Open Journal of Pain Medicine
Assessment of symptoms, quality of life and associated costs in a one-month follow-up of cancer patients with breakthrough pain: The IMDI Study
Concepción Pérez1, Antonio Javier Jiménez-López2, Almudena Sanz-Yagüe2 and Begoña Soler-López3*
2Medical Department, Kyowa Kirin Farmacéutica, S.L.U, Madrid, Spain
3Medical Department, E-C-BIO, S.L., Las Rozas (Madrid), Spain
Cite this as
Pérez C, Jiménez-López AJ, Yagüe AS, Soler López B (2020) Assessment of symptoms, quality of life and associated costs in a one-month follow-up of cancer patients with breakthrough pain: The IMDI Study. Open J Pain Med 4(1): 001-008. DOI: 10.17352/ojpm.000017Purpose: Breakthrough Pain (BTP) observed in up to 66% of cancer patients, is more common in patients with advanced cancer stages and is usually associated with a poor prognosis.
The objective of this study was to evaluate the quality of life and control of associated symptoms of cancer patients with breakthrough pain (BTP) after a one-month follow-up in pain and palliative care units and measure the associated cost savings after our intervention.
Methods/patients: A one-month observational prospective study was designed. Eight Spanish pain units, eight palliative care units, and one oncology department participated. On baseline and one-month visit, the Edmonton Symptoms Assessment Scale (ESAS), Brief Pain Inventory (BPI) and the quality of life (EORTC QLQ-C30, version 3) were assessed. The direct medical and non-medical costs fixed and variable and the indirect costs of the patient and the caregivers were evaluated and published previously. Factors related to cost and quality of life, were identified using Linear Generalized Models (LGM) type gamma and logistic link. Participants were oncologic patients with BTP, older than 18 years, with controlled background pain.
Results: A total of 152 patients with a mean age of 66.8 years (95% CI 64.8-68.8), and 65.8% males were included. All symptoms (ESAS) were significantly improved (p<0.05) from baseline to one month of follow-up. BPI dimensions and all functional and symptoms dimensions of EORTC QLQ-30 were also improved in one month (p<0.01). An improvement in EORTC QLQ-30 global health status-quality of life was associated with a reduction in overall BTP costs.
Conclusions: Cancer patients improved their quality of life and cancer associated symptoms in only one month of treatment in pain and palliative care units, and this improvement leads to significant BTP cost savings.
Abbreviations
BMI: Body Mass Index; BPI: Brief Pain Inventory; BTP: Breakthrough Pain; ESAS: Edmonton Symptom Assessment Scale; LGM: Linear Generalized Models; QOL: Quality of Life
Introduction
Breakthrough Pain (BTP) is observed in up to 66% of cancer patients but is also frequent in non-cancer patients with background pain of other etiologies. BTP is more common in advanced cancer stage patients and is usually associated with a poor prognosis [1-3].
BTP was defined in 1990 by Portenoy, et al., as a “transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain”[4]. The intensity of BTP is severe and of short duration, usually lasting fewer than 60 minutes and appearing 3-5 times a day [1,5,6].
BTP has a very significant impact on the quality of life of patients and has been described as associated with a high use of healthcare resources [7,8].
Real-life studies about the costs of BTP in cancer patients are scarce, and no prospective ones have been conducted in real world BTP patients [9-11]. Obtaining information about the real costs of BTP in cancer patients was the primary objective of the main study, and cost of illness results obtained were recently published [12]. The total BTP cost per patient was estimated at 2941.60 euros per month, where 88% resulted direct medical costs, 5% non-medical direct costs, and 7% indirect costs due to loss of productivity. From the multivariate analyses a better baseline EORTC QLQ-30 quality of life score, was associated with lower overall BTP costs [12].
The objective of this new analysis was to measure the improvement in symptom control and the EORTC QLQ-C30 quality of life after the intervention of pain and palliative units, and the relationship of both with cost savings [13,14].
Methods
Study design
This was a prospective observational study with one month of patient follow-up. Two visits (baseline and one-month) were completed. On days 1, 3, 5 and 7, the patient was either visited or contacted by phone, depending on their status.
