Open Journal of Pain Medicine
Consensus statement on the management of breakthrough cancer pain: Assessment, treatment and monitoring recommendations
María Dolores López Alarcón1*, Francisco Villegas Estevez2, Vicente Domingo Triado3, Pilar Blasco Segura1, Genoveva Hernández Comes4, Alfonso Berrocal Jaime1, Ana Blasco Cordellat1, Jorge Pastor Peidro1, José López Torrecilla1, Carlos Ferrer Albiach2, Almudena Ruiz Sastre1 and Manuel Hernández Peris3
2Consorcio Hospital General de Castellón, Spain
3Hospital Lluis Alcanyis. Xátiva. Valencia, Spain
4Hospital Doctor Peset. Valencia, Spain
Cite this as
López Alarcón MD, Estevez FV, Triado VD, Segura PB, Comes GH, et al. (2019) Consensus statement on the management of breakthrough cancer pain: Assessment, treatment and monitoring recommendations. Open J Pain Med 3(1): 008-014. DOI: 10.17352/ojpm.000011Objective: Breakthrough cancer pain (BTcP) is still underestimated and a percentage of patients are not adequately treated, affecting their quality of life. The aim of this review was to generate a series of expert recommendations based on clinical experience and current scientific data on the management of BTcP.
Methods: An interdisciplinary group of Spanish pain experts held face-to-face meetings to review existing protocols and the relevant scientific literature on the management of BTcP, sharing their clinical experience. The outcome was a consensus document, providing practical guidelines and suggestions on the diagnosis, treatment and follow-up of BTcP patients.
Results: The following recommendations were generated to assist healthcare professionals in their therapeutic decision-making in BTcP patients: personalized evaluation of the BTcP characteristics is recommended to implement the best possible therapeutic approach; the treatment plan for BTcP should also combine preventive measures with pharmacological and non-pharmacological methods, and should be individualized according to the patient’s characteristics, preferences and personal circumstances; and finally, a multidisciplinary team approach together with adequate patient health education are essential to prevent underdiagnosis and sub-optimal treatment.
Conclusions: These consensus recommendations based on recent scientific evidence will help healthcare professionals in assessing, treating and monitoring BTcP in oncology patients.
Abbreviations
BTcP: Breakthrough Cancer Pain; VAS: Visual Analogue Scale; NICE: National Institute for Health Care Excellence; ROOs: Rapid-Onset Opioids; ATC: Around the Clock.
Introduction
Pain is a complex health condition that affects both mental and physical abilities. It can be classified into acute pain (provoked by a specific disease or injury and lasting less than three months) and chronic pain (lasting more than three months, often idiopathic). Chronic pain is one of the most common reasons for seeking medical care, affecting between 2% and 40% of the adult population worldwide [1].
Cancer pain is a type of chronic pain, with 35% of oncology patients experiencing it at diagnosis [2]. Its pathophysiology is complex: it is mainly caused by the primary tumor, followed by associated cancer therapies including surgery, radiation therapy, chemotherapy, targeted therapy, supportive care therapies, and/or diagnostic procedures [3,4]. In many cases, however, the real cause is unknown [5,6].
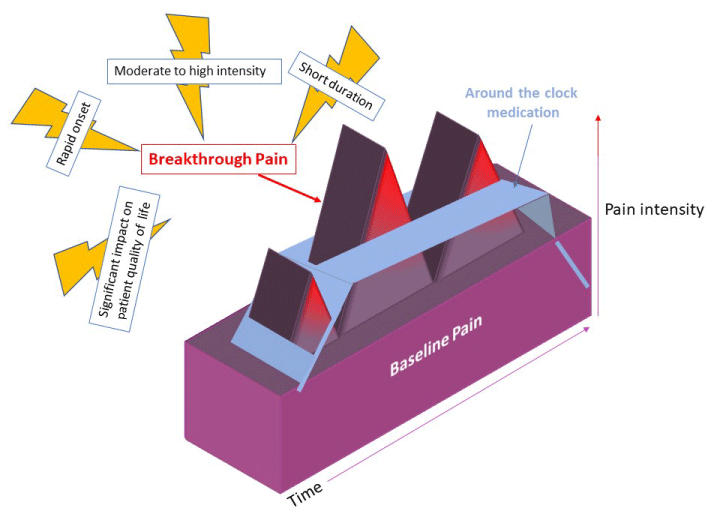
Most of the pain caused by cancer, known as baseline or background pain, can be controlled by long-term regular medical treatment, but even with good management, some patients still experience flares of short-term, intense pain. These transient pain events are called breakthrough cancer pain (BTcP), and are characterized by rapid onset (usually between 3 to 5 minutes), short duration (an average of 60 minutes) and moderate to high intensity (7 points on a 10-point pain Visual Analogue Scale [VAS]) (Figure 1) [7].
