldwide the leading cause of death is due to cardiovascular diseases (CVDs). It is responsible for deaths in more than 17 million people in 2008, including death before the age of 60 in more than 3 million which could have been easily prevented. The several risk factors affiliated with CVDs include: raised blood pressure, obesity, high blood sugar, less physical activity, smoking, [6] family history of heart disease in Ethnic background etc. The more the risk factors, the higher is the chance of developing CVDs. However, patients with CVDs are the most susceptible for DDIs, the reasons being the number and type of drugs they take and the impact of heart diseases on drug metabolism [7]. These drugs are more often involved in DDIs and have a higher involvement as shown in studies conducted worldwide [3]. Drug-drug interactions with Non-steroidal anti-inflammatory drugs (NSAIDs), cough and cold, migraine, weight loss medications etc. are common with cardiovascular drugs. For example, anticoagulant drugs such as aspirin and clopidogrel leading to recurrent infarction or bleeding is serious DDIs [8].
Some results of already done studies include 30.67% of potential drug interaction in hospitalized cardiac patients in India [9]. Another study shows more than one DDIs in 53% patients admitted to the Department of Internal Medicine, Nepal [10].
Despite having multiple disease states, prescriptions are written as if the patient has a single condition only and the cumulative effect of multiple clinical guidelines is not taken into account [11]. Hence, this study was conducted to evaluate the drug-drug interactions and prescription pattern of cardiologists among some patients admitted to the National Institute of Cardiovascular Diseases (NICVD), Bangladesh.
Methods and Materials
This randomized observational study was performed on the inpatients of the National Institute of Cardiovascular Diseases (NICVD), Bangladesh which is the pioneer and the largest cardiac institute in Bangladesh. It is situated in Sher-E-Bangla Nagar of Dhaka city.
The hundred prescriptions were chosen randomly from the inpatients of NICVD. These prescriptions were collected over a period of three months and analyzed. All of these patients, suffering from different cardiovascular diseases, were taking different cardiovascular and non-cardiovascular drugs concomitantly.
Results
It is very essential to assess properly the risk-benefit ratio while combining interacting drugs otherwise it may cause mild to disastrous effect to the patients. This study was conducted to evaluate the pattern of drug-drug interactions (DDI) found in the prescription of the cardiac patients admitted in the biggest cardiac institute in Bangladesh. Table 1 includes names of all the cardiovascular and non-cardiovascular drugs prescribed to cardiac patients.
Discussion
Prescribed drug interaction
In the present study, we have randomly collected 100 prescriptions of the inpatient who were admitted in that hospital. A total 28 different medicines were prescribed, among them 16 were cardiovascular drugs and 12 were non-cardiovascular drug. All drugs studied on this survey have been listed on the Table 1. All of these patients were suffering from cardiovascular diseases and they were different prescribed many cardiovascular along with some non-cardiovascular drug. Already several studies have shown that polypharmacy increases the chances of drug interactions [12-14].
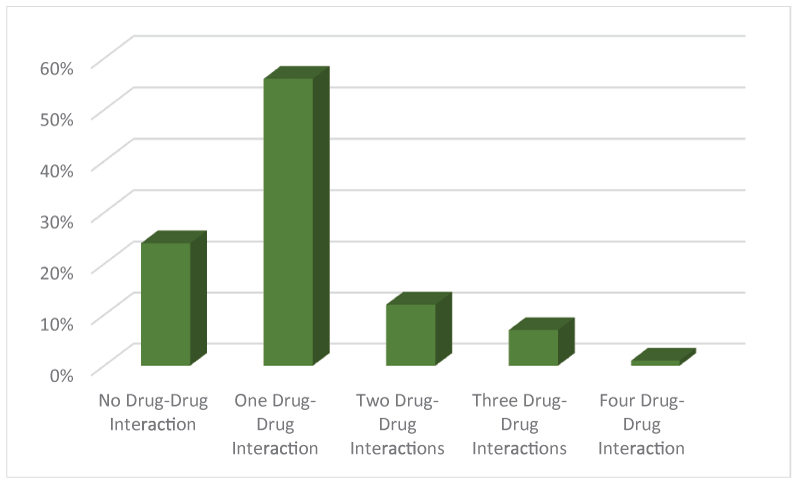
Upon analysis, we found 10 out of 16 cardiovascular drugs found in this study shows drug interactions with non-cardiovascular drug. From Figure 1, 24% prescriptions were identified with no cardiovascular and non-cardiovascular drug interactions. On other hand, almost 56% of the prescription had at least one drug- drug interactions. They were mainly the antiplatelet-PPI interaction, diuretic-antibiotic, lipid lowering agent-vitamin. Prescriptions containing two DDI or three DDI were found in 12% and 7% respectively. Fortunately, very few prescriptions have four DDI i.e. about 1% only. The patients whose prescriptions were collected, age was between 45 to 67 years.