Selection criteria
The patients were included between April 2015 and March 2016 in 17 hospitals from 16 Spanish provinces. The study was completed in eight pain units, eight palliative care units and one oncology department.
The investigators selected the first ten consecutive patients visiting the clinic who met the selection criteria.
Oncologic patients of any race and gender, older than 18 years with controlled background pain with a diagnosis of BTP and ambulatory when selected, were included in the study. BTP could be newly diagnosed (BTP naïve patients) or the patient could attend the visit with BTP on follow-up. Patients were excluded if they had cognitive impairment, were severely affected by their underlying disease or were uncooperative, or unable to complete the study questionnaires.
BTP was defined according to the criteria of Portenoy, et al., as: the presence of persistent background pain lasting twelve or more hours per day during the week before the evaluation or which would exist if treatment were not taken, which is adequately controlled, i.e., there is no pain or mild pain with an intensity score on the ten-point visual analogic scale of four points or fewer in the last week. The patients must exhibit transient exacerbations of pain [6].
Sociodemographic and clinical variables
The variables recorded were: date of birth, gender, weight, height, socioeconomic level (low: incomes of less than 2/3 of the mean salary, 15000Euros; middle: incomes of between 2/3 and twice the mean salary, >15000Euros and <45000Euros; or high: incomes of higher than twice the mean salary, >45000Euros), and Karnofsky Performance Status [15]. Information was collected about the patient’s clinical history, the type of cancer and date of cancer diagnosis. Information collected about the main characteristics of BTP are described in Table 1.
Assessment of the study objectives
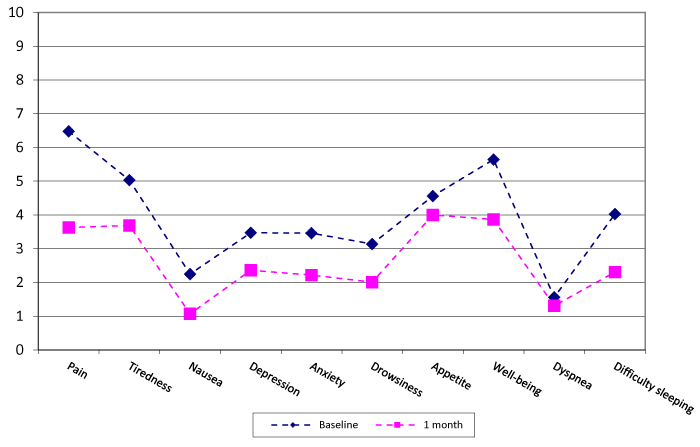
1) Patients were asked to complete four questionnaires at the baseline visit and at the one-month visit: The Edmonton Symptoms Assessment Scale (ESAS) evaluating 10 symptoms intensity, was used to measure other associated symptoms in the past week (Figure 1). The patient scored 0 to 10 points for each symptom and a higher score represented a worse status [16,17]. A difference of 1.2cm (95% CI 0.9-1.5) between the baseline and one-month score was considered of clinical significance [16]. It was considered the symptoms controlled when all ten symptoms scores were under five points in the ESAS scale.
2) Patients completed the Brief Pain Inventory (BPI) to assess the severity of pain and the impact on patient functionality. The patients completed the 12 BPI questions with scores between 0 and 10 points. BPI results were displayed in two dimensions: pain intensity dimension score, and the impact on daily living activities dimension. A higher score represented higher pain intensity or higher impact on daily living activities [18].
3) Quality of life of the patients was measured with the EORTC QLQ-C30 version 3 questionnaire specific for patients with cancer. The patients completed 30 questions, the first 28 with four possible answers, and questions 29 and 30 with seven possible answers. The scores on the questionnaire were calculated following the instructions and programming of the owners of the questionnaire (http://groups.eortc.be/qol/eortc-qlq-c30org). Results were obtained in a range from 0 to 100 points. In order to define the clinical significance of the changes observed, a mean score difference of 5-10 was considered as small but clinically noticeable change for patients, a change between 10 and 20 as moderate and above 20 as a large clinical change [19,20]. On the five functional scales and the global health score, a higher score represented a higher level of functioning and quality of life. A higher score in the eight symptoms scales and the financial difficulties question, meant a worse status [13,14,21].