Regardless of recent advances in diagnosis, assessment and treatment, BTcP is still a challenge for patients and healthcare professionals alike. Therapeutic approaches differ between countries because of a lack of uniform international guidelines, and indeed, a systematic literature review found that study designs and clinical settings were highly variable across regions. As a result, nearly 50% of patients were undertreated, affecting their daily life [8].
The main objective of this review was to present the current evidence on the management of BTcP, and to provide an action protocol to properly diagnose, treat and follow-up BTcP patients based on practical experience, to help healthcare specialists in making therapeutic decisions, thereby improving patient quality of care.
Methods
This paper is the result of a consensus document produced by a group of 12 Spanish experts in cancer pain therapy, based on their daily clinical experience and critical assessment of the current literature. The expert panel was composed of oncologists, pharmacists, pain specialists, radiation oncologists, in-home care physicians and emergency care doctors with extensive experience in treating cancer patients. A review of the existing literature was performed to obtain practical protocols for the management of BTcP. In order to identify as much information as possible, we searched the main international databases (MedLine/PubMed, Cochrane Library, ISI WOK [ISI Web of Knowledge], and SCOPUS), including Google scholar, for pertinent articles published in English and Spanish during the last 10 years; however, our search was unsuccessful.
The present consensus statement is the result of multiple face-to-face meetings where the panel experts discussed existing guidelines and relevant scientific studies on the management of BTcP. It includes a series of practical recommendations for the diagnosis, treatment and follow-up of BTcP, in order to aid other health professionals in making therapeutic decisions.
Results
Considerations on the definition of BTcP
Breakthrough cancer pain was first defined by Portenoy et al. in 1990 [7], as ‘a transitory exacerbation of pain experienced by patients undergoing long-term opioid treatment for cancer-related pain whose baseline pain is adequately controlled’, but there has been controversy with respect to this definition over the past two decades. Some international guidelines recommend the definition of BTcP derived from the original by Portenoy [9-12], whereas others have established their own definition [13-15]. The main discrepancy is the fact that BTcP can also occur in the absence of background cancer pain and regardless of opioid treatment [16,17]. So far, there has been no global agreement on the definition of BTcP [18]. This lack of consensus has generated different criteria for diagnosing cancer pain, which explains the large variability found in BTcP prevalence rates, ranging from 12% to 93%, depending on the study [8,19-22].
In 2012, an association of Spanish medical doctors, composed of palliative care, pain specialists, oncologists and radiation oncology experts, produced a consensus document with a series of guidelines to properly diagnose and treat BTcP patients. BTcP at that time was defined as ‘a transient, highly intense exacerbation of pain of short duration (less than 20-30 minutes) appearing through a stable pain baseline that can be controlled by opioid treatment’[13]. Although this definition is still valid, some clarifications are needed. First, how exactly is stable baseline pain defined? Some specialists consider that baseline pain is stable when the intensity is very low or there is no pain at all for more than 12 hours, but it can also be interpreted as pain with stable intensity for at least 48 hours, or when there are no more than five daily BTcP episodes. Second, we also need to delimit the length of a ‘transient episode’. BTcP episodes are extremely variable, both between and within individuals, and, although it is generally considered that the average duration of a BTcP event is around 30 minutes, it can last more than 1 hour [23]. Lastly, one should distinguish between BTcP and end-of-dose pain. End-of dose pain occurs just before the next scheduled dose of opioid treatment, indicating an inadequate dose or too prolonged interval between subsequent administrations.
All medical specialties involved in the management of BTcP should agree on a practical definition for BTcP, identifying all possible variables and drawing up an international consensus for the proper diagnosis and management of BTcP.
Recommendations for the treatment of BTcP
The following recomendations were generated with the goal of assisting healthcare professionals in their therapeutic decision-making in patients with cancer pain.
Diagnostic criteria
For patients with cancer, BTcP is associated with decreased functional status and increased levels of anxiety and depression, greatly affecting the patient’s quality of life [24]. In fact, a study by the American Pain Foundation showed that living with pain negatively influences emotional health and causes suffering in 82% of patients [25]. However, despite its prevalence and impact on patients, BTcP is sometimes unrecognized and often undertreated [26-28]. Prompt and efficient diagnosis is therefore essential.
