Open Journal of Pain Medicine
Breakthrough cancer pain: A delphi consensus study on expert recommendations for barriers that prevent the proper management of BTcP in Spain
Yolanda Escobar Álvarez1*, Javier Cassinello Espinosa2, Joaquín Montalar Salcedo3, Ramón de las Peñas4, Fernando Caballero Martínez5 and Ana Blasco Cordellat6
2Servicio de Oncología Médica, Hospital de Guadalajara, Guadalajara, Spain
3Servicio de Oncología Médica, Hospital Universitario y Politécnico La Fe, Valencia, Spain
4Servicio de Oncología Médica, Hospital Provincial de Castellón, Castellón, Spain
5Facultad Ciencias de la Salud, Universidad Francisco de Vitoria, Madrid, Spain
6Servicio de Oncología Médica, Hospital General Universitario de Valencia, Valencia, Spain
Cite this as
Álvarez YE, Espinosa JC, Salcedo JM, Las Peñas RD, Martínez FC, et al. (2020) Breakthrough cancer pain: A delphi consensus study on expert recommendations for barriers that prevent the proper management of BTcP in Spain. Open J Pain Med 4(1): 024-033. DOI: 10.17352/ojpm.000020Background: The management of Breakthrough cancer Pain (BTcP) remains unsatisfactory. Although many barriers to BTcP management have been identified, oncologists have not been able to overcome them. The aim of this study is to identify the barriers preventing proper BTcP management that Spanish medical oncologists have found, and to reach a consensus in order to draft the appropriate recommendations to overcome them.
Methods: This study is based on two surveys conducted by oncologists. The first survey was designed to reach a consensus on the main barriers (related to patients, physicians and healthcare organizations) that stand in the way of BTcP control. The second survey (a Delphi questionnaire) was based on the barriers evaluated in the first survey, including recommendations assessed using the two-round Delphi methodology.
Results: The identification of the main barriers to BTcP management to be assessed showed a high consensus regarding the need for greater involvement from health organizations. Eighty-eight experienced oncologists evaluated the proposed recommendations. A consensus was reached on 93% of these recommendations, always in terms of agreement. Only three recommendations did not reach a consensus, one in each block of barriers (patients, physicians and healthcare organizations).
Conclusion: Showing a high degree of consensus, the results of this study reflect that there are over-worked medical oncologists, which results in more time and training being taken away from proper BTcP management. Although oncologists considered cancer pain management to be suitable in oncologist consultations, they also revealed that more support and resources are necessary in order to improve BTcP control.
Abbreviations
BTcP: Breakthrough cancer Pain; CI: Confidence Interval; CPG: Clinical Practice Guidelines; MO: Medical Oncology; NC: No Consensus; PA: Primary Attention; PCP: Primary Care Physician; QoL: Quality of Life; RECs: Research Ethics Committees; SEOM: Spanish Society of Medical Oncology
Introduction
Breakthrough cancer Pain (BTcP) is commonly defined as the transient exacerbation of pain that occurs either spontaneously or in relation to a predictable or unpredictable trigger (an incident), despite stable, controlled background pain [1,2]. Currently, there is no universally accepted definition of BTcP [1,3]. This lack of worldwide agreement may make it difficult to adequately discriminate BTcP from uncontrolled background pain and lead to under diagnosis, despite the existence of diagnostic algorithms [1-4].
BTcP is a heterogeneous pain [5], that can be related to multiple causes. It can be a consequence of neoplasm (70%-80%) or a result of anticancer treatment (10%-20%) [1,6]. In less than 10% of all cases, the pain is not related to either the malignant disease or its treatment [6]. This variability complicates its diagnosis and treatment [4,5,7].
The prevalence of BTcP is high [8,9]. Recently, its prevalence has been reported at 59.2% [9], although previous prevalence rates ranged from 35% to 95% [1]. In Spain, BTcP is present in 48% of the patients with cancer-related pain [10].
