Open Journal of Pain Medicine
Lidocaine in prevention of pain on propofol anesthetic induction: A randomized double-blinded clinical trial to estimate the magnitude of the effect
Luíza Buffon1, Alexandre C Buffon2,3*, Paulo Freitas1, Nazaré O Nazário1, Jefferson Traebert3 and Anna Paula Piovezan3
2Head of Anesthesiology Service, Joana de Gusmão Children’s Hospital, Florianópolis, Santa Catarina, Brazil
3Post-Graduate Program in Health Sciences (PPGCS), UNISUL, Palhoça, Santa Catarina, Brazil
Cite this as
Buffon L, Buffon AC, Freitas P, Nazário NO, Traebert J, et al. (2020) Lidocaine in prevention of pain on propofol anesthetic induction: A randomized double-blinded clinical trial to estimate the magnitude of the effect. Open J Pain Med 4(1): 034-037. DOI: 10.17352/ojpm.000021Background and objectives: Sedation with propofol is a part of the anesthetic induction procedure because it provides anxiolysis, amnesia and hypnosis; however, up to 5% of patients have pain at propofol injection and of these, 1% of them have severe or excruciating pain. There are several techniques that are used to avoid it, including previous venous administration of lidocaine, a local anesthetic. Although it is widely used, this procedure is not absent of risks, since many adverse reactions are reported when using lidocaine, such as blurred vision, nausea, headache and many others Central Nervous System symptoms. The current randomized controlled study evaluated the Number Needed to Treat (NNT) of patients that receives lidocaine to reduce pain associated to propofol administration during anesthesia induction.
Methods: This is a prospective, double-blinded, randomized trial with 970 adult subjects who were submitted to propofol administration in the induction of anesthesia. Investigated groups were previously treated randomly, either with lidocaine or saline and then they were interrogated about pain during propofol injection.
Results: There were no differences in demographics between the study groups. Pain outcome was reduced in patients who received lidocaine (5%; 95% CI, 3.63–6.37) in relation to saline (14.2%; 95% CI, 12.0-16.4). However, the number needed to treat (NNT= 10.9) for prevention of this effect was considerably high.
Conclusion: This study suggests that the use of lidocaine prior to propofol application is not justified, if considered the risk factors for the patient and the savings related with the procedure.
Clinical trial registration: UTN 1111-1215-1557.
Introduction
Anesthesia is the procedure to ensure the absence of pain, the inhibition of autonomic reactions and a good surgical. Today, most anesthetic procedures involve the combination of different drugs, using anesthetics at considerably lower concentrations if compared to those that would be needed if they were used without association; modern anesthetic techniques typically involve the association of hypnotics, analgesics, and muscle relaxants [1].
The use of barbiturates as intravenous anesthetics have been tested in the last 70 years; among these, propofol (2,6-diisopropylphenol) was introduced clinically in 1977 and demonstrates many positive effects [2]. Because of its unique pharmacological properties [3], it is an anesthetic drug used in the induction and maintenance of anesthesia in adults, being popular for providing an easy induction and recovery is faster than other drugs, such as thiopental [4]. Sedation with propofol does not provide relief from pain, however it provides anxiolysis, amnesia, and hypnosis [5]. Despite these positive aspects, patients can experience pain at injection of propofol and of these [6]. When used in the back of the hand, this value may increase to 60-80% of patients receiving propofol peripherically [7].
There are several methods to prevent or reduce pain at the injection of propofol and there is an association between the site of injection and the prevalence of pain and, while there are rare reports of pain in the ulnar fossa, pain in the back of the hand is frequent [8]. The administration of propofol at low temperatures or a pre-treatment with saline at 4° C may generate a local anesthetic effect on the vein wall, a simple and safe method for reducing local pain [9]. There is also a significant reduction in pain due to previous administration of intravenous opioid analgesics [10]. It has been shown that the use of pregabalin may also be useful in reducing pain at its injection, besides requiring less use of opioids for this purpose [7]. Another way to prevent this pain is with the administration of lidocaine, a local anesthetic drug, which can be administered immediately before or mixed with the injection of propofol, with doses up to 100mg in total [4].
However, besides lidocaine has been used in anaesthesiology for a long time to reduce the injection pain of propofol, this process is not risk-free since adverse reactions to lidocaine are commonly reported [11]. Among its most common side effects are mild CNS-related symptoms, being that patients may experience drowsiness, dizziness, metallic taste, headache, blurred vision, paresthesia, dysarthria, euphoria and nausea; however, in larger doses or if given rapidly, it may cause tinnitus, tremor, and agitation, whereas cardiovascular changes are usually minimal with the usual doses [12].
The benefit that mainly justifies its use relates to the fact that in patients who report propofol pain, the experience of anesthetic induction is remembered as the most painful part of the perioperative period. However, few studies have quantified the magnitude of the benefit of this therapy in reducing this unwanted outcome. Taking this into account, the main objective of this study was to investigate the magnitude of the effect of the lidocaine in order to prevent an undesired outcome of pain on propofol anesthetic induction. This is an important and original issue to be addressed, since randomized controlled trial is usually considered the gold standard for determining the efficacy of an intervention from which doctors can choose a safe and effective treatment for their patients.
