Open Journal of Pain Medicine
Clinical practice evaluation of opioids induced constipation management in patients with cancer: The EIO-50 project
Vicente Guillem1, Francisco Javier Salvador Bofill2, Margarita Feyjoo3, Antonio Antón4, Enrique Aranda5, Carlos Camps6, Alfredo Carrato7, Manuel Constenla8, Juan Jesús Cruz-Hernández9, Eduardo Díaz-Rubio10, Jesús García-Foncillas11, Pere Gascón12, Rafael López13, Begoña Soler14* and Yolanda Escobar15
2Medical Oncology Department, Hospital Virgen del Rocío, Sevilla, Spain
3Fundación ECO, Medical Oncology Department, Hospital Universitario La Moraleja. Sanitas, Madrid, Spain
4Fundación ECO, Medical Oncology Department, Hospital Universitario Miguel Servet, Zaragoza, Spain
5Fundación ECO, Medical Oncology Department, Hospital Reina Sofía, Córdoba, Spain
6Fundación ECO, Medical Oncology Department, Hospital General de Valencia, Universidad de Valencia, CIBERONC, Valencia, Spain
7Fundación ECO, Medical Oncology Department, Hospital Ramón y Cajal, Madrid, IRYCIS, CIBERONC, Universidad de Alcalá de Henares, Spain
8Fundación ECO, Medical Oncology Department, Hospital Provincial de Pontevedra, Spain
9Fundación ECO, Medical Oncology Department, Hospital Asistencial Universitario de Salamanca (CAUSA), Universidad de Salamanca (USAL), Instituto de Investigación Biomédica de Salamanca (IBSAL), Spain
10Fundación ECO, Vicepresident of the Royal Academy of Medicine, IdISCC, CIBERONC, Spain
11Fundación ECO, Medical Oncology Department, Fundación Jiménez Díaz, Madrid, Spain
12Fundación ECO, Director Laboratory of Molecular & Translational Oncology-CELLEX, Madrid, Spain
13Fundación ECO, Medical Oncology Department, Hospital Clínico Universitario e Instituto de Investigación Sanitaria de Santiago de Compostela, CIBERONC, Spain
14E-C-BIO, S.L., Medical Department, Las Rozas (Madrid), Spain
15Department Medical Oncology, Hospital Universitario Gregorio Marañón, Madrid, Spain
Cite this as
Guillem V, Salvador Bofill FJ, Feyjoo M, Antón A, Aranda E, et al. (2019) Clinical practice evaluation of opioids induced constipation management in patients with cancer: The EIO-50 project. Open J Pain Med 3(1): 034-040. DOI: 10.17352/ojpm.000016Introduction: Opioid-Induced Constipation (OIC) is a common distressing symptom associated with cancer pain treatment. Consensus guidelines for management of OIC are limited and little is known about the decision making of Health Care Professionals (HCP) for the management of OIC. The aim of this study was to learn about the diagnostic and treatment criteria of OIC management in clinical practice in patients with cancer.
Methods: A survey was designed with 50 OIC specific questions and was completed by 135 HCP: 122 medical oncologists (90.4%) and 13 palliative care specialists (9.6%).
Result: OIC was considered a relevant side effect by 97% of the specialists. HCP declared differences in the characteristics of OIC depending on whether the patients were treated with major or minor opioids: the percentage of patients developing OIC (54.5% vs 29.8%), OIC intensity (severe in 17% vs 10.8%) and the time to symptoms onset in 1-4 weeks (92.6% vs 73.3%). Only 14% HCP used algorithms for the diagnosis or treatment of OIC. Healthy life-style was considered first-line treatment for OIC by 85.2%. Laxatives were prescribed by 99.3% of HCP, but 32.6% of patients did not achieve a response. Peripherally Active µ-Opioid Receptor Antagonists (PAMORAs) were considered the most effective specific treatment of OIC. Naloxegol was the PAMORA most often used for the treatment of OIC in patients with cancer.
Conclusion: The narrow effectiveness of traditional therapies and the emergence of more effective pharmacological approaches suggest the need for standardized and updated guidelines for OIC diagnosis and management in patients with cancer.
Abbreviations
BFI: Bowel Function Index; ESMO: European Society for Medical Oncology; HCP: Health Care Professional; OIC: Opioid-Induced Constipation; PAMORA: Peripherally-Acting μ-Opioid Receptor Antagonists; WHO: World Health Organization.