BTcP is a major indicator of poor clinical outcome and lower efficacy of opioid treatment [4]. Moreover, it promotes functional deterioration and has a negative impact on Quality of Life (QoL) [5] and bears a significant physical, psychological and economic burden [9]. Therefore, BTcP should be adequately identified and treated (along with anti neoplastic treatment) to minimize the intensity and severity of the episodes and to lessen the impact on patients’ QoL [1].
BTcP is still a little-known problem with serious consequences on patients’ health; it is not well researched and may be incorrectly treated [8,11]. Various evidence suggests that it is often managed suboptimally [4]. Several barriers that prevent proper BTcP management have been identified [1], which arise from healthcare professionals, patients themselves and healthcare settings [5]. Even so, diagnostic and therapeutic inertia makes it necessary to identify more barriers and find solutions to eliminate the deficiencies or problems detected in BTcP management.
The objectives of the BARDIO consensus were to explore and identify the main barriers preventing the correct management of BTcP in standard Spanish clinical practice, and to provide solutions to the highest-priority problems by developing recommendations.
For this purpose we used the Delphi method, an accepted methods available for attaining expert consensus [12]. It is a structured process that starts defining a problem, and then involve: developing questions for experts to resolve, selecting a panel of experts, using open-ended questionnaires, performing controlled assessment and feedback (qualitative and quantitative analysis), and follow-up (reassessment) using multiple rounds of surveys until a consensus is reached [12].
Materials and methods
This study was carried out through a survey of doctors’ opinions (the Delphi method). The validity of the Delphi method is supported by the participation of a large number of experts who have knowledge and an interest in the topic and the use of successive rounds of the questionnaire [13,14]. This justify that it is one of the reasonably well accepted methods available for attaining expert consensus [12].
In Spain, this type of study is not among those that require the approval or written consent of Research Ethics Committees (RECs).
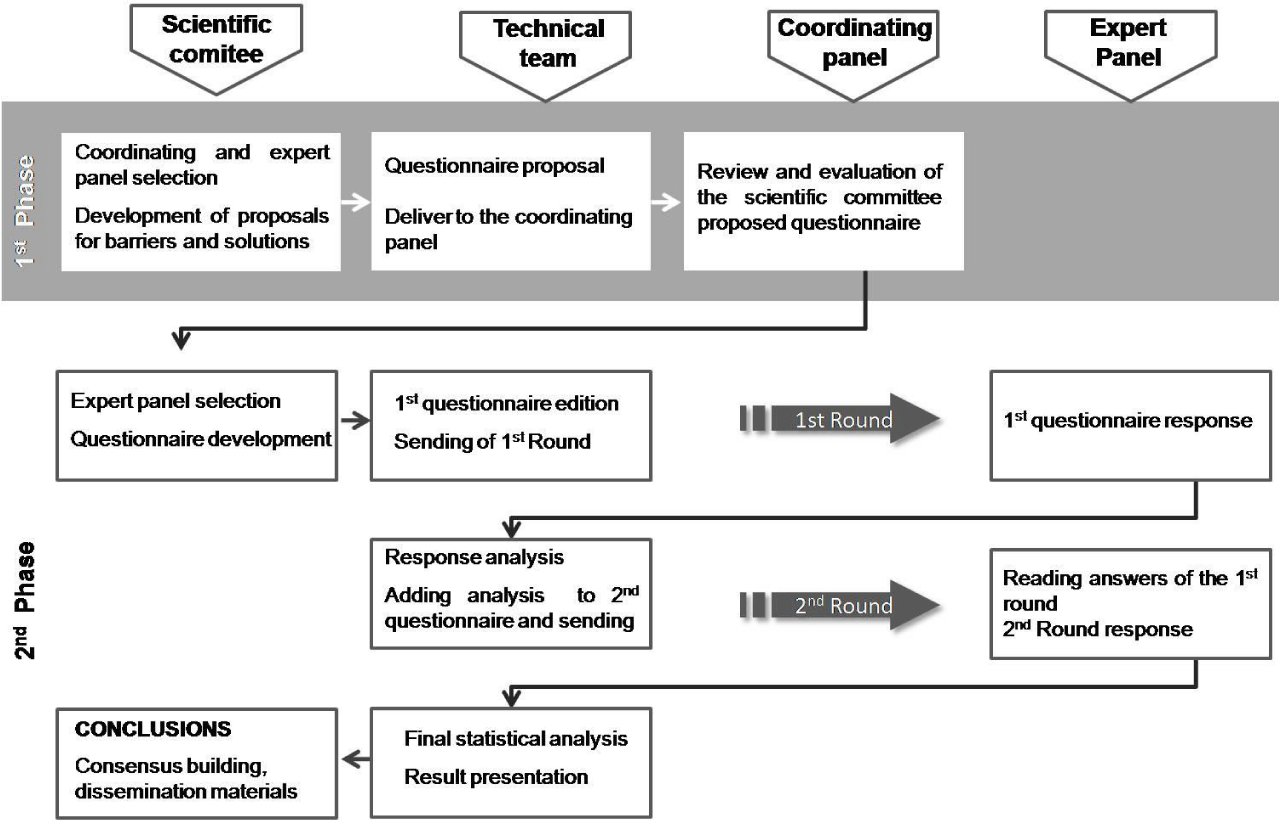
A scientific committee comprised of five leading oncologists in this field reviewed the objective of the study and developed an initial questionnaire concerning the main barriers of BTcP management (which were dependent on patients, physicians or healthcare professionals and health organizations).