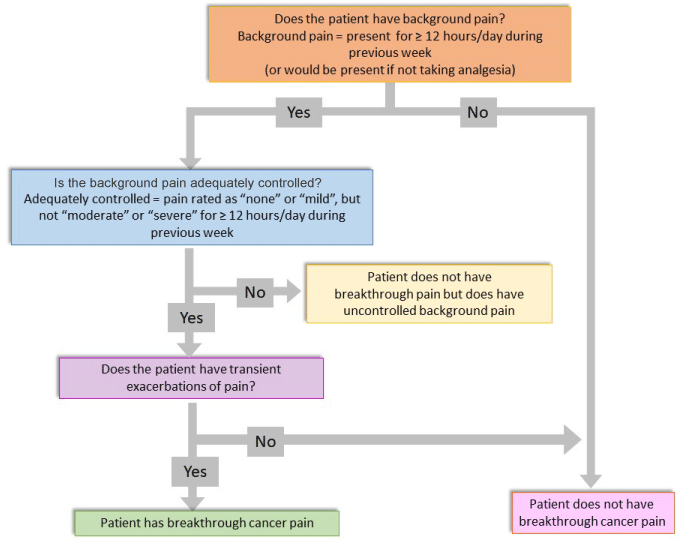
Pain levels can currently be evaluated using different scales and questionnaires, including a visual analogue scale (VAS), the SF-36 pain scale and a neuropathic pain diagnostic questionnaire (DN4) [29,30]. There are different tools for the diagnosis of BTcP [31-33], although the standard one used is the Davies algorithm. Figure 2 shows the most widely used algorithm, modified from the original published in 2009 [23,34,35]. Although it has a good positive predictive value, its sensitivity is still limited due to patient variability. A complete clinical evaluation is still the preferred method to diagnose BTcP [36].
Therapeutic management
Given the wide inter- and intra-individual variability that characterizes BTcP patients [12,23], it is crucial to perform an individualized and very exhaustive initial evaluation to provide them with the best possible management approach. The etiology of the cancer pain will determine the best treatment option. However, regardless of the treatment, therapeutic management should be multimodal, combining preventive measures with both pharmacological and non-pharmacological methods. Support from family and health professionals will also be essential for a successful outcome.
Non-pharmacological approaches: Interventional strategies can be used prior to or alongside pharmacological therapy. Specialists have traditionally followed the original analgesic stepladder algorithm (WHO), in which pharmacological measures are first applied and non-pharmacological techniques are used only when the analgesic ladder fails [37,38]. More recently, though, some clinicians are advocating the early application of non-pharmacological techniques, arguing that it could be beneficial for pain control [39]. Regardless of the time of application, professionals should know and consider the use of interventional, non-pharmacological methods in the management of BTcP. These include:
• Palliative radiotherapy
• Orthopedic surgery
• Spinal reinforcement measures
• Analgesia techniques, including local infiltrations and nerve block.
• Physical measures
Pharmacological approaches: The management of BTcP is primarily based on pharmacological treatment. Treatment options include:
• Addition of co-analgesics such as antidepressant and anticonvulsant medication, corticosteroids and others.
• Increase the daily basal analgesic dose (25% to 50%), to minimize the intensity and number of BTcP episodes [40]. Previous studies on patient tolerability and adverse events at higher levels of basal analgesics should be performed before applying this measure.
• Prescription of opioid analgesics as rescue medication for BTcP. Strong opioids, such as immediate-release morphine, have traditionally been used to treat BTcP [41], and indeed it is still the first treatment option for some scientific societies, including the National Institute for Health and Care Excellence (NICE) [42]. However, the pharmacokinetic/pharmacodynamic profiles of opioids do not always follow the temporal characteristics of most breakthrough pain episodes [43]. Oral opioids need around 30–40 minutes to take effect, while the maximum pain intensity of BTcP is reached very fast (often in less than 10 minutes) [44]. Rapid-onset opioids (ROOs), specifically fentanyl formulations, are the current gold standard in BTcP treatment because they act very quickly, and are very effective [45,46]. In fact, the European Society for Medical Oncology (ESMO) guidelines for the management of cancer pain recommends the use of fentanyl forms for the treatment of BTcP (level of evidence I, degree of recommendation A), whilst morphine is given a level of evidence of II and degree of recommendation of B [34]. Moreover, fentanyl forms are also generally better tolerated, which is particularly important in patients with renal and hepatic disease, since they cannot tolerate strong opioids due to known accumulation of toxic metabolites [47]. Thus, fentanyl formulations are presently the first option in the treatment of BTcP recommended by most medical oncology societies [34,48].