Introduction
Pain is the main reason for consultation among patients with cancer. Pain occurs in 50% of all patients with cancer, and this figure may increase to 70-90% as the disease progresses [1]. The recommendations of the World Health Organization (WHO) pain management ladder should be followed for the appropriate treatment [2]. The use of second and third step opioids in patients with cancer is increasingly common due to the greater pain intensity experienced by an increasingly larger number of patients with cancer, the growing number of long-surviving patients with chronic residual pain, the availability of new opioids and improved knowledge of their use by the health care professionals (HCP) [3].
Opioid use for months or even years is often seen in clinical practice in patients with cancer. The growing use of opioids also increases the incidence of their adverse effects, with gastrointestinal disorders being the most common problem [4-6]. Such side effects may include dry mouth, oesophageal reflux, nausea, vomiting, abdominal discomfort, swelling, abdominal pain and symptoms of constipation (straining, hard stools, sensation of incomplete evacuation, rectal tenesmus, pain during bowel movements, etc.). Constipation is the most common and generally also the most bothersome symptom, forming part of what is known as opioid-induced constipation (OIC) [5,7].
OIC can occur from the start of treatment and may persist for the full duration of therapy. Unlike other adverse effects caused by opioids such as nausea, vomiting, and sedation that disappear over time, OIC is not characterized by the development of tolerance [7]. In a large percentage of cases, the adoption of hygiene-dietary measures and the use of laxatives are unable to achieve symptom relief with the only effective management measure being opioid withdrawal.
The medical definition of OIC has been introduced only very recently (2016) [8-9]: A change in bowel habit and defecation pattern when opioid treatment is started, characterized by any of the following conditions: decreased stool frequency; development or worsening of straining; sensation of incomplete evacuation; or patient perceived alterations related to bowel habit.
No specific international guidelines are currently available for the management of OIC in patients with cancer. The guidelines for the management of constipation in the palliative care setting were published in 2008 [10]. In Spain the clinical practice guide “Irritable bowel syndrome with constipation and functional constipation in adults” was produced with the participation of different scientific societies, and which has already been updated in line with the new Rome IV criteria, but do not addressed OIC management in patients with cancer [11]. The European Society for Medical Oncology (ESMO) has recently published a guide for the management of constipation in patients with cancer [12]. However, the physiopathology of functional constipation and OIC is different and therefore require different approach. Despite the growing interest on the morbidity of OIC and the development of new therapeutic options for OIC, there is still a need for specific and updated recommendations for the management of OIC in patients with cancer. In this context, the present study was designed with the primary objective of assessing how HCP manage OIC in patients with cancer in our setting.
Materials and Methods
A survey was designed with 50 specific closed questions related to OIC in 8 pages. The scientific committee of the study selected the questions of the survey based as far as possible on clinical recommendations and routine clinical practice. Questions referred to the diagnosis of OIC and current treatment of OIC were selected [8-11].
The Clinical Research Ethics Committee of Hospital Universitario Puerta de Hierro (Majadahonda, Madrid, Spain) approved the study (13 February 2016). The survey was completed between March 9, 2017, and May 21, 2017.
A convenience sample of 138 HCP from Oncology or Palliative Care Units with previous experience in OIC management in patients with cancer were involved. The response rate was 135/138 (97.8%). The participants were selected by the sponsor from a database of Spanish hospitals, and contacted in personal visits, where the questionnaire was supplied. The participants signed an informed consent form to be part of the study and completed a registry form where a personal password was given. The participants transcribed the answers into the study-specific website, with restricted and individual access. This was a voluntary and closed password- protected survey. Consistency and completeness checks were programmed by JAVA Script highlighting items before submission. A monetary incentive was offered after completing the survey.
A descriptive analysis was completed of the variables included in the study, based on the distribution of frequencies and calculation of percentages for qualitative variables, and the calculation of the mean, 95% confidence interval and median for the quantitative variables. The SPSS 25.0 statistical package was used for the analysis.
Results
Type of centres
A total of 135 HCP from 39 provinces in 16 out of 17 Spanish autonomous communities participated in this study. The response rate was 135/138 (97.8%).
Of these participants, 90.4% (n=122) were medical oncologists, and 9.6% (n=13) from Palliative Care Units, working in public care centres (91.1%, n=123), mixed centres (5.9%, n=8) and private centres (3%, n=4).