Subsequently, a coordinating panel (made up of 23 oncology specialists selected by the scientific committee) reviewed and validated the questionnaire and proposed solutions to the barriers. The scientific committee then reviewed the results and comments and used them to develop a Delphi questionnaire, which would later be answered by an expert panel to reach a consensus on the proposed solutions. The scientific committee also selected the members of the expert panel (n=88 oncologists) using the snowball sample technique. This panel, stratified among autonomous communities based on the group size in each territory, participated without remuneration.
A technical team was responsible for the method implementation (editing and dissemination of the questionnaires, analysis of responses and statistical interpretation of the consensus reached). The study design and all participants are shown in Figure 1.
Each questionnaire item was formulated as an assertion and assessed on a 9-point, single, ordinal, Likert-type scale: 1-3= disagree; 4-6= neither agree nor disagree; 7-9= agree. Individual observations and new proposals for consideration could be added.
We used the modified Delphi method (a technique of professional consensus performed through written surveys) in two rounds [15]. The Delphi questionnaire (an online survey) had 41 items distributed in proposals for improvement on 1) patient-dependent barriers (9 items); 2) barriers dependent on the physician/healthcare personnel (22 items); and 3) barriers dependent on the health organization (10 items). The survey rounds were performed between May and June 2017.
The median score of each item was evaluated. Consensus was considered to be reached when at least two-thirds of the panel ranked the item within three points of the median: 1-3 points in the case of “disagreement” and 7-9 in the case of “agreement.” Items with a median score located in the region of 4-6 were considered “indeterminate.” When the scores of a third or more of the panelists were within the region of 1-3 and the scores of another third or more were within the region of 7-9, the item was considered “without consensus.”
After the first Delphi round, panelists were informed of aggregate-level summary statistics of the individual responses (mean, median, percentage of distribution of the respondents situated outside the region of median) and the type of consensus reached. This summary also included any written comments made by panelists. The items without consensus, those with a high dispersion of opinions and those marked “indeterminate” were considered for reassessment in the second Delphi round. The panelists then submitted a new individual assessment on these items.
After the second round, the results were analyzed according to the same criteria of the first round. Items without consensus were analyzed descriptively in order to distinguish those that reflected opinions that were markedly different between the panelists from those that fell within the “indeterminate” region.
The mean score of each item was also calculated, with a 95% Confidence Interval (CI). The lower amplitude of CI is explained by greater unanimity of opinions in the group. A more extreme mean score indicated a more evident consensus in terms of agreement or disagreement.
Results
Of the 27 items included in the first proposal of the questionnaire about the barriers preventing BTcP management, the coordinating panel reached consensus in six items (Table 1); none of them in the block of patient-dependent barriers. The consensus was in terms of “disagreement” in one item (11, regarding the specialist’s lack of interest in controlling the patient’s symptoms), and in terms of agreement in the remaining items (14, 17, 23, 25 and 26). The highest degree of agreement was reached in the items on the health organization barriers.