Fentanyl selection: Administration options and dose titration procedure
As previously mentioned, rescue treatment for BTcP involves taking an extra dose (usually at 5%–20% of the total daily dose) of the opioid used around-the-clock (ATC) to relieve baseline pain [47,49,50]. This approach, which is based on years of clinical experience, might not always work because the pharmacokinetics of ATC opioids might not match the onset of the BTcP episode [43]. For this reason, ROOs, specifically fentanyl formulations, are the treatments of choice at present.
Clinicians should take into consideration different variables to choose the correct transmucosal fentanyl form from among buccal, sublingual or intranasal preparations [51]. Patient preferences and personal circumstances should first be considered (Table 1). For instance, in the case of patients with frequent vomiting or mucositis, intranasal or sublingual forms of fentanyl administration are recommended. Patients taking buccal tablets or films are advised not to eat or drink anything until the medication has completely dissolved inside the buccal cavity. Having a dry mouth, as well as breaking or sucking the tablets, will decrease their clinical efficacy. Previous moisturization of the oral mucosa has been proven to effectively improve fentanyl absorption [52]. Additionally, the pain specialist should also evaluate the drug pharmacokinetic characteristics and possible contraindications, especially in polymedicated patients.
For adequate BTcP analgesia, it is important for fentanyl forms to be titrated over time to meet the patient’s individual needs [35,53]. To do so, physicians should consider their clinical needs, age, presence of comorbidities and appearance of adverse events upon treatment initiation. The final aim is to provide individually tailored BTcP treatment by identifying the optimum fentanyl dose and minimizing the risk of side effects.
The fentanyl dose should be gradually adjusted starting with the lowest concentration possible, as indicated in the Summary of product characteristics. The algorithm for fentanyl dose-titration is shown in Figure 3. If, for any reason, patients do not obtain sufficient relief, the concentration should be gradually increased until the pain is adequately controlled without side effects, with an interval of at least two hours between doses of sublingual fentanyl tablets and four hours in other fentanyl forms. In older adults, the method for trialing the opioid treatment is not exactly the same [54]. These patients are usually more susceptible to the medication effects, and many analgesic guides recommend a “start slow and go slow” approach [55]; however, this dosing strategy is often misinterpreted as “start low, stay low”, entailing the risk of inadequate analgesia [56]. To avoid excess sedation, it is recommended to use half the starting dose used for younger adults and proceed with smaller dose increments when needed [56]. If patients of any age present adverse effects or tolerability issues, it is advised to change to another fentanyl formulation. In these cases, a new dosage adjustment protocol should be drawn up, because each fentanyl preparation has different absorption profiles.
To evaluate treatment efficacy and tolerability, patients should be advised to keep track of their in-home fentanyl use by keeping a daily diary [13]. They should record all the variables that could affect medication performance, as summarized in Table 2. Keeping this pain diary can be helpful to reinforce patient treatment adherence. Ultimately, the objective is that the patients identify the most appropriate dosage of analgesic therapy by themselves, according to their individual characteristics and type of pain. Patients should be closely supervised until the optimal dose of BTcP analgesia is achieved.
Follow-up
Physicians should do an early assessment by telephone 48 hours after treatment initiation [11,57]. It is also recommended that patients contact their specialist if they have more than four BTcP episodes per day. Subsequent follow-up can be done through scheduled visits or appointments at the patient’s request.
Optimal cancer pain management will require continuous monitoring and patient reevaluation. Physicians should check for the following factors:
• Treatment efficacy.
• Any kind of change in the BTcP profile.
• Duration, frequency and intensity of BTcP episodes.
• Rescue medication dose.
• Pain relief level and duration after treatment.
• Side effects.
• Patient functionality.
Treatment withdrawal should start when patients achieve complete remission. Furthermore, patients with hard-to-control BTcP events should be referred to a pain specialist or to palliative medicine.