Effect of drug interaction
Table-2 shows the possible fifteen DDI pairs found on this survey. Most frequent DDI pair found was antiplatelet drugs and PPIs which was nearly 64% beside; it is one of the severe DDI which increases the likelihood of myocardial infarction (MI) hospitalization and death [15]. Clopidogrel and omeprazole DDI pair was found in 33.5% of the total prescriptions. Similarly, Clopidogrel and esomeprazole DDI pair was found in around 27.8% of the total prescriptions. Omeprazole and esomeprazole when used concomitantly with clopidogrel, it lowers the therapeutic activity of clopidogrel [25]. Various pharmacodynamic laboratory testsclaimed that the omeprazole and esomeprazole interacts with clopidogrel by competitive inhibition of the CYP2C19 isoenzyme.
Furosemide, which is a diuretics and cephalosporin, was the second most prevalent DDI pair present in 10.6% of the total prescriptions. Cephalosporin itself has nephrotoxic nature. In addition, a study illustrate that the furosemide decreases the clearance of cephalondine, first generation cephalosporin C, is reduced. In this report, furosemide (80 mg) found to prolong the serum half-life of cephalondine by 25%. Thus combined use of furosemide and cephalosporin exacerbates the cephalosporin induced nephrotoxicity [16].
Combination of lipid lowering drugs, atorvastatin, and vitamin B may develop myopathy and rhabdomyolysis [17]. However, this severe DDI pair was found in almost 8% of the total prescription. Another frequent DDI pair found was aspirin and calcium which decreases the efficacy of aspirin and this DDI has proven in a study earlier [17]. The incidence of this drug interaction was around 6%. Aspirin also interact with glicazide, an anti-diabetic drug, due to two features of aspirin – intrinsic glucose reducing effect and high affinity for plasma protein helps to displace the highly plasma protein bound drug like glicazide. This pharmacokinetic interaction may prevent efficient control of glucose in blood and potentiate the hypoglycemic action [18]. This DDI pair was present in approximately 3% of the total prescription.
Antibiotics like amoxicillin and moxifloxacin eliminate the intestinal flora that are responsible to produce vitamin K, and it also inhibit CYP450 enzyme which metabolizes warfarin. Consequently, high serum concentration of warfarin results excessive bleeding disorder. Occurrence of this DDI pair in the prescription was about 2%, around 1% for amoxicillin and 1% for moxifloxacin [19]. Total 2% were found with theophylline DDI. Among them nearly 1% was with metoprolol another 1% was with diltiazem. After the introduction of verapamil and nefidipine, toxicity of theophylline has been reported in many patients [20,21].
One of the DDI pair is nitrate and corticosteroids (2.0%).Nitrates results vasodilatation which give symptomatic relief from cardiovascular disease like angina. However, the hypotensive effect due to the vasodilatation could be counteracted by the concomitant administration of corticosteroids [22]. Another drug interaction of nitrates can be possible with paracetamol. Paracetamol, similarly like NSAIDs, reduce the activity of sublingual nitrate because dry mouth could not dissolve the drug properly [23]. Ramipril and losartan interact with paracetamol (2.0%) to worsen the renal function [24]. Other DDI pair found less frequently i.e. nearly 1% of the total prescriptions. However, the DDI was identified by the mechanism of action of the drugs present in the prescriptions not by their clinical occurrences.
Conclusion
This study concludes that some cardiovascular drug give severe drug-drug interaction with some non-cardiovascular drug. Thus, our study shows the frequent severe interacting pairs, which might results major serious health hazards. Concomitant use of a proton pump inhibitor (omeprazole, esomeprazole) and clopidogrel increases the risk of MI. Combination treatment with gliclazide and aspirin has not proven efficient in controlling blood glucose level. Co-administration of Furosemide and cephalosporin might increase the risk of nephrotoxicity. Moxifloxacin and amoxicillin interfere with warfarin’s metabolism. Aspirin interact with calcium results decreased anticoagulant effectiveness due to reduced drug absorption. Nearly, most of the DDI obtained from the prescriptions, seemed to exacerbate the cardiovascular disease condition via increased MI hospitalization and excessive bleeding problems. Besides that, some of the DDI are likely to develop the comorbid conditions by resulting renal dysfunction. Additionally, few of the DDI eliminates the therapeutic effect of the medications owing to pharmacokinetic interactions. It also highlights that proper therapeutic planning might reduce the possible drug interactions. It is already proven that deficiency of true role of pharmacist in less developed countries; the chances of adverse effects are higher [25]. So, introducing pharmacists could be way to minimize this problem.
Authors are grateful to the National Institute of Cardiovascular Diseases (NICVD). We also wish to express our sincere gratitude to our Department of Pharmaceutical Sciences, and North South University for optimistic support while carrying out this study.