Taking into account the results and the comments obtained about the proposed barriers, the number of recommendations was adjusted and included in the Delphi questionnaire that was sent to the expert panel. Eighty-eight experienced oncologists were surveyed. Response rates were high: 97.8% (n=88/90) in the first round of the Delphi questionnaire and 100% (n=88) in the second round.
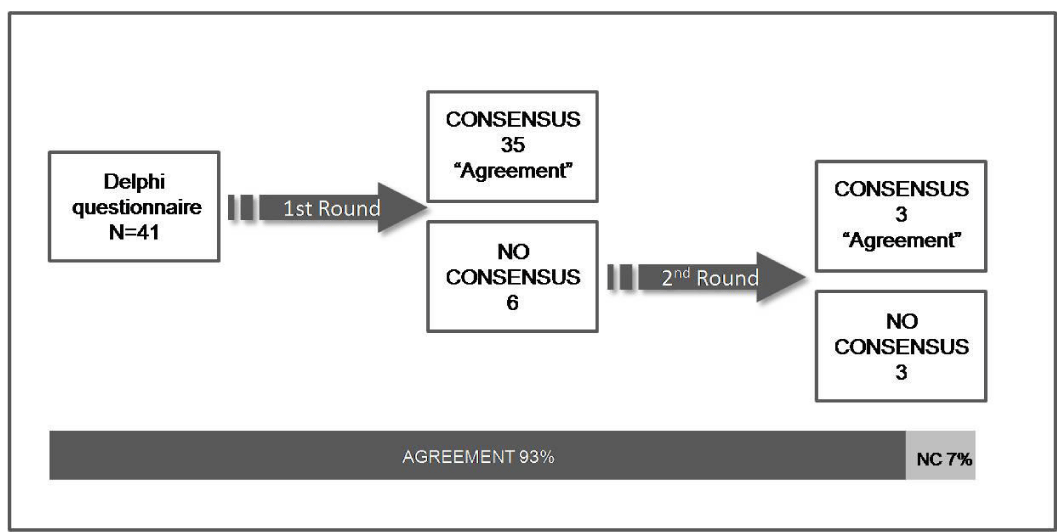
In the first round, panelists reached consensus in 35 out of 41 items/barriers, in terms of agreement. The six items without consensus were: Block 1) item 9, regarding educational campaigns about pain control for the general population; Block 2) item 25, about the availability of multidisciplinary consultations for supportive treatment in oncology, and items 29 and 30, regarding the lack of appropriate drugs for BTcP treatment in the hospitals’ pharmacy services; Block 3) item 34, about distance medication adjustments (for example, remote titration by telephone), and item 39, regarding the existence of a healthcare professional who acts as a bridge between the primary attention (PA) physician and the oncologist. These items were proposed for reconsideration in the second Delphi round, and consensus was reached on 3 items (25, 30 and 39). In the remaining items (n=3 [6, 29 and 34], each in a different block; 7% of the total) there was no consensus due to disparity of professional opinion or lack of criteria (Table 2).
Eventually the expert panel reached consensus on 38 of 41 items (93%), all of them in terms of agreement with the assertion of the barrier (Figure 2) (Table 2).
The proposed items in the block of the patient-dependent barriers were those with the highest degree of consensus: 4 of 9 items (3, 4, 6 and 7) with less than 10% of the panelists’ scores outside the median region.
With regard to the results, the scientific committee gathered in a face-to-face work session during the meeting of the Spanish Society of Medical Oncology (SEOM; Oct 25-27, 2017) to establish some recommendations (Table 3).
Discussion
Although BTcP and its proper management have been widely researched, evidence shows that it is still managed suboptimally [4-6,16-18]. The aim of this study was to establish consensus on the barriers present in Spanish clinical practice for BTcP management, and to suggest recommendations to address them. The use of the Delphi technique allowed for the anonymous participation of a large number of experts distributed throughout Spain, thus avoiding the risk of some experts dominating responses, and without the time/geographical restraints of other methods [19].