Treatment
The effect of protocolized intervention of pain and palliative units on symptom control and improvement of quality of life was measured. This protocol consists on systematic measurement of symptoms (ESAS) and measurement of patient’s quality of life and pain control (BPI). Information was collected on current treatments for BTP to allow the cost of illness to be evaluated [12]. Additionally, any medications, even for chronic pain, the patient was receiving at the time of the visit and during the month of follow-up were recorded.
Recording of costs
Patients completed a resource use diary card for the 30-day follow-up. The number of visits and hospital admissions, and the consumption of non-healthcare resources and treatments due to or related to breakthrough pain were collected. The doctors confirmed the data recorded by patients in the diary and transcribed them to the study case report form. Results of this analysis were previously published [12].
Sample size calculation
The principal objective of the project was to calculate the cost of illness of BTP in oncologic patients. In one study on patients with breast cancer, monthly cost was estimated at 1489euros. For a standard deviation of 250euros, a 95% confidence interval and a precision of 41.68euros, it was estimated that a sample of 140 patients yield a power of 80% [22]. Data detailed in this manuscript refers to the analysis of clinical evolution of the patients in the same sample for what the sample size was appropriate.
Statistical analysis
A descriptive analysis was completed. Comparisons between qualitative variables were made using the Fisher test or Chi2 test. The Student t-test was used to compare independent groups in the case of quantitative variables. When the differences in the quality of life as a function of different characteristics were evaluated, the factorial analysis of variance model was applied, implementing the Bonferroni or Games-Howell correction, depending on the homogeneity of the variances, for the control of the error from multiple comparisons.
The cost of illness study was performed by measuring direct medical costs (hospital and drug costs), direct nonmedical costs, and indirect costs. Medical costs were associated with resource consumption and were calculated by multiplying the number of resources used by unit cost. Unit costs of healthcare resources, diagnostic tests and treatments were obtained by taking the average value of the prices obtained in the official bulletins of public prices and tariffs of the autonomous communities of Andalusia, Castile and Leon, Catalonia, Galicia, Madrid and the Basque Country, and the Oblikue health care cost database [23]. Results of the cost of illness analysis were previously published [12].
A multivariate analysis was performed to identify factors related to cost and quality of life, and baseline characteristics of the patients that could be associated independently with an increase or decrease in these factors. Generalized Linear Models (GLM) were used, a generalization of least-squares linear regression that allows the response variable to follow non-normal distributions [24]. The GLM family was selected by the AIC statistic using the R statistical package. Thus, multivariate analyses were carried out with GLM in which total cost was included as the dependent variable, using sociodemographic and clinical information (gender, age, initial QoL, QoL increase, main drug, type of onset of BTP) as co-variables. The values of the dependent variable, total cost, were log-transformed to adjust them to the models. The statistical significance level was set as 0.05. The SPSS version 23.0 statistical package was used for the analysis.
Ethical standards
The study was approved by the Clinical Research Ethics Committee of Hospital Universitario de La Princesa, Madrid (Spain) with code number 2488. All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committees and with most recent version of the Helsinki declaration. Written informed consent was obtained from all individual participants included in the study.
Results
A total of 152 patients were included in the study. Fourteen patients withdrawn from the study: by medical decision in two cases (1.3%); in two cases the patient decided not to continue in the study (1.3%); four patients (2.6%) were lost to follow-up and six patients died during the study (3.9%). Each center included an average of nine patients (95% CI 7-11).
Demographic data and cancer history
In table 2 the demographic and medical history characteristics are described. The mean age was 66.8 years (95% CI 64.8-68.8). The Body Mass Index (BMI) was 24.6kg/m2 (95% CI 23.9-25.2). Cachexia was observed in 10.3% (15 patients), those with BMI <20kg/m2 [25].