The physicians had a mean of 12.2 years of experience in pain treatment (95% CI 11.3-13.1). According to the results of this survey, the HCP prescribed analgesic treatment in the form of major opioids to an average of 36 patients a month (95%CI 30-41). About 44.5% (95% CI 40.4-48.7) of the patients attended in one month of full activity, received treatment with major opioids. The prescription of minor opioids was an average of 15 patients a month (95%CI 10-19) representing a total of 18.6% of attended patients (95%CI 16.3-21).
According to 71.2% of participants, the mean length of treatment with opioids in patients with cancer was over 6 months. A smaller proportion (25.2%) reported an estimated duration of 3-6 months, while 3.7% reported a duration of less than three months.
Characteristics of OIC
According to the participants, OIC developed in 54.5% of all patients with cancer receiving major opioids and in 29.8% of those receiving minor opioids. In addition, 61.5% of the participants (n=83) considered that the symptoms of OIC differed depending on the analgesic potency of the opioid. In most patients, the symptoms of OIC appear within the first month of opioid therapy (Table 1). Indeed, 40.7% of the HCP (n=55) considered that the symptoms could develop in the first week of treatment with major opioids. Table 1 shows the characteristics of OIC reported by the participants according to whether the patients were receiving treatment with major or minor opioids.
Management of OIC
97% of the HCP (n=131) considered OIC to be an important health issue, with a mean attributed importance score of 7.8 points (95%CI 7.5-8.1) on a ten-point scale. In addition, 69.6% of the participants (n=94) recognized OIC as a new gnoseological entity.
A total of 85.9% (95%CI 83.9-87.8) of the patients with OIC were treated directly by the oncologists themselves, without referral to other specialists or health professionals. In this regard, it was seen that in 57.8% of the sites (n=78), the nursing staff did not participate in the evaluation and management of patients with OIC. Most of the HCP (96.3%; n=130) routinely reported the possible occurrence of OIC to patients starting treatment with opioids. The HCP proactively asked in 71.4% of the cases (95%CI 66.7-76) about bowel habits.
Diagnosis and assessment of OIC
Only 14.8% of the participants (n=20) used diagnostic algorithms, and of these, the most widely used were the Rome criteria (n=7). Despite this, the HCP (n=116) considered OIC to be well diagnosed in 60.9% of the cases (95% CI 57-64.7). Most of the investigators requested complementary tests for the diagnosis of OIC: imaging techniques (37.3%), anorectal exploration (24%) and laboratory tests (12.9%). In 18.4% of the cases no tests were requested, since the patients were considered adequately evaluated.
More than half of the investigators (62.9%; n=85) did not used assessment tool for the evaluation of their patients with OIC. Among those who used assessment tools, the Bristol scale (21.1%), the Rome criteria (16.8%) and the Bowel Function Index (BFI) (4.3%) were the most frequently reported. Despite such low frequency of use, 59.3% of the investigators (n=80) claimed to know the Rome criteria.
Treatment of OIC
Only 14.1% of the HCP (n=19) used an algorithm for the treatment of OIC.
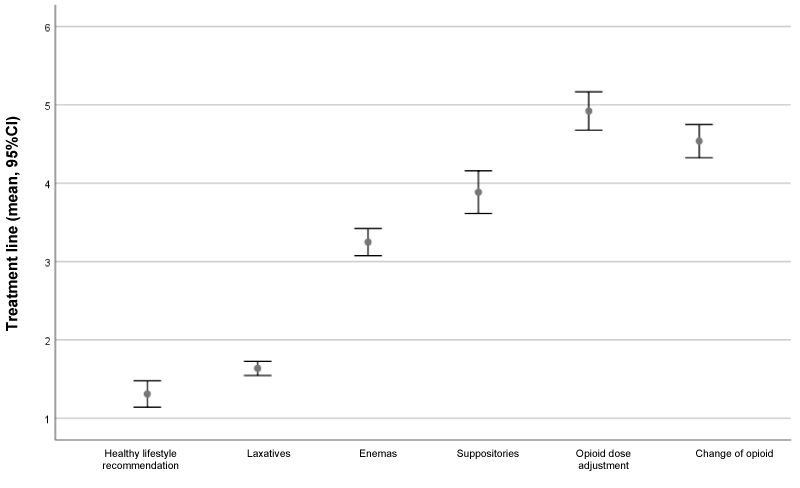
The most commonly used therapeutic measures and their order of use according to the investigators are shown in Figure 1. Table 2 shows the proportion of patients failing to respond to each treatment or intervention for the management of OIC.