Among the barriers preventing BTcP control that were initially proposed by the scientific committee, those related to patients were met with the most doubt. It is known that patient assessment is poor in oncologist consultations [20]. Effective physician-patient communication and the promotion of patient participation in consultations are very important [7] in order to better understand patient-dependent barriers. However, the estimated reduced consultation time available in Spain (first and second visit: 60-90 minutes; successive visits: 15 minutes; follow-up/check-up visits: 20 minutes; hospitalization: 20 minutes; interconsultation: 30-60 minutes) [21] and the large amount of information that physicians must provide to patients (about the disease, treatment, side effects, etc.) [22], justify this situation. The lack of time available during the consultation was one of the two proposed physician-related barriers that demonstrated agreement in the coordinating panel reflection. It has been previously reported [23] and identified as one of the reasons for the non-implementation of recommendations from clinical practice guidelines in Spain [24].
The other physician-related barrier that demonstrated agreement was the lack of adequate BTcP anamnesis, which is essential for BTcP diagnosis [7] and has been highlighted in previous Spanish consensus recommendations [25]. The only proposed barrier with consensus in terms of disagreement was the lack of the specialist’s interest in the control of BTcP symptoms. Supporting this consensus, a recent study reflects how Spanish oncologists are increasingly guided by evidence-based Clinical Practice Guidelines (CPG) for BTcP management [24]. With reference to health organization barriers, the coordinating panel stated its agreement with the lack of support outside of scheduled oncology consultations, the absence of contact and coordination between levels of care, and the training deficiencies in PA and ambulatory settings.
Cancer pain management required a multidisciplinary team involving many healthcare professionals (oncologists, pharmacists, nurses, etc.) in different clinical settings (inpatient-outpatient [ambulatory and primary care]). However, in this study the role of pharmacists was not mentioned, despite the fact that they can provide a broad scope of services that may be very useful for cancer pain management [26]. As stated by the panel, effective interactions between specialists are crucial for adequate pain management [26].
When the expert panel assessed the recommendations suggested by the scientific committee (using the Delphi questionnaire), a high degree of consensus was observed, always in terms of agreement. Only three recommendations did not reach consensus: one in the block of patient-dependent barriers (about the execution of educational campaigns for the general population), another in the block of physician/healthcare personnel barriers (about problems related to the non-availability of all effective BTcP drugs in hospitals) and the last one in the block of health organization barriers (about the inappropriateness of remote titration of drugs for BTcP control).
The lack of consensus on the execution of educational campaigns for the general population may reflect the questionable utility of these campaigns for cancer patients without pain or with multifactorial pain, despite the fact that pain management education has been shown to rectify patients’ misconceptions of pain, reduce pain and improve QoL [5]. When the recommended educational/informative programs were meant for patients and caregivers, the degree of consensus was very low (with dispersed opinions). The difficulty in carrying out this program, due to time and space constraints and a shortage of professionals available for sessions, could justify this result [5]. On the other hand, the recommended patient-dependent barriers with the highest degree of consensus were those on the need for structured interviews including pain-specific anamnesis. Considering the importance of this, the latest Spanish Society of Medical Oncology (SEOM) guideline for the treatment of cancer pain listed the minimum information to be included in each medical history for the evaluation and management of BTcP [1]. The need for more time in consultations and the importance of the oncological nurse also showed a high degree of consensus. However, nurses’ understanding of BTcP is currently considered insufficient and, despite the existence of specific guidelines [27], more training is needed [28].