At study entry 59.8% (91 patients) were on cancer treatment. Time from diagnosis of the cancer to study entry was 2.4 years (95% CI 1.9-3), with a median of 1.2 years. This information was not recorded in 18 patients (11.8%). No differences were observed in the time from cancer diagnosis between newly BTP diagnosed patient and patients on BTP follow-up (p=0.361).
Characteristics of BTP
A total of 69 patients (45.4%) presented their first BTP episode when they were included in the study (naïve). The mean number of episodes of BTP per day was 3.1(95% CI 2.8-3.4), with a median of three episodes. The mean duration of BTP was 30.6minutes (95% CI 24.8-36.4), with a median of 20 minutes. Table 1 summarizes the characteristics of BTP.
The treatment for BTP was in 81.2%(173 patients) fentanyl, morphine was administered to 8%(17 patients), metamizole in 4.2%(9 patients), tramadol in 2.8%(6 patients), oxycodone in 1.4%(3 patients), paracetamol in 1.4%(3 patients), and diclofenac and desketoprofen in 0.5%(1 patient). In nine patients the treatment was modified in the follow up to increase the dose or to use fentanyl.
Evaluation of symptoms intensity-ESAS scale
Prevalence of ESAS symptoms were: Pain 98%(144); tiredness 95.9%(141); nausea 56.8%(83); depression 74.8 (110); anxiety 74.8%(110); drowsiness 73.5%(108); lost appetite 88.4%(130); well-being affected 99.3%(146); dyspnea 46.9%(69); difficulty sleeping 82.3%(121). All ESAS symptoms were controlled in 3.4%(5) patients at baseline, and in 32.6(33) at one month of follow-up.
Comparing males and females at baseline visit, significant worst scores (p<0,01) were observed in females in tiredness (mean difference 2.1, 95% CI 1.2-3), nausea (mean difference 1.7, 95% CI 0.8-2.6), depression (mean difference 2, 95% CI 1-3), anxiety (mean difference 2.3; 95% CI 1.3-3.4), drowsiness (mean difference 1.4; 95% CI 0.5-2.2) and appetite (mean difference 1.3; 95% CI 0.2-2.3).
Figure 1 shows the mean ESAS scores at baseline visit and one month of follow-up. All the symptoms significantly improved in one month of follow-up (p<0.05) with no difference by gender. The differences were calculated in 136 valid cases.
Brief pain inventory (BPI)
The baseline mean score on the BPI pain intensity dimension was 4.9 points (95% CI 4.7-5.1). Significant reduction of the pain intensity dimension score was observed at the one-month visit, with a mean improvement of 1.8 points (95% CI 1.5-2.2), with 136 valid cases (p<0.0001). All items on BPI pain intensity dimension significantly improved (p<0,0001), maximum intensity in the last 24 hours (2.9; SD 2.6), minimum intensity in the last 24hours (1; SD 2.4), mean intensity (1.5; SD 2.2), and actual pain intensity (1.8; SD 3.2).
The baseline mean score on the impact on the daily living activities dimension of the BPI was 6.1 points (95% CI 5.8-6.5). The score also improved at the one-month of follow-up, with a mean of 1.7 points (95% CI 1.3-2.1), p<0.0001.
In patients with first episode of BTP and patients observed in the visit on follow-up, BPI evolution was compared. On baseline visit the scores were comparable on pain intensity BPI dimension (p=0.644) and impact on daily living activities BPI dimension (p= 0.187). In the BPI pain intensity dimension the improvement was significantly higher in naïve patients (p<0.0001) 1.5 points better (95% CI 0.8-2.2). In the BPI impact on daily living activities dimension the improvement was also significantly better for naïve patients, 1.5 points better (95% CI 0.8-2.3), p<0.0001.
EORTC QLQ-30 version 3 questionnaire
Figure 2 shows the scores on the five functional scale of the quality of life on baseline and one-month visit in BTP naïve patients and those on BTP follow-up, as well as the reference values for patients with any type of cancer (reference EORTC cancer patients, n= 6709) aged from 60 to 69 years [13,14]. The figures were calculated over 136 valid cases.