The need for a multidisciplinary approach
Cancer pain can be extremely difficult to manage because it is very heterogenous and can vary significantly from one patient to another [12,23]. It is essential that patients and their families receive appropriate health education. Physicians should focus on explaining the different treatment options and the importance of early treatment initiation, to prevent low adherence rates to medication due to lack of confidence in the treatment or fear of possible side effects.
The most successful way to diagnose, treat and monitor patients suffering from BTcP is to establish a multidisciplinary approach. Since cancer pain occurs throughout the course of the disease, it is essential that all specialists who treat cancer patients are familiar with its detection and management. Close collaboration between medical specialists, nurses and primary care physicians will result in a more accurate diagnosis and optimal management of BTcP to the benefit of patients and their families. Primary care health professionals and nurses are in the best position to identify problems and plan care accordingly. They have a crucial role in patient follow-up and in improving their quality of life, providing them with psychological support.
Discussion
Breakthrough cancer pain affects a large proportion of cancer patients, decreasing their quality of life. However, management of the disease is often unsatisfactory because it is based on old definitions and treatment algorithms. A European nursing survey performed in 2014 [58] showed that there is still high variability in the definition of BTcP used in different countries, resulting in poor patient management. A standardized, global consensus definition of BTcP and its symptomatology is needed to help doctors to correctly diagnose it. Similarly, treatment and monitoring guidelines should be standardized to provide patients worldwide with the best possible care. At present, different tools have been created to facilitate the correct diagnosis of BTcP [31-33]. Nonetheless, these instruments have only been validated in some countries, and need to be further approved to be introduced in other populations. There is therefore still a long way to go in order to standardize procedures, and to have a universal action protocol for the management of BTcP.
Although some controversy remains, enough clinical data has been generated to suggest that ROOs, specifically fentanyl formulations, should be the analgesic of choice for the pharmacological treatment of BTcP. However, they must be titrated carefully according to the patient’s needs, age or presence of comorbidities in order to minimize the risk of clinically significant adverse effects. Many countries, including Spain, have national programs and guidelines for the safe use of ROOs [59]. Moreover, patients, doctors and pharmacists must receive proper education to be aware of all their possible risks.
Conclusions
In this paper, a group of Spanish experts in BTcP provide a series of practical recommendations to assist practitioners and clinicians in the current management of BTcP, based on a critical review of the evidence and their own extensive clinical experience. These recommendations include: performing a personalized initial evaluation of the patient, treating cancer pain by combining preventive measures with both pharmacological and non-pharmacological methods based on the patient’s individual circumstances, and providing them with adequate health education from a multidisciplinary team.
Conflict of Interest
Mª Dolores López Alarcón has received payments for consultancies and lecture fees from Kyowa Kirin, Takeda, Gebro Pharma, Lab Esteve, Teva and Grunenthal. Francisco Villegas declares that he has received honoraria scientific advice from Esteve and acted as speaker for Takeda, Gebro Pharma, Grünenthal, Teva, Mylan, and Kyowa Kirin. Vicente Domingo Triadó reports having received payments for consultancies and lecture fees from Kyowa Kirin and Grunenthal. Ana Blasco Cordellat declares that has collaborated with Kyowa Kirin, Takeda and Bristol-Myers Squibb as a consultant. Jorge Pastor Peidro has received payments for consultancies and advisory role from Grünenthal, Astellas Pharma, GP Pharm and Janssen Cilag. Carlos Ferrer Albiach has received payments for consultancies and lecture fees from Astellas Pharma, Janssen Cilag and Kyowa Kirin. Alfonso Berrocal reports having received consulting fees from Bristol-Myers Squibb, Novartis, Roche, Pierre Fabre, Pfizer, Merk, MSD, Amgen, Sanofi and acted as speaker for Bureau and Bristol-Myers Squibb. Almudena Ruiz, Genoveva Hernández Comes, Manuel Hernández Peris, Jose López Torrecilla and Pilar Blasco Segura have no conflicts of interest to declare.
Medical writing and editing services were provided by Medical Statistics Consulting S.L.(MSC) and funded by a grant from Kyowa Kirin Farmacéutica S.L.U.
All authors, as members of the scientific committee, were responsible and participated in data collection and interpretation and had access to full data and reports. In addition, all authors revised the manuscript versions and approved the final draft.
The authors thank Lucía Perez Carbonell and Vanessa Marfil Vives at MSC S.L. (Valencia, Spain) for editorial support in writing of this manuscript.
- Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA (1999) The epidemiology of chronic pain in the community. Lancet 354:1248-1252. Link: http://bit.ly/2HlyKss
- Gomez-Batiste X, Madrid F, Moreno F, Gracia A, Trelis J, et al. (2002) Breakthrough cancer pain: prevalence and characteristics in patients in Catalonia, Spain. J Pain Symptom Manage 24: 45-52. Link: http://bit.ly/2ZypuLX
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, et al. (2007) Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 18: 1437-1449. Link: http://bit.ly/2ZpaWxR
- Gutgsell T, Walsh D, Zhukovsky DS, Gonzales F, Lagman R (2003) A prospective study of the pathophysiology and clinical characteristics of pain in a palliative medicine population. Am J Hosp Palliat Care 20: 140-148. Link: http://bit.ly/33UNaJx
- Vuorinen E (1993) Pain as an early symptom in cancer. Clin J Pain 9: 272-278. Link: http://bit.ly/2NuJQPu
- Twycross RG, Fairfield S (1982) Pain in far-advanced cancer. Pain 14: 303-310. Link: http://bit.ly/30u3w9P
- Portenoy RK, Hagen NA (1990) Breakthrough pain: definition, prevalence and characteristics. Pain 41: 273-281. Link: http://bit.ly/2TYOVkp
- Deandrea S, Corli O, Consonni D, Villani W, Greco MT, et al. (2014) Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage 47: 57-76. Link: http://bit.ly/2NsiPfx
- Nientemale DEI, Vellucci R, Fanelli G, Pannuti R, Peruselli C, et al. (2016) What to Do, and What Not to Do, When Diagnosing and Treating Breakthrough Cancer Pain (BTcP): Expert Opinion. Drugs 76: 315-330. Link: http://bit.ly/2L6mFZa
- Wengstrom Y, Geerling J, Rustoen T (2014) European Oncology Nursing Society breakthrough cancer pain guidelines. Eur J Oncol Nurs 18: 127-131. Link: http://bit.ly/33VRatm
- Porta-Sales J, Perez C, Escobar Y, Martinez V (2016) Diagnosis and management of breakthrough cancer pain: Have all the questions been resolved? A Delphi-based consensus assessment (DOIRON). Clin Transl Oncol 18: 945-954. Link: http://bit.ly/2P8AOds
- Mercadante S, Costanzo BV, Fusco F, Butta V, Vitrano V, et al. (2009) Breakthrough pain in advanced cancer patients followed at home: a longitudinal study. J Pain Symptom Manage 38: 554-560. Link: http://bit.ly/2ZfuaGS
- Escobar Álvarez Y, Biete i Solà A, Camba Rodríguez M, Gálvez Mateos R, Mañas Rueda A, et al. (2013) Diagnóstico y tratamiento del dolor irruptivo oncológico: recomendaciones de consenso. Revista de la Sociedad Española del Dolor 20: 61-68. Link: http://bit.ly/2L5DcfP
- Mercadante S, Marchetti P, Cuomo A, Mammucari M, Caraceni A, et al. (2016) Breakthrough pain and its treatment: critical review and recommendations of IOPS (Italian Oncologic Pain Survey) expert group. Support Care Cancer 24: 961-968. Link: http://bit.ly/2Zfb1VB
- Lohre ET, Klepstad P, Bennett MI, Brunelli C, Caraceni A, et al. (2016) From "Breakthrough" to "Episodic" Cancer Pain? A European Association for Palliative Care Research Network Expert Delphi Survey Toward a Common Terminology and Classification of Transient Cancer Pain Exacerbations. J Pain Symptom Manage 51: 1013-1019. Link: http://bit.ly/2HoTNdq
- Petzke F, Radbruch L, Zech D, Loick G, Grond S (1999) Temporal presentation of chronic cancer pain: transitory pains on admission to a multidisciplinary pain clinic. J Pain Symptom Manage 17: 391-401. Link: http://bit.ly/2Mz0kGT
- Caraceni A, Davies A, Poulain P, Cortes-Funes H, Panchal SJ, et al. (2013) Guidelines for the management of breakthrough pain in patients with cancer. J Natl Compr Canc Netw 11: S29-36. Link: http://bit.ly/2Mz0QEP
- Mercadante S, Portenoy RK (2016) Breakthrough cancer pain: twenty-five years of study. Pain 157: 2657-2663. Link: http://bit.ly/2ZoQcGp
- Bruera E, Fainsinger R, MacEachern T, Hanson J (1992) The use of methylphenidate in patients with incident cancer pain receiving regular opiates. A preliminary report. Pain 50: 75-77. Link: http://bit.ly/2ZpCHm3