With respect to the second recommendation without consensus—the impact of the non-availability of all effective BTcP drugs in hospitals on residents’ learning and patients’ self-confidence—it must be taken into account that there are different options and a wide variety of formulations [16,29], and that the new galenic preparations are considerably more expensive than existing alternatives [30]. Each delivery system required the patients to be trained by the physicians, and the experts did not reach consensus on whether the resident physicians would find it difficult to learn about the systems not available in hospitals, nor whether it might affect the patients’ trust in their treatment. However, there was agreement, albeit with dispersed opinions, on the difficulty entailed by this unavailability for treatment titration and toxicity evaluation. A program for healthcare professionals (other than the oncologists) to support patients outside of the hospital could solve these problems, although the recommendation of drug titration in a quick outpatient consultation also had high dispersion. There was controversy about the personnel involved (nurses, primary care, etc.) and the duration, perhaps because some forms of titration demand high levels of expertise and knowledge of the drugs involved, making them very difficult for non-pain-specialists to manage [7,30]. Other recommendations with a low degree of consensus in the block of physician/healthcare personnel barriers included several that dealt with addressing the lack of time in consultations. Panelists agreed that it is recommended the early referral of patients from the palliative care units and the creation of specific palliative medical consultations (either face-to-face or via phone). These recommendations indicate the relevance of palliative care for oncologist in these setting but the dispersion of opinions reflects the doubts of the panelists on the possibility of improperly overburdening the palliative care service with patients that should still be subject to follow-ups by oncologists. Furthermore some doubts on the term “multidisciplinary” consultation was also observed.
The recommendations with the highest degree of consensus in the physician/healthcare personnel block regarded the need for specific BTcP education right from the beginning of specialty oncology training, and the presence of caregivers during consultations for patients with cognitive deterioration. The need for education on cancer pain management has been extensively reported in the past [31] and this need still persists [1]. Therefore, an early and specific BTcP education program could improve the situation. On the other hand, the fact that the patient was the main source of information for the BTcP assessment, coupled with the need to educate patients and relatives in order to maximize its control [4,5,29], reflect the requirement of the patient’s adequate cognitive functioning and the presence of a caregiver (when necessary). The high degree of consensus that was also reached on the need for accurate anamnesis and medication reassessment clearly demonstrates the panelists’ knowledge of the relevance of information collection during consultations, and also the need to improve it. Better physician-patient communication and greater implementation of BTcP guidelines could address these needs, requiring more time and physician education [24].
The last recommendation without consensus was the inappropriateness of remote titration of drugs for BTcP control. The suitability of telephone assessments for the titration of drugs for BTcP control (such as fentanyl) has already been described in the literature [32,33]. However, various aspects led to non-consensus, such as the availability of personnel to perform this task, the need to carefully select the appropriate patients and the possibility of bias in the interpretation of information. Of the recommendations with consensus in the block of health organization barriers, the one with the lowest degree of consensus was that which regarded the figure of the primary care physician as a reference for the patient with BTcP. The dispersion of opinions once again reflected the lack of time and insufficient BTcP-specific training, this time in the primary care setting. On the other hand, the recommendations with the highest degree of consensus included the need for the health administration to facilitate more resources between the scheduled oncology consultations. The results of this study reflect the needs of the oncologists (time and training) and the usefulness of support outside of consultations to improve BTcP control. In concordance with this, panelists reached a high degree of consensus on the need for hospital support and coordination with primary care, including through digital technologies that cancer patients are already using to support personalized symptom monitoring and communication between patients and healthcare professionals [34].
The main strength of this study is the fact that it is based on responses (response rate: first round 97.8%, second round 100%) from a national panel of experts. However, limitations must be recognized; there could be a disparity between the responses of the oncologists from the different Spanish autonomous communities, yet our findings aim to be representative of the overall population. Additionally, it should be noted that the study has been addressed to oncologists; it could be appropriate to discuss this subject with other healthcare professionals (primary care services, palliative care units and other hospital teams).
Conclusions
Our results demonstrate that Spanish oncologists are aware of the main barriers for BTcP management. A strong consensus was reached on most of the proposed recommendations that were evaluated, reflecting the oncologists’ opinions of the convenience of BTcP management that is centralized in oncologist consultations. However, due to lack of time and training, oncologists consider more support (including trained personnel outside of the oncologist consultations, such as nurses, primary care physicians, etc.) and more resources to be necessary in order to improve BTcP control.
The authors gratefully acknowledge all experts who have participated in this study.
Funding
This project was funded by Kyowa Kirin Farmacéutica, SLU, as were all article processing charges. Kyowa only provided the funds necessary to develop the study, without intervention in the design and management of the study. All authors had full access to all of the data in this study and take full responsibility for the integrity of the data and accuracy of the data analysis.