Global health status (quality of life) and all five EORTC QLQ-C30 questionnaire dimensions significantly improved in one-month of follow-up (p<0.001). A mean of 17% (95% CI 12.4-21.7) of improvement in EORTC-QLQ-C30 was observed. The improvement in quality of life in naïve patients was 24.5% (95% CI 18.5-30.5), and in patients in follow-up BTP the improvement was of 10.2% (95% CI 3.4-17).
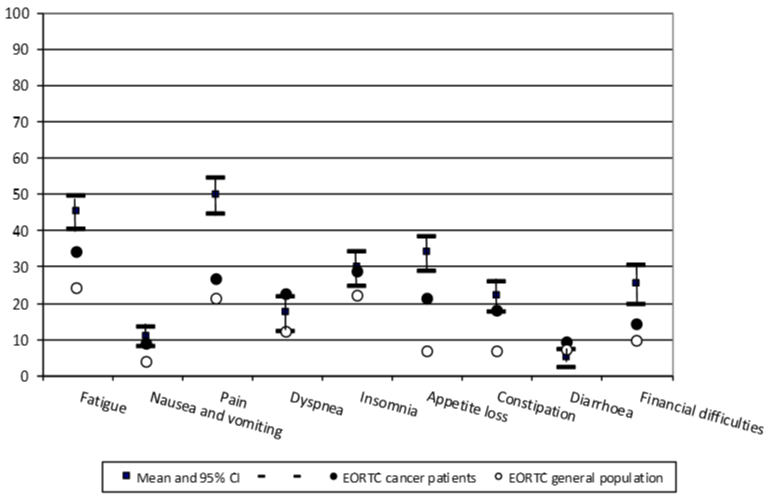
Figure 3 shows the scores on symptoms scales and financial difficulties at the one-month visit compared to the reference values for patients with any type of cancer (reference EORTC cancer patients, n=6709) aged from 60 to 69 years and general population scores (reference EORTC general population, n= 7802) [13,14].
Cost of illness (BTP) summary
In the previously published article about the study, a total BTP cost of 2941.60 euros was obtained, with 2572.50euros for direct medical costs (88% of the cost per patient), 5% (168.50euros) in direct nonmedical costs and 200.70euros for indirect costs due to loss of productivity (7% of the cost per patient) [12]. In the GLM analysis of the total BTP cost per patient adjusted by the co-variables, no significant differences were observed in demographic factors. As no relationship was observed between each symptom improvement from baseline and cost, these variables were not included in the equation. It was observed that the better the overall score on quality of life, the lower the cost of treatment of BTP in the patient. For every ten points of improvement in quality of life during the month of follow-up, the odds ratio was 0.91, and therefore the reduction in BTP cost was 9% (Table 3).
Discussion
Significant improvements in quality of life and symptoms control of cancer patients with BTP were observed in only one month of follow-up, after the protocolized intervention of pain and palliative care units, and the objective of this publication was to show the extent of this finding and the repercussion on BTP cost.
BTP was the illness of interest, and newly diagnosed BTP patients (naïve) could be included, but also those in BTP treatment follow-up. Differences between these groups were observed; naïve patients showed better quality of life improvements (Table 3). Patients on BTP follow-up may have more advanced cancer but no differences in time from cancer diagnosis were observed between them. As no information was collected about cancer stage, this could not be analyzed. Patients were ambulatory when selected, 73% had a Karnofsky of 60 or higher, and about 60% were on cancer treatment. This was an elderly population with a mean age of 66.8 years old, and the BTP characteristics (Table 1) were comparable with those already published [1,5,6].
Relating the intensity of symptoms measured by the ESAS scale, females showed worse baseline scores in tiredness, nausea, depression, anxiety, drowsiness and appetite. Perhaps this difference could be due to different cancer treatments in women, with special mention of aromatase inhibitors used for breast cancer. All ESAS symptoms significantly improved after one month of follow-up (p<0.05), and this reflects the good quality of care received by patients and maybe a good quality control indicator to be used in our consultations that can be easily used for this purpose (Figure 1). Depression, drowsiness, appetite and dyspnea significantly improved but without clinical relevance. The improvement in the control of all ESAS symptoms from 3.4% of patients to 32.3% after one month in our study was significant but less effective than the improvement observed in one study completed in palliative care units in cancer patients with BTP and similar characteristics and age, that changed from 2.5% to 52.6% [26]. The difference could be due to the number of participant units in our study (17) versus only one unit in the referenced study, where the procedures for symptoms management could be more consistent. The control of all ESAS symptoms was found as an independent factor related to quality of life improvement, so getting this control we could obtain better patient prognosis [26].
