Open Journal of Pain Medicine
Observational study to analyse the opioid titration process in the treatment of breakthrough pain of cancer patients in clinical practice
Lara Iglesias-Docampo1, Paola Pimentel-Cáceres2, Mª Paola García-Coves3, José Luis Firvida-Pérez4, Julio José Lambea-Sorrosal5, Antonio Andrés Rueda-Ramos6, Iker López-Calderero7, Juan Antonio Núñez-Sobrino8, Antonio Javier Jiménez-López9, Ana Cabezón-Álvarez10 and Begoña Soler-López11*
2MD, Oncology Department, Hospital Santa Lucía, Cartagena (Murcia), Spain
3MD, Oncology Department, Hospital de San Juan, Alicante, Spain
4MD, Oncology Department, Cosaga-Ourense, Ourense, Spain
5MD, Oncology Department, Hospital Clínico Lozano Blesa, Zaragoza, Spain
6MD, Oncology Department, Hospital Puerta del Mar, Cádiz, Spain
7MD, Oncology Department, Dr. López Calderero Clinic, Ibiza, Spain
8MD, Oncology Department, Hospital Universitario 12 de Octubre, Madrid, Spain
9MD, Medical Department, Kyowa Kirin Farmacéutica, S.L.U, Madrid, Spain
10PhD, Medical Department, Kyowa Kirin Farmacéutica, S.L.U, Madrid, Spain
11MD, Medical Department, E-C-BIO, S.L., Las Rozas (Madrid), Spain
Cite this as
Iglesias-Docampo L, Pimentel-Cáceres P, García-Coves MP, Firvida-Pérez JL, Lambea-Sorrosal JJ, et al. (2019) Observational study to analyse the opioid titration process in the treatment of breakthrough pain of cancer patients in clinical practice. Open J Pain Med 3(1): 001-007. DOI: 10.17352/ojpm.000010Background: During the oncological disease, patients may suffer a special type of pain called breakthrough pain (BTP), defined as a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain. Opioids are an essential part of BTP treatment, and its dosing adjustment should be performed irrespective of the basal dose of analgesia. However, in clinical practice it is unknown how opioids titration is completed.
The context and purpose of the study: The objective of the study was to assess opioid titration process for the treatment of breakthrough pain (BTP) in cancer patients in usual clinical practice.
An observational study was completed in 17 Medical oncology departments. Information was collected on 165 cancer patients over 18 years of age, with controlled baseline pain and BTP, in whom an opioid was prescribed for BTP. The sequence of the titration process of the new opioid prescribed to the patient were recorded.
Results: The mean age of the patients was 64.8 years (95% confidence interval: 63 to 66.5), and 69.1% were males (114). The active ingredients analysed were: Fentanyl (93% of patients), Morphine (4%), and Oxycodone (2%). Titration required: One step in 86.1% (142) of patients; two steps in 9.1% (15), three steps in 4.2% (7), and four steps in 0.6% (1). The mean time to next step in titration was 22.04 minutes (95% confidence interval: 17 to 27), with a median of 15 minutes; no significant differences were observed between the products administered.
Conclusions: Most patients (86.1%) require only one step for opioid titration for BTP treatment. The conclusion is that titration of immediate-acting opioid in patients with BTP is a rapid process, adequate to the characteristics of the BTP, and is achieved at the doses recommended in the summaries of product characteristics.
Abbreviations
BTP: Breakthrough Pain
Background
Most cancer patients experience moderate to severe chronic pain during their disease. It occurs in 30% to 40% of patients at the time of diagnosis, and in up to 75% of cases of advanced disease. Control of this chronic pain can be achieved in most cancer patients with adequate analgesic treatment [1-6].
During the oncological disease, patients may also present a special type of pain called breakthrough pain. This is defined as “a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain”. It typically occurs acutely, reaching, in less than three minutes, its maximum intensity is usually higher than seven points on a ten-point visual analogue scale for pain intensity, and lasts about 30 minutes, occurring one to five times daily [5,7-11].