- Jara C, Del Barco S, Gravalos C, Hoyos S, Hernandez B et al. (2018) SEOM clinical guideline for treatment of cancer pain (2017). Clin Transl Oncol 20: 97-107. Link: https://bit.ly/2OdXLZv
- Mercadante S, Portenoy RK (2016) Breakthrough cancer pain: twenty-five years of study. Pain 157: 2657-2663. Link: https://bit.ly/2Walmi5
- Deandrea S, Corli O, Consonni D, Villani W, Greco MT, et al. (2014) Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage 47: 57-76. Link: https://bit.ly/2ZXSBX9
- Vellucci R, Mediati RD, Gasperoni S, Mammucari M, et al. (2017) Assessment and treatment of breakthrough cancer pain: from theory to clinical practice. J Pain Res 10: 2147-2155. Link: https://bit.ly/38NDIL6
- Koh SJ, Keam B, Hyun MK, Ju Seo J, Uk Park K, et al. (2018) Cancer Pain Management Education Rectifies Patients' Misconceptions of Cancer Pain, Reduces Pain, and Improves Quality of Life. Pain Med 19: 2546-2555. Link: https://bit.ly/328e8z6
- Margarit C, Julia J, Lopez R, Anton A, Escobar Y, et al. (2012) Breakthrough cancer pain - still a challenge. J Pain Res 5: 559-566. Link: https://bit.ly/300aR1P
- Working Group Nientemale DEI, Vellucci R, Fanelli G, Pannuti R, Peruselli C, et al. (2016) What to Do, and What Not to Do, When Diagnosing and Treating Breakthrough Cancer Pain (BTcP): Expert Opinion. Drugs 76: 1063-1065. Link: https://bit.ly/2ZihM7t
- Breivik H, Cherny N, Collett B, de Conno F, Filbet M, et al. (2009) Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 20: 1420-1433. Link: https://bit.ly/2W7QcId
- Cortesi PA, D'Angiolella LS, Vellucci R, Allegri M, Casale G, et al. (2017) Cost-effectiveness analysis of oral fentanyl formulations for breakthrough cancer pain treatment. PLoS One 12: e0179523. Link: https://bit.ly/38MExUr
- Pérez-Hernández C, Blasco A, Gándara Á, Mañas A, Rodríguez-López MJ, et al. (2019) Prevalence and characterization of breakthrough pain in patients with cancer in Spain: the CARPE-DIO study. Scientific reports 9: 17701. Link: https://bit.ly/32dMpwC
- Gomez-Batiste X, Madrid F, Moreno F, Gracia A, Trelis J et al. (2002) Breakthrough cancer pain: prevalence and characteristics in patients in Catalonia, Spain. J Pain Symptom Manage 24: 45-52. Link: https://bit.ly/3emGJ5Y
- Hohmann E, Brand JC, Rossi MJ, Lubowitz JH (2018) Expert Opinion Is Necessary: Delphi Panel Methodology Facilitates a Scientific Approach to Consensus. Arthroscopy 34: 349-351. Link: https://bit.ly/2ZjcatR
- Njuangang S, Liyanage C, Akintoye A (2017) Application of the Delphi technique in healthcare maintenance. Int J Health Care Qual Assur 30: 737-754. Link: https://bit.ly/2Odf9xk
- Hasson F, Keeney S, McKenna H (2000) Research guidelines for the Delphi survey technique. Journal of advanced nursing 32: 1008-1015. Link: https://bit.ly/2CmcizN
- Dalkey N, Brown B, Cochran S (1969) The Delphi Method, III: Use of Self Ratings to Improve Group Estimates, vol. RM-6115-PR. Santa Monica (California): Rand Corporation. Link: https://bit.ly/3iOsudI
- Mercadante S (2018) Treating breakthrough pain in oncology. Expert Rev Anticancer Ther 18: 445-449. Link: https://bit.ly/3fi2K7i
- Mercadante S (2018) Non pharmacological interventions and non-fentanyl pharmacological treatments for breakthrough cancer pain: A systematic and critical review. Crit Rev Oncol Hematol 122: 60-63. Link: https://bit.ly/2W8bbKW