The Brief Pain Inventory was developed to measure pain control in cancer patients, but nowadays it is recommended for use in any clinical trial where chronic pain is evaluated (www.immpact.org) [27,28]. The two dimensions of the BPI significantly improved. Starting from homogeneous baseline scores, improvement was higher in BTP naïve patients than in BTP in follow-up. Perhaps this difference could be due to the adjustment of pain treatments in the first BTP visit.
EORTC-QLQ-C30 functional and symptom scores were improved in all patients but were greater in those who were BTP naïve (Figures 2,3). Improvements were clinically significant in the six functional scales, but small to moderate (Figure 2). In the symptom scales there were small improvements in tiredness, nausea and vomiting, insomnia, appetite and constipation, and the improvement in dyspnea and diarrhea had no clinical relevance. Improvements in pain were large (20.8%), (Figure 3). In only one month of follow-up global health status/quality of life in naïve patients improved to be like the EORTC cancer population of the same age group and also emotional and cognitive function improved to these terms (Figure 2). In fact, naïve patients got similar scores to those of the EORTC general population of any age in the emotional function and the cognitive function dimensions (Figure 2). The group of patients with BTP on follow-up also improved although after one month, scores were between 10%-30% lower than the reference population. This can be explained as these patients’ cancer could be more evolved. The differences with the reference EORTC scores could be explained by the presence of BTP in the patients of this study. It may also be due to different type of cancers, as in EORTC 21% were gastrointestinal, 17% lung, 8% breast, prostate 20%, and 54% metastatic, or due to shorter time of cancer evolution or different cancer stages that could explain the differences [14].
In terms of quality of life, a mean of 17% (95% CI 12.4-21.7) of improvement in EORTC-QLQ-C30 was observed and this was considered a moderate clinical improvement [13]. As for every ten points of improvement in quality of life during the month of follow-up, the odds ratio was 0.91(reduction in BTP cost 9%, Table 2), for the patients in the study the BTP cost reduction was about 15.3%, which means 450euros monthly. Now analyzing the improvement in quality of life in naïve patients was 24.5(95% CI 18.5-30.5), this was a large clinical improvement, and it represents 22.1% of reductions in BTP costs, that means 648.60 Euros monthly. On the other hand, for patients in follow-up BTP, the improvement was of 10.2(95% CI 3.4-17). This was considered a moderate clinical improvement and represented 9.18% in reductions of costs, or 270euros monthly on BTP treatment.
When intensity of symptoms is observed in Figure 3, compared to reference EORTC cancer patients, and with EORTC reference general population, the most marked differences in the study participants were shown in pain scores, perhaps this could be related to BTP control during the study [14].
One of the limitations of the study could be that patients with different cancer were included and in different stages. Although it is not described that BTP could be different depending of the cancer, this factor could affect the evolution of the quality of life and symptoms, but we had not enough sample to analyze this issue by cancer type. Although not described in this manuscript we did not found differences in pain intensity between different types of cancer. The short duration of the study could be a limitation, but we have been able to demonstrate that in only one month of intervention in pain and palliative care units, the quality of life and symptoms improve, so longer studies could analyze the evolution of this improvement. The strength is that we have observed the patients prospectively and collected the information about the resource use and pharmacological costs prospectively also.
In a recent pooled analysis in cancer patients, baseline EORTC global health status has been found as an independent prognostic indicator of survival (hazard ratio, 0.97; 95% CI 0.95-1; p<0.0001) [29]. In addition, dyspnea (hazard ratio 1.04; 95% CI 1.02-1.06; p<0.0002) and appetite loss (hazard ration 1.06; 95% CI 1.04-1.08; p<0.0001) were found independent prognostic factors. Regular quality of life and symptom assessments during treatment could be used for early detection of deterioration of patients and could allow timely additional interventions thereby ultimately improving quality of life and survival [29,30].