- Swanwick M, Haworth M, Lennard RF (2001) The prevalence of episodic pain in cancer: a survey of hospice patients on admission. Palliat Med 15: 9-18. Link: http://bit.ly/2ZatEdr
- Mercadante S, Zagonel V, Breda E, Arcara C, Gebbia V, et al. (2010) Breakthrough pain in oncology: a longitudinal study. J Pain Symptom Manage 40: 183-190. Link: http://bit.ly/31Sunge
- López Castro R (2015) Prevalencia del dolor en enfermos oncológicos. Dolor irruptivo. Medicina Paliativa 22: 2-9. Link: http://bit.ly/2Zq1J8z
- Davies A, Buchanan A, Zeppetella G, Porta-Sales J, Likar R, et al. (2013) Breakthrough cancer pain: an observational study of 1000 European oncology patients. J Pain Symptom Manage 46: 619-628. Link: http://bit.ly/33XJDKm
- Burton B, Zeppetella G (2011) Assessing the impact of breakthrough cancer pain. Br J Nurs 20: S14, S16-19. Link: http://bit.ly/2P8VLVz
- American Pain F (2011) Breakthrough cancer pain: mending the break in the continuum of care. J Pain Palliat Care Pharmacother 25: 252-264. Link: http://bit.ly/30BtJ6I
- Deandrea S, Montanari M, Moja L, Apolone G (2008) Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 19: 1985-1991. Link: http://bit.ly/2PlScMa
- Shen WC, Chen JS, Shao YY, Lee KD, Chiou TJ, et al. (2017) Impact of Undertreatment of Cancer Pain with Analgesic Drugs on Patient Outcomes: A Nationwide Survey of Outpatient Cancer Patient Care in Taiwan. J Pain Symptom Manage 54: 55-65. e1. Link: http://bit.ly/33VVybO
- Camps Herrero C, Reina Zoilo JJ, Monge Martin D, Caballero Martinez F, Guillem Porta V, et al. (2019) Active study: undetected prevalence and clinical inertia in the treatment of breakthrough cancer pain (BTcP). Clin Transl Oncol 21: 380-390. Link: http://bit.ly/2ZpuIp4
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, et al. (2008) Assessment of pain. Br J Anaesth 101: 17-24. Link: http://bit.ly/2zgJOSX
- Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, et al. (2005) Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114: 29-36. Link: http://bit.ly/2Nui1Hk
- Hagen NA, Stiles C, Nekolaichuk C, Biondo P, Carlson LE, et al. (2008) The Alberta Breakthrough Pain Assessment Tool for cancer patients: a validation study using a delphi process and patient think-aloud interviews. J Pain Symptom Manage 35: 136-52. Link: http://bit.ly/2ZjeqCb
- Webber K, Davies AN, Zeppetella G, Cowie MR (2014) Development and validation of the breakthrough pain assessment tool (BAT) in cancer patients. J Pain Symptom Manage 48: 619-631. Link: http://bit.ly/2Zkgea1
- Samolsky Dekel BG, Remondini F, Gori A, Vasarri A, Di Nino G, et al. (2016) Development, validation and psychometric properties of a diagnostic/prognostic tool for breakthrough pain in mixed chronic-pain patients. Clin Neurol Neurosurg 141: 23-29. Link: http://bit.ly/2zkHQRO
- Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, et al. (2018) Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Annals of Oncology 29: iv166–iv191. Link: http://bit.ly/2L8UzMI
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G (2009) The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 13: 331-338. Link: http://bit.ly/30vwuX3
- Webber K, Davies AN, Cowie MR (2015) Accuracy of a Diagnostic Algorithm to Diagnose Breakthrough Cancer Pain as Compared With Clinical Assessment. J Pain Symptom Manage 50: 495-500. Link: http://bit.ly/2L8ZddC
- Max M (1986) World Health Organization cancer pain relief program: Network news. JPSM 1: 53-57. Link: http://bit.ly/31Wbfha
- Vargas-Schaffer G (2010) Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 56: 514-517, e202-205. Link: http://bit.ly/2TYWGH0
- Candido KD, Kusper TM, Knezevic NN (2017) New Cancer Pain Treatment Options. Curr Pain Headache Rep 21: 12. Link: http://bit.ly/33QqslX