- Olarte JM (2017) Breakthrough cancer pain and rational drug use. Support Care Cancer 25: 11-17. Link: https://bit.ly/2OgAbeI
- de Meyrick J (2003) The Delphi method and health research. Health Education 103: 7-16. Link: https://bit.ly/2ZXQgvl
- Muller-Schwefe G, Ahlbeck K, Aldington D, Alon E, Coaccioli S, et al. (2014) Pain in the cancer patient: different pain characteristics CHANGE pharmacological treatment requirements. Curr Med Res Opin 30: 1895-1908. Link: https://bit.ly/2W9NodE
- Gravalos C, Salvador J, Albanell J, Barnadas A, Borrega P, et al. (2012) Functions and workload of medical oncologists in Spain. Clin Transl Oncol 14: 423-429. Link: https://bit.ly/3gNssRp
- Rivera F, Andres R, Felip E, Garcia-Campelo R, Lianes P, et al. (2017) Medical oncology future plan of the Spanish Society of Medical Oncology: challenges and future needs of the Spanish oncologists. Clin Transl Oncol 19: 508-518. Link: https://bit.ly/2W9zjNh
- Boceta J, De la Torre A, Samper D, Farto M, Sanchez-de la Rosa R (2016) Consensus and controversies in the definition, assessment, treatment and monitoring of BTcP: results of a Delphi study. Clin Transl Oncol 18: 1088-1097. Link: https://bit.ly/3ek4A6c
- Lopez Lopez R, Camps Herrero C, Khosravi-Shahi P, Guillem Porta V, Carrato Mena A, et al. (2017) Oncologist's knowledge and implementation of guidelines for breakthrough cancer pain in Spain: CONOCE study. Clin Transl Oncol. Link: https://bit.ly/2W8oonc
- Escobar Y, Biete A, Camba Rodríguez M, Galvez R, Mañas A, et al. (2013) Diagnosis and treatment of breakthrough cancer pain: Consensus recomendations. Rev Soc Esp Dolor 20.
- Ratka A (2002) The role of a pharmacist in ambulatory cancer pain management. Curr Pain Headache Rep 6: 191-196. Link: https://bit.ly/2Of73V8
- Wengstrom Y, Geerling J, Rustoen T (2014) European Oncology Nursing Society breakthrough cancer pain guidelines. Eur J Oncol Nurs 18: 127-131. Link: https://bit.ly/325MyT1
- Porta-Sales J, Perez C, Escobar Y, Martinez V (2016) Diagnosis and management of breakthrough cancer pain: Have all the questions been resolved? A Delphi-based consensus assessment (DOIRON). Clin Transl Oncol 18: 945-954. Link: https://bit.ly/3007nwh
- Mercadante S, Cuomo A (2016) Breakthrough Cancer Pain: Ten Commandments. Value Health 19: 531-536. Link: https://bit.ly/2Cmbo6n
- Hans GH (2013) Treatment of breakthrough cancer pain: to titrate or to proportionate? Curr Med Res Opin 29: 1523-1526. Link: https://bit.ly/3egbGsx
- Von Roenn JH, Cleeland CS, Gonin R, Hatfield AK, Pandya KJ (1993) Physician attitudes and practice in cancer pain management. A survey from the Eastern Cooperative Oncology Group. Ann Intern Med 119: 121-126. Link: https://bit.ly/38Q6muP
- Rauck R, Bull J, Parikh N, Dillaha L, Stearns L (2015) Effective Dose Titration of Fentanyl Sublingual Spray in Patients With Breakthrough Cancer Pain. Pain Pract 16: 1012-1018. Link: https://bit.ly/2ZfMKgu
- Alberts DS, Smith CC, Parikh N, Rauck RL (2016) Fentanyl sublingual spray for breakthrough cancer pain in patients receiving transdermal fentanyl. Pain Manag 6: 427-434. Link: https://bit.ly/3iW7fH8
- Adam R, de Bruin M, Burton CD, Bond CM, Giatsi Clausen M, et al. (2017) What are the current challenges of managing cancer pain and could digital technologies help? BMJ Support Palliat Care 8: 204-212. Link: https://bit.ly/3iV1clR
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