- Lee A, Stockley IH (2003) Drug interactions. In: Clinical Pharmacy and Therapeutics. Walker R, Edward C, (eds). 3rd ed. Philadelphia,Churchill Livingstone 21–31. Link: https://goo.gl/kU9rgE
- Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, et al. (2007) Drug-related problems in hospitals: a review of the recent literature. Drug safety 30: 379-407. Link: https://goo.gl/qshp8F
- Baxter K, Preston CL, Stockley IH (2013) Stockley's drug interactions: a source book of interactions, their mechanisms, clinical importance, and management. Baxter K, Preston CL (eds). 10th ed. London: Pharmaceutical Press. NLM ID: 101606996.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, et al. (2004) Adverse drug reactions as a cause for admission to hospital: prospective analysis of 18820 patients. Bri Med J 329: 15-19. Link: https://goo.gl/BRwCf8
- Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, et al. (2007) Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiology and drug safety 16: 641-651. Link: https://goo.gl/6EktIo
- Global Atlas on Cardiovascular Disease Prevention and Control (2011) World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization 3–18. Link: https://goo.gl/itukvo
- Gholami K, Ziaie S, Shalviri G (2008) Adverse drug reactions induced by cardiovascular drugs in outpatients. Pharm Pract 6: 51-55. Link: https://goo.gl/O9sUl8
- Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, et al. (2009) A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. Can Med Assoc J 180: 713-718. Link: https://goo.gl/F3ZmZZ
- Patel VK, Acharya LD, Rajakannan T, Surulivelrajan M, Guddattu V, et al. (2011) Potential drug interactions in patients admitted to cardiology wards of a south Indian teaching hospital. Australasian Med J 4: 9-14. Link: https://goo.gl/DS1CNh
- Bista D, Saha A, Mishra P, Palaian S, Shankar PR (2009) Impact of educational intervention on the pattern and incidence of potential drug-drug interactions in Nepal. Pharm Pract 7: 242-247. Link: https://goo.gl/RhVCqS
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, et al. (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 380: 37-43. Link: https://goo.gl/iUs8GH
- Guthrie B, McCowan C, Davey P, Simpson CR, Dreischulte T, et al. (2011) High risk prescribing in primary care patients particularly vulnerable to adverse drug events: cross sectional population database analysis in Scottish general practice. Br Med J 342: d3514. Link: https://goo.gl/q8UxiB
- Bourgeois FT, Shannon MW, Valim C, Mandl KD (2010) Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiology and drug safety 19: 901-910. Link: https://goo.gl/JJwhVO
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, et al. (2005) Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. J Am Assoc 294: 716-724. Link: https://goo.gl/XTyJpP
- Rassen JA, Choudhry NK, Avorn J, Schneeweiss S (2009) "Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome." Circ120: 2322-2329. Link: https://goo.gl/K70yBH
- Harada Y, Teshima K, Okamoto T (1981) [Comparative nephrotoxicity of latamoxef and other cephalosporins in rabbits. Combined administration with furosemide or tobramycin. (author's transl)]. The Japanese journal of antibiotics 34: 1549-1570. Link: https://goo.gl/kF5Cbz
- Yetley EA (2007) Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions. Am J ClinNutr 85: 269S–76S. USA. Link: https://goo.gl/yspsCS
- Bag S, Das S, Bagchi C, Tripathi SK (2014) Aspirin potentiates blood glucose lowering effect of glimepiride-pioglitazone combination in streptozotocin-induced diabetic rats. Indian J Pharmacol 46: 562-564. Link: https://goo.gl/oKimm5
- Baillargeon J, Holmes HM, Lin YL, Raji MA, Sharma G, et al. (2012) Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med 125: 183-189. Link: https://goo.gl/WFeK51
- Christopher MA, Harman E, Hendeles L (1989) Clinical relevance of the interaction of theophylline with diltiazem or nifedipine. Chest 95: 309-313. Link: https://goo.gl/cCB5dF
- Shannon M, Borron S, Burns M (2007) Haddad& Winchesters clinical management of poising and drug overdose. 4th ed. Saunders. Link: https://goo.gl/iXLD2l
- Longemore M, Wilkinson I, Baldwin A, Wallin E (2014) Oxford Handbook of clinical Medicines. 9th ed. Oxford, England, Oxford University Press. Link: https://goo.gl/BfektQ
- Gualtierotti R, Zoppi A, Mugellini A, Derosa G, D'Angelo A, et al. (2013) Effect of naproxen and acetaminophen on blood pressure lowering by ramipril, valsartan and aliskiren in hypertensive patients. Expert Opin Pharmacother 14: 1875-1884. Link: https://goo.gl/F6gkR7
- Macaione F, Montaina C, Evola S, Novo G, Novo S (2012) Impact of Dual Antiplatelet Therapy with Proton Pump Inhibitors on the Outcome of Patients with Acute Coronary Syndrome Undergoing Drug-Eluting Stent Implantation. ISRN Cardiol 2012: 692761. Link: https://goo.gl/VQbQSI
- Albadr Y, Bohassan AK, Ming LC, Khan TM (2014) An exploratory study investigating the potential drug–drug interactions in internal medicine department, Alahsa, Saudi Arabia. J Pharma Health Services Res 5: 237-241. Link: https://goo.gl/MplKMH
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