Recommendations for a healthy lifestyle (adequate hydration, dietary fibre intake, regular meal times and physical activity) were regarded as first-line treatment by 85.2% of the participants (n=115).
99.3% of the HCP (n=134) prescribed laxatives, as first-line treatment by 38.5% (n=52) and as second-line by 59.3% (n=80). 58.5% of the participants (n=79) considered osmotic laxatives to be the most effective option in OIC, followed by stool softening laxatives (13.3%; n=18), stimulating laxatives (11.1%; n=15) and bulk-forming laxatives (6.7%; n=9). 10.4% of the participants (n=14) considered none of the laxative mechanisms of action to be effective for the treatment of OIC. Laxative use in monotherapy for OIC was recommended by 71.1% (n=96), two laxatives by 25.9% (n=35) and three laxatives by 3% (n=4).
Most of the participants (97%; n=131) sometimes prescribed enemas for patients with OIC, while 2.2% did so in all cases, and 0.7% never prescribed enemas.
Opioid dose adjustment was scarcely used to control OIC in patients with cancer in only 20.1% of the patients on average.
Eighty percent of the investigators considered the new drug therapies, specifically PAMORAs (peripherally-acting μ-opioid receptor antagonists), to be a good alternative for the treatment of OIC in patients with cancer. A total of 48.9% (n=66) prescribed PAMORAs, with naloxegol being the most widely used drug of this class (92.4%). A total of 34.1% (n=46) of the participants had been able to assess the efficacy of PAMORAs. Of these, 78.3% considered that over 50% of the patients responded. On the other hand, 66% (n=89) had not been able to assess the proportion of patients who failed to respond to PAMORAs.
Efficacy of OIC treatment
The mean number of bowel movements per week below which OIC treatment was considered ineffective was estimated to be 3.3 (95%CI 3.1-3.5).
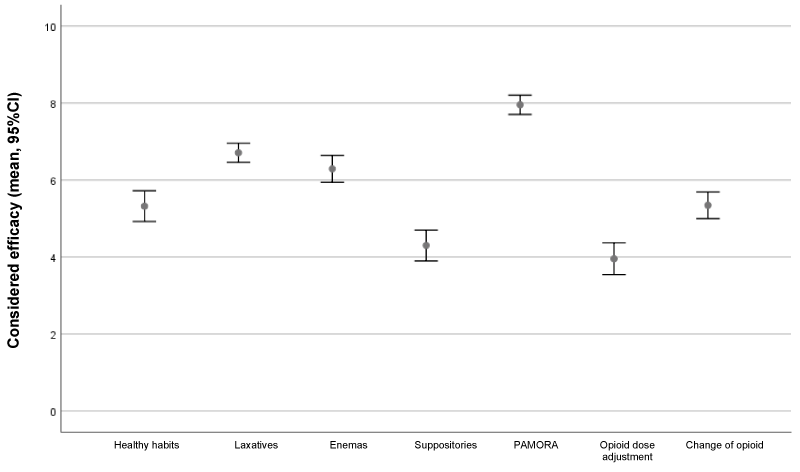
The use of PAMORAs was considered the most effective treatment option for OIC in patients with cancer, with a score of 8/10. Figure 2 shows the results referred to assessment of the efficacy of the treatments and interventions for the management of OIC.
Discussion
The EIO-50 project reveals how HCP deal with OIC in patients with cancer in clinical practice, evaluating the diagnostic process, clinical assessment and current treatment of OIC. From the results we remark that there is a need for updated international and national clinical guides to standardize both the diagnosis and treatment of OIC in patients with cancer. The use of OIC diagnostic criteria and assessment tool among HCP in clinical practice would help minimize the burden of OIC in patients with cancer.
Until very recently, the management of OIC was based on recommendations for the treatment of functional constipation. In the last years, major contributions have been made to characterize this clinical entity and to elucidating its complex pathophysiology differentiating it from other causes of constipation, thus leading to the development of new, more specific therapeutic options targeted to the cause underlying OIC like PAMORAs [9,13-15].
The Rome IV criteria presented the OIC as a new clinical entity for the first time, differentiating it from functional constipation [9, 15]. However, increasing clinician awareness of OIC need to be fostered since, despite 69.9% of the participants acknowledged OIC as a new entity, only 59.3% were aware of the Rome criteria, and very few (14.8%) used diagnostic criteria to identify OIC in their clinical practice.