Prevalence of breakthrough pain have been reported in 59.2% of cancer patients, with great variability ranging from 39.9% in outpatients to 80.5% in hospitalized patients [12]. Figures of 23% [13] and 41% [14], of BTP in cancer patients have been reported in Spain. The reason for this great variability in prevalence is due to the relatively recent recognition of breakthrough pain as a clinical condition and to differences in its definition up to that time (1990) [7]. Also due to prevalence differences depending on the clinical setting where this is determined. It is known that breakthrough pain is not usually recognized as a clinical condition, and therefore it is not adequately treated in up to 77% of cases [15].
Breakthrough pain can negatively affect the patient’s psychological and functional prognosis, so its adequate control and management may significantly improve the patient’s quality of life [16].
Breakthrough pain treatment need a multiple approach with drugs, especially opioids, being an essential part of the treatment success. Opioids have been described as the primary factor of relief for BTP in 44.61% of patients, while 26%-44% of patients find relief with the avoidance of the movement causing them, and 12-20% do not appear to find adequate relief with pharmacological or non-pharmacological measures [17,18].
The pharmacological treatment should be adapted to the special characteristics of the breakthrough pain, i.e., it should be potent enough to alleviate such a severe pain, but it should also be rapid in its action, since breakthrough pain episodes usually last approximately 30-45 minutes, and are mostly unpredictable.
Opioids are an essential part of breakthrough pain treatment. At present, transmucosal fentanyl, in its different formulations is the drug that best adapts to the clinical characteristics of breakthrough pain due to the immediate onset of action. The selection of the appropriate formulation will mainly depend on the clinical condition of the patient and the previous experience of the physician. Other opioids that do not conform to the profile of breakthrough pain are sometimes also used for the treatment of oncological breakthrough pain [19].
Dose adjustment for breakthrough pain should be performed irrespective of the basal dose of analgesia [17,20]. In clinical practice, the patient’s specific situation, the treatment for cancer pain that the patient is receiving, and the presence of concomitant diseases, could condition both the initial dose prescribed to the patient and the opioid titration process. It is unknown how is completed the titration of the immediate-onset opioids in daily clinical practice.
The objective of this study was to assess opioid titration regimens for the treatment of breakthrough pain in cancer patients in standard clinical practice.
Materials and Methods
Study design and ethical standards
An observational study was designed. The Clinical Research Ethics Committee of Hospital Universitario 12 de Octubre in Madrid (Spain) approved the study on 22 December 2015 (15/349).
The study was completed following the International Society for Pharmacoepidemiology (ISPE) International Guidelines for Good Epidemiology Practice, the national guidelines on observational studies, and the basic ethical principles contained in the Declaration of Helsinki.
The first patient was included in the study on 22 January 2016 and the last patient on 14 October 2016, participating 17 medical oncology departments.
Patients were screened by consecutive sampling from among those referred to the clinic who met the screening criteria. The study population consisted of patients who, at the time of the screening visit, presented cancer pain and breakthrough pain requiring treatment with a new opioid.
The operative definition of breakthrough pain was [7,9]: 1) Presence of baseline pain: persistent pain 12 hours or more per day, during the week prior to evaluation (or pain that would exist if no analgesics were taken); 2) Controlled baseline pain: The patient is considered to be adequately controlled when there is no pain or if the pain is mild (not moderate or severe) for 12 or more hours a day, during the week prior to evaluation; 3) Existence of transient exacerbations of severe or unbearable pain, with a score on the visual analogue scale pain intensity scale higher than seven points, that appear spontaneously or that are related to a concrete, predictable or unpredictable trigger. Its duration can be of 15-30 minutes, reaching maximum pain intensity within three to five minutes, and occurring 1 to 5 times daily.
The patients screened for the study must comply with the following screening criteria: 1) Males and females over 18 years of age; 2) Patients with controlled oncological pain at baseline with a score of ≤ 4 on the ten-points visual analogue scale for pain intensity; 3) Patients with a diagnosis of breakthrough pain; 4) Patients who were prescribed a new opioid to treat their breakthrough pain; 5) Patients who signed a written informed consent to participate in the study.