Conclusions
To conclude, we remark that cancer patients with BTP can improve their quality of life and cancer associated symptoms in only one month of follow-up, when they are treated in pain and palliative care units and that this improvement leads to significant cost savings. The systematic measurement of symptoms and quality of life again demonstrate its usefulness in controlling the best clinical and costs improvements.
Declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Hospital Universitario de La Princesa de Madrid (Spain) with code number 2488. All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee and with most recent version of the Helsinki declaration. Written informed consent was obtained from all individual participants included in the study.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
Antonio Javier Jiménez and Almudena Sanz-Yagüe belong to the Medical Department of Kyowa Kirin Farmacéutica, S.L.U.
Begoña Soler was hired by Concepción Pérez-Hernández and Kyowa Kirin Farmacéutica S.L.U., to conduct the design, monitoring, statistical analysis, and management of the publications of the study.
Funding
Concepción Pérez-Hernández received funding from Kyowa Kirin Farmacéutica, S.L.U. as an Investigator Sponsored Study, for the completion of the study and article processing charges.
Authors’ contributions
Concepción Pérez participated in the design, inclusion of patients and drafting of the paper. Antonio Javier Jiménez and Almudena Sanz-Yagüe revised critically the paper for intellectual content. Begoña Soler designed the study, complete the quality control and the statistical analysis of the study and drafted the paper. All authors approved the version to be published and agree to be accountable for all aspects of the work.
The participation of the following researchers in the study is appreciated: IMDI study group: Pilar Anadon Senac, Alvaro Deblas Sandoval, Adela Delgado Alvarez de Sotomayor, Ernesto Delgado Cidranes, Diego Díaz Cárdenas, Diego Díaz Rodríguez, Silvia Forcano Sanjuan, María López Gómez, Mercedes López Luque, Raquel Marsé Fabregat, Marcelino Mosquera Pena, Mercedes Mozas Calabaza, Ferran Nonell i Gregori, Miguel Ángel Núñez Viejo, Manuel Ojeda Martín, Rogelio Sánchez de las Matas Peña.
- Escobar Álvarez Y, Biete i Solà A, Camba Rodríguez M, Gálvez Mateos R, Mañas Rueda A, et al. (2013) Diagnóstico y tratamiento del dolor irruptivo oncológico: Recomendaciones de consenso. Med Paliat 20: 150-157. Link: http://bit.ly/2L5DcfP
- Deandrea S, Corli O, Consonni D, Villani W, Greco MT, et al. (2014) Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage 47: 57-76. Link: http://bit.ly/2NsiPfx
- Nekolaichuck CL, Fainsinger RL, Lawlor PG (2005) A validation study of a pain classification system for advanced cancer patients using content experts: the Edmonton Classification System for Cancer Pain. Palliat Med 19: 466-476. Link: http://bit.ly/2TqHxAf
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G, et al. (2009) The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 13: 331-338. Link: http://bit.ly/30vwuX3
- Mercadante s, Marchetti P, Cuomo A, Caraceni A, Mediati RD, et al. (2018) Factors influencing the clinical presentation of breakthrough pain in cancer patients. Cancer 10: pii: E175. Link: http://bit.ly/30cJ7XD
- Portenoy RK, Hagen NA (1990) Breakthrough pain: definition, prevalence and characteristics. Pain 41: 273-281. Link: http://bit.ly/2TYOVkp
- Fortner BV, Demarco G, Irving G, Ashley J, Keppler G, et al. (2003) Description and predictors of direct and indirect costs of pain reported by cancer patients. J Pain Symptom Manage 25: 9-18. Link: http://bit.ly/2R87bGT
- Fortner BV, Okon TA, Portenoy RK (2002) A survey of pain-related hospitalizations, emergency department visits, and physician office visits reported by cancer patients with and without history of breakthrough pain. J Pain 3: 38-44. Link: http://bit.ly/381SZ9w
- Abernethy AP, Wheeler JL, Fortner BV (2008) A Health Economic Model of Breakthrough Pain. Am J Manag Care 14: S129-S140. Link: http://bit.ly/39VUGXL
- Kuo KL, Saokaew S, Stenehjem DD (2013) The pharmacoeconomics of breakthrough cancer pain. J Pain Palliat Care Pharmacother 27: 167-175. Link: http://bit.ly/36WavvL
- Velucci R, Mediati RD, Gasperoni S, Piergallini LM, Valentino MC, et al. (2015) Pharmacoeconomic considerations about breakthrough cancer pain. Value in Health 18: A665-A666. Link: http://bit.ly/36MajPl
- Pérez-Hernández C, Jiménez-López AJ, Sanz-Yagüe A, Mar-Medina J, Larrañaga I, et al. (2018) Observational study evaluating the economic impact of breakthrough pain in cancer patients in clinical practice in Spain: The IMDI study. Pain Ther 7: 227-240. Link: http://bit.ly/36PiYR2
- Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, et al. (2001) On behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual (3rd Edition). Published by: European Organisation for Research and Treatment of Cancer Brussels.
- Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, et al. (2008) EORTC QLQ-C30 Reference Values. Link: http://bit.ly/3a5HDmK
- Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncology 2: 187-193. Link: http://bit.ly/2Tg2r4F
- Kelly AM (2001) The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 18: 205-207. Link: http://bit.ly/2QK3wQy
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7: 6-9. Link: http://bit.ly/3a16Qi2
- Badia X, Muriel C, Gracia A, Nuñez-Olarte JM, Perulero N, et al. (2003) Validation of the Spanish version of the brief Pain Inventory in patients with oncological pain. Med Clin (Barc) 120: 52-59. Link: http://bit.ly/2Nkmprc
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality of life scores. J Clin Oncol 16: 139-144. Link: http://bit.ly/2tMB7QZ
- Cocks K, King MT, Velikova G, de Castro G Jr, Martyn St-James M, et al. (2012) Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cance 48: 1713-1721. Link: http://bit.ly/2QLOmKT
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, et al. (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365-376. Link: http://bit.ly/388ZWWn
- Arrospide A, Soto-Gordoa M, Acaiturri T, López-Vivanco G, Abecia LC, et al. (2015) Coste del tratamiento del cáncer de mama por estadio clínico en el País Vasco. Rev Esp Salud Pública 89: 93-97. Link: http://bit.ly/2TfqHnD
- Spanish Cost Database (2019) e-salud- eSalud - Información económica del sector sanitario Oblikue Consulting. Link: http://bit.ly/36LpQyN
- Glick HA, Doshi JA, Sonnad SS, Polsky D (2014) Economic evaluation in clinical trials. Oxford; New York: Oxford University Press 272. Link: http://bit.ly/2TqJhth
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489-495. Link: http://bit.ly/2t0ptlF
- Núñez Viejo MA, Areses Manrique C, Iglesias Rey L, Iglesias Rey A, Jiménez López AJ, et al. (2019) Quantification of quality of life improvement after appropriate symptoms control in cancer patients with breakthrough pain: A four-week follow-up study in a palliative care unit. Trends Med 19: 4-8. Link: http://bit.ly/2sgZLZH
- Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, et al. (2003) Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 106: 337-345. Link: http://bit.ly/30cJIZn
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9-19. Link: http://bit.ly/2uHv9RV
- Ediebah DE, Quinten C, Coens C, Ringash J, Dancey J, et al. (2018) Quality of life as a prognostic indicator of survival: A pooled analysis of individual patient data from Canadian Cancer Trials Group Clinical Trials. Cancer 124: 3409-3416. Link: http://bit.ly/2Ng1CFu
- Ambroggi M, Biasini C, Toscani I, Orlandi E, Berte R, et al. (2018) Can early palliative care with anticancer treatment improve overall survival and patient-related outcomes in advanced lung cancer patients? A review of the literature. Support Care Cancer 26: 2945-2953. Link: http://bit.ly/3a2hXY0
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