As evidenced by most of the participants (97%), OIC is a serious health issue. This is particularly so in patients with cancer, who are already affected by a neoplastic disease itself [16,17].
OIC can affect many patients with cancer receiving analgesic treatment in the form of major and minor opioids. A broad range of prevalence has been published, from 50% to over 90% [18] or even higher (70-94%) among hospitalized patients [4, 19]. In this study, the participants considered that 54.5% of all patients with cancer treated with major opioids developed OIC. This may be underestimated, since very few HCP used diagnostic criteria to identify their patients. Controversies on OIC prevalence has been described earlier [15,19].
OIC is attributed to activation of enteric μ-opioid receptors, which decrease bowel tone and contractility and increase colonic fluid absorption and anal sphincter tone while reducing rectal sensation. This leads to harder stools, which can be difficult to pass. In patients with cancer, OIC is often under-diagnosed due to the common opioid induced adverse effects are commonly accepted by medical physicians to get an effective analgesic treatment.. Both major and minor opioids can cause OIC [13,15]. However, the participants considered that patients receiving minor opioids presented a lower incidence of OIC (29.8%) and milder symptoms than patients receiving major opioids. A possible explanation for this could be related to the characteristics of the patients and the underlying disease condition. Constipation in patients with cancer pain is multifactorial, and several causes may concur in one patient [16, 17, 20]. Constipation may also be due to the cancer itself, the cancer treatment provided, or other conditions inherent to the patient and unrelated to the neoplastic disease. Patients treated with major opioids usually experience more severe pain and are often at more advanced stages of the disease, with greater impairment of their general condition. Under these conditions, the probability of experiencing constipation due to other causes in addition to OIC increase [9].
Patients with OIC need to be regularly evaluated. OIC symptoms may be assessed using validated tools that are reliable and easy to use. In this regard, the Bowel Function Index (BFI) is one of the most widely recommended tool due to its simplicity and applicability in clinical practice [15,21]. For a more comprehensive evaluation, the BFI may be complemented with additional items such as the number of spontaneous bowel movements, or stool consistency (Bristol scale). In the present study, 62.9% of the investigators did not use any assessment tool. The Bristol scale was the most widely used (21.1%), and only 4.3% of the HCP used the BFI. The adoption of assessment tools in routine clinical practice should be encouraged for a more effective OIC approach.
Only 14.1% of the participants used an algorithm for the treatment of OIC. The prophylactic use of laxatives and a healthy lifestyle are recommended for the prevention of OIC in patients with cancer [12,15]. In this study, 85.2% of the participants cited a healthy lifestyle as first-line treatment but it was considered to be ineffective in 52% of the cases.
A total of 99% of participants prescribed laxatives for the treatment of OIC. Despite their widespread use, laxatives are often not effective, and patients are not satisfied with the results of the treatments prescribed [5,19,22]. In the present study, the investigators considered that 33% of the patients did not respond to laxatives.
At present, no laxative has been shown to be more effective than any another for the treatment of OIC [23], and randomized clinical trials with laxatives for the treatment of OIC are very limited [24,25]. Bulk-forming laxatives and fibre intake are not recommended for the treatment of OIC, because they can cause intestinal obstruction [26]. Furthermore, sugars and sugar alcohols such as lactulose, lactose and sorbitol should not be used in patients with OIC, since sugar and sugar alcohol metabolism by the intestinal microbiota generates short-chain carbonic acids and gas, and the consequent bloating effect can aggravate the distension caused by OIC [27].
About 97% of the participants sometimes prescribed enemas, and 57% recommended their use on an occasional basis. Enemas are recommended in the case of faecal impaction but are not advised in patients with cancer at risk of suffering thrombocytopenia or leukopenia, or in individuals with recent pelvic radiotherapy or colorectal or gynaecological surgery. Likewise, they are not recommended in cases of paralytic ileus, intestinal obstruction or rectal or anal trauma [12,28].
The impact of OIC on the quality of life of patients with cancer is significant. Patients often preferred to discontinue opioid use to avoid constipation, thus sacrificing pain control [16]. It has been described that up to one third of all opioid-treated patients do not take their opioid dose, or they decrease it, because of the intestinal effects [5]. Decreasing the opioid dose does not relief the symptoms and interferes with pain control - since the dose at which constipation occurs is approximately four times lower than the dose required to produce analgesic effects [29]. In our study, the investigators recommended a dose reduction in 20.1% of the patients, though they considered the efficacy of this measure to be low (Figure 2).