Methods
This study is a double-blind randomized clinical trial, conducted in Florianópolis, Santa Catarina, Brazil, at the clinic of endoscopic diagnostic exams - Instituto de Medicina do Sistema Digestivo Ilha de Santa Catarina.
Study subjects
Patients aged 18 years or older, of both genders, who agreed to participate in the study were included in it. These were divided into two groups: group 1, which received the preparation of propofol preceded by injection of lidocaine and group 2, which received the preparation of propofol, preceded by injection of saline solution. A sample of 970 patients (485 in each group) was calculated as sufficient to detect a 75% decrease in pain incidence (RR = 25%), with reference to an incidence of pain in the non-exposed group of 4%, with a significance level of 95% and statistical power of 80%. Patients with a known allergy to propofol or lidocaine were not included in the study, as well as the patients who were excluded because the procedure was not possible after their consent to participate in the study.
Procedures
The randomization of the subjects was guaranteed by the allocation of patients in both groups in a sequence made from the use of a random number table generated by the Open Epi program. Both the anesthesiologist and the patient were blinded, since the preparation of the medication was performed by a nurse technician specialized in anesthesiological care, properly trained and qualified for such activity, in a separate environment and employing the same identical solution volume condition (lidocaine or saline solution) and injectable device. Patients had a punctured antecubital fold vein and 2% lidocaine at a dose of 1 mg/kg (mean weight 73.9 kg, then 73 mg lidocaine, on average) was injected prior to propofol injection in the patients in the group 1 or saline (same conditions), in patients in group 2. Propofol was injected at a rate of 1 ml/sec and patients were questioned before loss of consciousness regarding pain outcome.
Study outcome
The responses were recorded as positive or negative for the pain outcome at propofol injection, and this was confirmed after patient awakening. The data were tabulated using the Windows Excel software, later analyzed through the Statistical Package for the Social Sciences program (SPSS, version 18.0, Chicago, SPSS Inc 2009). Incidence of pain in the treated (prior administration of lidocaine) and untreated (prior administration of saline) groups was calculated. Bivariate analyzes were performed to test the homogeneity of proportions between the groups by means of the chi-square test, in which values of P <0.05 indicated statistically significant differences. Relative risks (RR) and their respective 95% confidence intervals (95% CI) were estimated. The measures of effect were also calculated: absolute risk reduction (ARR), relative risk reduction (RRR) and number needed to treat (NNT). The research project was approved by the Research Ethics Committee-UNISUL, consubstantiated report with number CAAE 59325716.4.0000.5369.
Results
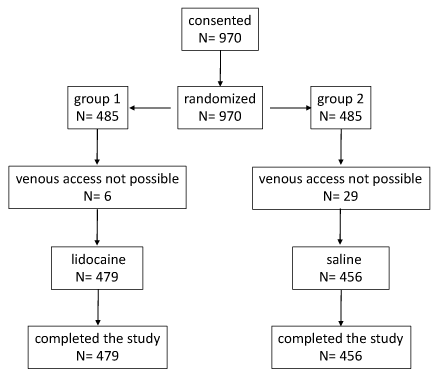
A total of 970 patients were allocated on one of the two study groups, as it can be seen in the flowchart of Figure 1; a small number of patients was excluded from the study (total n= 37), due to the difficulty in venous access in the antecubital fold.
The incidence of pain in the lidocaine group was 5.0% (95% CI 3.63, 6.37), while in the non-treated group it was 14.2% (95% CI 12.0, 16.4). These and other descriptive results are shown in Table 1. The results of the bivariate and multivariate analyzes can be seen in Table 2.
The Absolute Risk Reduction (ARR), which indicates the difference between the two groups in relation to the intervention was 9.2%. Relative Risk Reduction (RRR), which indicates the percent reduction of outcome (pain) in the treated versus untreated group was 36%. The Number Needed to Treat (NNT), which indicates in how many patients it is needed to intervene to obtain an outcome, is calculated as NNT= 1/Pt - Pc (Pt= proportion of successes in the treated group; Pc= proportion of successes in the control group) and in this study was 10.9.
Discussion
Our findings confirmed that a previous infusion of 2% lidocaine, 1 mg/kg, was shown to be a protective factor for the pain caused by the injection of propofol into the antecubital fold vessels during anesthesia induction procedure, when compared to the control group. These results reinforce those recorded by a systematic review followed by meta-analysis [13] which showed the efficacy of various pharmacological and non-pharmacological interventions for pain relief followed by propofol injection in adults. Despite this, the dose used in the present study (average weight of subjects equal to 73.9 kg, equivalent to an average dose of 73.9 mg per patient) was greater than the higher dose described in the review of these latter authors.
It is thus observed that in the literature the idea of the efficacy of prior administration of lidocaine in the prevention of pain associated with propofol anesthetic induction seems to be well accepted. However, the most important point of the present study relates to the results for NNT, which quantifies the magnitude of the benefit of this therapy in reducing this unwanted outcome. According to our findings, with an NNT of 10.9, it is emphasized that there is a need to intervene in about 11 patients in order to obtain change in the outcome of pain in only one individual.