- Mercadante S, Villari P, Ferrera P, Casuccio A (2004) Addition of a second opioid may improve opioid response in cancer pain: preliminary data. Support Care Cancer 12: 762-766. Link: http://bit.ly/2Hnrf4i
- Hanks GW, Conno F, Cherny N, Hanna M, Kalso E, et al. (2001) Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 84: 587-593. Link: http://bit.ly/2PsmzAD
- National Institute for Health and Care Excellence (NICE) (2012) Palliative care for adults: strong opioids for pain relief. Link: http://bit.ly/30v7wqT
- Jandhyala R, Fullarton JR, Bennett MI (2013) Efficacy of rapid-onset oral fentanyl formulations vs. oral morphine for cancer-related breakthrough pain: a meta-analysis of comparative trials. J Pain Symptom Manage 46: 573-580. Link: http://bit.ly/31Zw77q
- Zeppetella G (2008) Opioids for cancer breakthrough pain: a pilot study reporting patient assessment of time to meaningful pain relief. J Pain Symptom Manage 35: 563-567. Link: http://bit.ly/2MA0hKP
- Zeppetella G, Davies A, Eijgelshoven I, Jansen JP (2014) A network meta-analysis of the efficacy of opioid analgesics for the management of breakthrough cancer pain episodes. J Pain Symptom Manage 47: 772-785.e5. Link: http://bit.ly/2TYSWVY
- Zeppetella G (2011) Opioids for the management of breakthrough cancer pain in adults: a systematic review undertaken as part of an EPCRC opioid guidelines project. Palliat Med 25: 516-524. Link: http://bit.ly/2ZdJbcf
- Mercadante S (2015) Opioid metabolism and clinical aspects. Eur J Pharmacol. 769:71-78. Link: http://bit.ly/2KRPARB
- Jara C, Del Barco S, Gravalos C, Hoyos S, Hernandez B, et al. (2018) SEOM clinical guideline for treatment of cancer pain (2017). Clin Transl Oncol 20: 97-107. Link: http://bit.ly/2KRoPg6
- Hagen NA, Fisher K, Victorino C, Farrar JT (2007) A titration strategy is needed to manage breakthrough cancer pain effectively: observations from data pooled from three clinical trials. J Palliat Med 10: 47-55. Link: http://bit.ly/2ZleRaP
- Cherny NI, Portenoy RK (1993) Cancer pain management. Current strategy. Cancer 72: 3393-3415. Link: http://bit.ly/33U7Mlb
- Meriggi F, Zaniboni A (2013) Fentanyl for breakthrough cancer pain: where are we? Rev Recent Clin Trials 8: 42-47.
- Davies A, Mundin G, Vriens J, Webber K, Buchanan A, et al. (2016) The Influence of Low Salivary Flow Rates on the Absorption of a Sublingual Fentanyl Citrate Formulation for Breakthrough Cancer Pain. J Pain Symptom Manage 51: 538-545. Link: http://bit.ly/2HnWXOB
- Kleeberg UR, Filbet M, Zeppetella G (2011) Fentanyl buccal tablet for breakthrough cancer pain: why titrate? Pain Pract 11: 185-190. Link: http://bit.ly/2PaaKP7
- Guerriero F (2017) Guidance on opioids prescribing for the management of persistent non-cancer pain in older adults. World J Clin Cases 5: 73-81. Link: http://bit.ly/33XRdoA
- Hanlon JT, Backonja M, Weiner D, Argoff C (2009) Evolving pharmacological management of persistent pain in older persons. Pain Med 10: 959-961. Link: http://bit.ly/2zknhF2
- McLachlan AJ, Bath S, Naganathan V, Hilmer SN, Le Couteur DG, et al. (2011) Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol 71: 351-364. Link: http://bit.ly/33YZx7l
- Boceta J, De la Torre A, Samper D, Farto M, Sanchez-de la Rosa R (2016) Consensus and controversies in the definition, assessment, treatment and monitoring of BTcP: results of a Delphi study. Clin Transl Oncol 18: 1088-1097. Link: http://bit.ly/2zg6YJr
- Wengstrom Y, Rundstrom C, Geerling J, Pappa T, Weisse I, et al. (2014) The management of breakthrough cancer pain--educational needs a European nursing survey. Eur J Cancer Care 23:121-128. Link: http://bit.ly/30uebkR
- Sociedad española de farmacia hospitalaria (2015) Prácticas seguras para el uso de opioides en pacientes con dolor crónico. Link: http://bit.ly/2HnUJii
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