The introduction of PAMORAs as the only treatment option acting directly on the cause of OIC offers new hope for patients who fail to respond to the traditional treatments. These new drugs do not act at central level and therefore do not affect opioid analgesic efficacy. Most of the HCP (80%) considered PAMORAs to be a good alternative for the treatment of OIC in patients with cancer, and described them as the most effective option, with a score of 8/10 points. The low use of PAMORAs observed during the study (49%) is probably due to the fact that access to PAMORAs in Spain was limited at the time of the study; naloxegol was the PAMORA most frequently used for the treatment of OIC in patients with cancer in Spain (92.4%). Naloxegol is a pegylated naloxone derivative indicated for the treatment of OIC in adult patients with cancer and non-cancer pain who fail to respond adequately to laxative(s) [30].
The study conducted has the limitations inherent to a survey, in which the results correspond to physician recall based on their personal experience, so the results cannot be conclusive but can serve to explore practical needs in the knowledge and management of OIC. To our knowledge, this was the first survey conducted with specialists in Spain relating the management of OIC in patients with cancer. Next steps should be to explore real data related to OIC management from patients treated by the specialists, and to compare them with the physician’s recall data.
Conclusion
The EIO-50 project reveals the routine clinical practice in the management of OIC in the Spanish oncological setting. Despite OIC is regarded as a serious condition by most specialists and can affect many patients with cancer receiving analgesic treatment with major and minor opioids, OIC diagnosis and assessment are frequently suboptimal. Furthermore, the conventional treatments are considered ineffective in a large percentage of cases, whereas the emerging therapies targeting the origin of OIC, such as PAMORAs, are regarded as more effective options for OIC. Taken together, a paradigm shift is expected, and need to be included in updated clinical guidelines with specific recommendations for the diagnosis, assessment and treatment of OIC in patients with cancer.
Conflict of interest
VG, SB, MF, AA, AC, MC, JJCH, JGF, PG, RL and YE declared no conflict of interest. EA declared an advisory role outside the submitted work with Amgen, Bayer, Celgene, Merck, Roche and Sanofi. CC declared an advisory role outside the submitted work with BMS, Roche, Bayer and Angelini, and research funding by BMS, Astra Zeneca and Sysmex. EDR declared an advisory role outside the submitted work with Merck-Serono, Amgen, Bayer, Genomica, Servier and MSD. BS was contracted by Fundación ECO.
This study was sponsored by ECO Foundation (Excellence and Quality in Oncology), Fundación ECO, Madrid, Spain that received a grant from Kyowa Kirin Farmacéutica, S.L.U. for the study.
The participation of the following researchers in the study is appreciated: Investigators of the study EIO-50: Alfonso Acero Caballero; Ana Albert Balaguer; Vicent Alcolea Fuster; Iñaki Alvarez Busto; Renata Carola Alvarez Llosa; Margarita Alvaro Pardo; Eider Azkona Uribelarrea; Lorena Bellido Hernández; Gretel Benítez López; Reyes Bernabe Caro; Isabel Blancas López-Barajas; Ana Blasco Cordellat; Sara Blasco Mollá; Pablo Borrega García; Miguel Ángel Cabrera Suárez; Núria Calvo Vergés; Marc Campayo Guillaumes; Arturo Candal Gómez; Francisco Carabantes Ocon; Núria Cárdenas Quesada; Fernando Carmona Espinazo; Alberto Carral Maseda; Sergio Carrera Revilla; Raúl Carrillo; Victoria Casado Echarren; Javier Cassinello Espinosa; Beatriz Castelo Fernandez; Diego Cayuela Lopez; Sara Cerezo Gonzalez; Raquel Cervera Calero; Beatriz Cirauqui Cirauqui; Manuel Constenla Figueiras; Manuel Conti Jimenez; Nazaret Cordero Franco; Juan Felipe Cordoba Ortega; Almudena Cotes Sanchís; Sara Cros Costa; Sara Custodio