This data is important because it leads to reflection on the clinical relevance of using such a procedure. This is because it is necessary to consider whether it is rational to expose more than 10 patients to the risk of injecting non-inert drugs to avoid pain in a reduced number of cases. Propofol is extensively used for sedation in the modern practice of digestive system exams, with a frequency of use varying from 8 to 53% in different regions of the United States [14]. Local anesthetics can cause both type I (immediate hypersensitivity) allergic reactions and type IV (contact dermatitis) [15], but the number of patients that is affected by these reactions is unknown. In addition, even topical use of lidocaine can lead to systemic toxicity, both in children and in adults [16].
Another aspect to be considered, although not so relevant, is the economic point of view, for which the costs of being associated with the anesthetic procedure plus a drug should be questioned. To better illustrate, approximately 4 to 5 thousand anesthetics per month (data obtained by the author with the clinical directors of these) are performed in large clinics in the region of Greater Florianópolis, where this study was done, and about 75% use some type of sedation/general anesthesia with the use of propofol. Thus, taking into account the average cost of a 20 ml vial of 2% lidocaine without vasoconstrictor, which is sufficient to prepare solution for approximately six patients, the savings of not using the local anesthetic in the procedures of anesthetic induction in the municipality would be taken into consideration if we considered only the costs of the drug; these would be even greater when considering the expenditures with the devices and technical team required for the procedures.
Conclusion
Data from the present study suggest that the use of lidocaine prior to the administration of propofol during the anesthetic induction procedure is not justified, considering the high NNT value observed in our study. The need to treat 11 patients to obtain therapeutic benefit in only one of them may expose a large number of patients to the risk of unwanted drug reactions as well as raise the cost of the procedure to health institutions.
- Urban BW, Bleckwenn M (2002) Concepts and correlations relevant to general anaesthesia. Br J Anaesth 89: 3-16. Link: https://bit.ly/2KEPL5b
- Robinson DH, Toledo AH (2012) Historical development of modern anesthesia. J Investig Surg 25: 141-149. Link: https://bit.ly/2HD2yUz
- Ambesh SP, Dubey PK, Sinha PK (1999) Ondansetron pretreatment to alleviate pain on propofol injection: a randomized, controlled, double-blinded study. Anesth Analg 89: 197-199. Link: https://bit.ly/378QUcN
- Euasobhon P, Dej-arkom S, Siriussawakul A, Muangman S, Sriraj W, et al. (2016) Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. In: Euasobhon P, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd. Link: https://bit.ly/2JeP5TH
- Kendall MC, Pisano DV, Cohen AD, Gorgone M, McCormick ZL, et al. (2018) Selected highlights from clinical anesthesia and pain management. J Clin Anesth 51: 108-117. Link: https://bit.ly/39ooQoN
- Picard P, Tramèr MR (2000) Prevention of pain on injection with propofol: A quantitative systematic review. Anesth Analg 90: 963–969. Link: https://bit.ly/3lduRqq
- Choi E, Kim D, Jeon Y (2016) Comparative study between 2 different doses of pregabalin and lidocaine on pain following propofol injection: A double-blind, randomized clinical consort study. Medicine 95: e5153. Link: https://bit.ly/3m9tgU3
- Tan CH, Onsiong MK (1998) Pain on injection of propofol. Anaesthesia 53: 468-476. Link: https://bit.ly/368TaS3
- Barker P, Langton JA, Murphy P, Rowbotham DJ (1991) Effect of prior administration of cold saline on pain during propofol injection. Anaesthesia 46: 1069-1070. Link: https://bit.ly/3o50Ued
- Lee JR, Jung CW, Lee YH (2007) Reduction of pain during induction with target-controlled propofol and remifentanil. Br J Anaesth 99: 876-880. Link: https://bit.ly/375q8SI
- Corbo MD, Weber E, DeKoven J (2016) Lidocaine allergy: do positive patch results restrict future use? Dermatitis 27: 68-71. Link: https://bit.ly/368HIpN
- Oliveira CMB de, Issy AM, Sakata RK (2010) Lidocaína por via venosa intraoperatória. Rev Bras Anestesiol 60: 325-332. Link: https://bit.ly/33BcQN1
- Jalota L, Kalira V, George E, Shi YY, Hornuss C, et al. (2011) Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ 342: d1110. Link: https://bit.ly/2HKaRy8
- Wernli KJ, Brenner AT, Rutter CM, Inadomi JM (2016) Risks Associated with anesthesia services during colonoscopy. Gastroenterology 150: 888-894. Link: https://bit.ly/2V3g8Ue
- Trautmann A, Stoevesandt J (2018) Differential diagnosis of late-type reactions to injected local anaesthetics: Inflammation at the injection site is the only indicator of allergic hypersensitivity. Contact Dermatitis 80: 118-124. Link: https://bit.ly/3686JB8
- Tran AN, Koo JY (2014) Risk of systemic toxicity with topical lidocaine/prilocaine: a review. J Drugs Dermatol 13: 1118-1122. Link: https://bit.ly/2Jg0v9C
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