Cabello; Cristina Victoria Del Pino Hernández; Lorena Del Rio Pazos; Ignacio Delgado Mingorance; Arancha Dueñas Comino; Sara Estalella Mendoza; Emilio Esteban González; María Aránzazu Fernández Orgiler; Marta Ferrer Cardona; María José Flor Oncala; José Fuentes Pradera; José Fuster Salva; María Isabel Gallegos Sancho; Laura Gálvez Carvajal; Silvia García Adrián; María Paola García Coves; Regina García Galindo; Jorge García González; Francisco Javier García Navalón; Emilio Julio García Ortega; José García Sánchez; Nieves Gascón Costoso; Ariadna Gasol Cudós; María Regina Gironés Sarriá; Ana Godoy Ortiz; María De Las Nieves Gómez Camacho; Roberto Gómez Diaz; César Gómez Raposo; Beatriz González Astorga; Inés González Barrallo; Marta González Cordero; Alejandro González Forastero; María Belén González Gragera; Raquel Guardeño Sánchez; Plácido Guardia Mancilla; María Guirado Risueño; Carmen Hererro Vicent; Alba Hernández García; Ana Herrero Heras; Iratxe Intxaurbe Echevarría; Berta Maria Jiménez Rubiano; Asunción Juárez; Francisco Javier Lacueva Guallar; Rodrigo Lastra; Martín Lázaro; Guillermo López Vivanco; Julián Lorca Chapa; Natalia Lupion Morales; Julia Madani Pérez; Margarita Majem Tarruella; Aranzazu Manzano Fernández; Gema Marín Zafra; Braulio Martín Calero; Lola Martín De Barberá; Mireia Martinez Kareaga; Natividad Martinez-Banaclocha; María Miranda Serrano; Fernando Molano Criollo; Raquel Molina Villaverde; Sofía Maria Montenegro Luis; Ángela Moreno Martí; Diana Moreno Muñoz; Idoia Morilla Ruiz; José Muñoz Langa; Adolfo Murias Rosales; Esteban Nogales Fernández; Beatriz Nuñez García; Sebastián Ochenduszko; Amaya Olaverri Hernández; Francisco Jesús Olmo Montes; Santiago Olmos Antón; Ana Laura Ortega Granados; Eugenio Palomares García; Juan Gabriel Pérez De Miguel; Núria Piera Molons; María Inmaculada Raja Casillas; Avinash Ramchandani Vaswani; María Ramírez Rotger; Daniel Ramos Pollo; Silvia Remesal Blanco; Alejandra Rodríguez Capote; María Purificación Rodríguez Cernuda; Mercedes Rodríguez Garrote; Dulce Rodríguez Mesa; Silverio Ros Martínez; Fernando Rosillo Fernández; Isabel Ruiz Cabrero; Pedro Sánchez Mauriño; José Luis Sánchez Sánchez; Inmaculada Sánchez Simón; Sergio Sandiego Contreras; Salvador Saura Grau; Tamara Sauri Nadal; Olbia Serra Solá; Garbiñe Unanue Oyarbide; Cristina Vicente Martin; Vicente Villarreal Rivas; Gemma Viñas Villaro; Marta Zafra Poves.
- Virizuela JA, Escobar Y, Cassinello J, Orrega P, SEOM (Spanish Society of Clinical Oncology) (2012) Treatment of cancer pain: Spanish Society of Medical Oncology (SEOM) recommendations for clinical practice. Clin Transl Oncol 14: 499-504. Link: http://bit.ly/2PqyA7t
- Organización Mundial de la Salud (OMS) 1996 (2019) Documento de Ginebra. 2ª ed. Alivio del dolor en el cáncer. Link: http://bit.ly/2Pvkbae
- Cicero TJ, Inciardi JA, Muñoz A (2005) Trends in abuse of oxycontin and other opioid analgesics in the United States: 2002-2004. J Pain 6: 662-672. Link: http://bit.ly/2PT7ftG
- Sykes NP (1998) The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 12: 375-382. Link: http://bit.ly/2PRpaRx
- Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, et al. (2009) The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 10: 35-42. Link: http://bit.ly/2M1Ka6G
- Galvez R, Provencio M, Cobo M, Pérez C, Pérez C, et al. (2014) Prevalencia y severidad de la disfunción intestinal inducida por opioides. Aten Primaria 46: 32-39. Link: http://bit.ly/36NWfol
- Panchal SJ, Müller-Schwefe P, Wurzelmann JI (2007) Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 61: 1181-1187. Link: http://bit.ly/2YUZlUv
- Drossman DA, Hasler WL (2016) Rome IV – Functional GI Disorders: Disorders Gut-Brain Interaction. Gastroenterology 150: 1257-1261. Link: http://bit.ly/2En2enB
- Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, et al. (2016) Bowel Disorders. Gastroenterology 150: 1393-1407. Link: http://bit.ly/36NWnnP
- Larkin PJ, Sykes NP, Centeno C, Ellershaw JE, Elsner F, et al. (2008) The management of constipation in palliative care: clinical practice recommendations. Palliat Med 22: 796-807. Link: http://bit.ly/36IpU1U
- Mearin F, Ciriza C, Mínguez M, Rey E, Mascort JJ, et al. (2016) Clinical Practice Guideline: Irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enf Dig 108: 332-363. Link: http://bit.ly/2Pqekmi
- Larkin PJ, Cherny NI, La Carpia D, Guglielmo M, Ostgathe C, et al. (2018) Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol 29:iv111-iv125. Link: http://bit.ly/38UUebM
- Poulsen JL, Brock C, Olesen AE, Nilsson M, Drewes AM (2015) Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap Adv Gastroenterol 8: 360-372. Link: http://bit.ly/2M36PQ7
- Jara C, Del Barco S, Grávalos C, Hoyos S, Hernández B, et al. (2018) SEOM clinical guideline for treatment of cancer pain (2017). Clin Transl Oncol 20: 97-107. Link: http://bit.ly/2sxDjv1
- Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, et al. (2014) Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 26: 1386-1395. Link: http://bit.ly/2rKEY0q
- Rumman A, Gallinger ZR, Liu LWC (2016) Opioid induced constipation in cancer patients: pathophysiology, diagnosis and treatment. Expert Rev Qual Life Cancer Care 1: 25-35. Link: http://bit.ly/38LpTw9
- Abramowitz L, Beziaud N, Labreze L, Giardina V, Caussé C, et al. (2013) Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: a cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J Med Econ 16: 1423-1433. Link: http://bit.ly/2PTjpCH
- Coyne KS, Sexton C, Lo Casale RJ, King FR, Margolis MK, et al. (2016) Opioid-induced constipation among a convenience sample of Patients with cancer Pain. Front Oncol 6: 131. Link: http://bit.ly/35pAWsK
- Droney J, Ross J, Gretton S, Welsh K, Sato H, et al. (2008) Constipation in cancer patients on morphine. Support Care Cancer 16: 453-459. Link: http://bit.ly/2M37cdt
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, et al. (2001) Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 93: 247-257. Link: http://bit.ly/34tcSnA
- Argoff CE, Brennan MJ, Camilleri M, Davies A, Fudin J, et al. (2015) Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Medicine 16: 2324-2337. Link: http://bit.ly/2Ps69Wx
- Freedman M, Schwartz H, Roby R, Fleisher S (1997) Tolerance and efficacy of polyethylene glycol 3350/electrolyte solution versus lactulose in relieving opiate induced constipation: a double-blinded placebo-controlled trial. J Clin Pharmacol 37: 904-907. Link: http://bit.ly/38MuvSP
- Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, et al. (2014) American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Ame J Gastroenterol 109: S2-S26. Link: http://bit.ly/38Kyf7g
- Candy B, Jones L, Goodman ML, Drake R, Tookman A (2011) Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database of Syst Rev CD003448. Link: http://bit.ly/35tDjLf
- Kumar L, Barker C, Emmanuel A (2014) Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract 2014: 141737. Link: http://bit.ly/36BL9Cy
- Müller-Lissner S, Bassotti G, Coffin B, Drewes AM, Breivik H, et al. (2017) Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med 18: 1837-1863. Link: http://bit.ly/34oDGFD
- Emmanuel A, Johnson M, McSkimming P, Dickerson S (2017) Laxatives Do Not Improve Symptoms of Opioid-Induced Constipation: Results of a Patient Survey. Pain Medicine 18: 1932-1940. Link: http://bit.ly/2r0eA2b
- Instituto Nacional del Cáncer (INC) (2016) Complicaciones gastrointestinales (PDQ®)–Versión para profesionales de salud. Link: http://bit.ly/38NKVdl
- Green AF (1959) Comparative effects of analgesics on pain threshold, respiratory frequency and gastrointestinal propulsion. Br J Pharmacol Chemother 14: 26-34. Link: http://bit.ly/36Sj6iD
- Chey W, Webster L, Sostek M, Lappalainen J, Barker PN, et al. (2014) Naloxegol for opioid-induced constipation in patients with non-cancer pain. N Engl J Med 370: 2387-2396. Link: http://bit.ly/2LZExpW
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