Patients with absolute contraindications to opioid treatment, with cognitive involvement, severely affected by their underlying disease or uncooperative were not included in the study.
The primary objective of the study was to assess opioid titration regimens for the treatment of breakthrough pain in cancer patients in standard clinical practice. Information was collected on opioids used, their starting dose, how many modifications to the dose or type of drug were needed to achieve analgesic efficacy, time to efficacy of the prescribed regimen, and the final doses with which efficacy was achieved.
Patient age, sex, weight and height, socioeconomic status (low: incomes less than 2/3 of mean salary, 15,000 Euros; middle: incomes between 2/3 and twice the mean salary, >15,000 Euros and < 45,000 Euros; or high: incomes higher than twice the mean salary, >45,000 Euros)) and performance status of the patient at the time of consultation using the Karnofsky index were recorded in the case record form [21]. Patients completed the Edmonton Symptom Assessment System (ESAS) [22,23] for evaluating the symptoms reported in the last 24 hours. The ESAS scale consists of 10 visual analogue scales (VAS) ranging from zero to ten points, with zero being the absence of the symptom and the value ten being the maximum intensity, and assesses: pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, wellbeing, dyspnea, and difficulty sleeping. Information was collected on the presence of other medical history in the patients and the date of diagnosis of the cancer and the primary organ affected.
Characteristics of the breakthrough pain were described: reason for consultation (first episode of breakthrough pain or patient in follow-up), location, number of episodes per day, form of onset (gradual or sudden), intensity (mild, moderate, severe or unbearable), total duration of the episode (minutes), whether spontaneous or incidental, and the events that intensified the breakthrough pain (movement, coughing, eating, bowel movements, etc.), when it predominantly appeared (at night, during the day or unrelated), whether it was unpredictable or predictable, and the type of pain (somatic, visceral, neuropathic or mixed). Information was collected on the treatments received by the patient for the control of the chronic pain (active ingredient, dose and daily frequency, administration route and start date of treatment). The trade name, dose and daily frequency, administration route of the prescribed opioids for the treatment of breakthrough pain and start date was collected. Trade names were collected due to possible differences in formulations that could affect the safety.
Patients could be included if they had their first breakthrough pain episode, or if they attended the clinic to swift to a new treatment. The sequence of steps needed to achieve the titration-efficacy, and waiting time until the decision to take the next step for titration were recorded. When the first BTP episode appeared, the patient completed the visual analogue scale of pain intensity. The decision for the next titration step was made based on the response of the breakthrough pain episode measured every 5 minutes: it was considered effective if the score on the visual analogue scale of intensity of pain measured after opioid administration was greater than 33% of initial measurement, in which case the dose was maintained and the titration-efficacy was considered to be achieved; if the score was less than 33% of initial visual analogue scale, increasing the dose of the opioid or modifying the opioid administered was considered; if the dose was not well tolerated by the patient, then the physician decided to decreasing the opioid dose, or modifying the opioid, or discontinuing the treatment with opioids. Time from dosing to efficacy o to next titration step was recorded in every step.
As BTP is of short duration, the titration process was observed in consecutive BTP episodes if titration was not gotten at the first episode, in those patients who suffer more than one daily BTP episode. In those patients with only one daily episode, titration was completed on next episode.
Statistical analysis
No sample size calculation was possible due to the characteristics of the objective.
Data quality control was performed through 100% validation of the cases and 100% of the data, which was entered into an electronic database designed for the study, whose access was restricted to participating investigators.
A descriptive analysis was completed of the variables included in the study, based on the distribution of frequencies and calculation of percentages for qualitative variables, and the calculation of the mean, 95% confidence interval, median, minimum and maximum for the quantitative variables.
Comparisons between qualitative variables were made using Fisher’s test or the Chi2 test. The Student’s t-test or Mann Whitney U test was used to compare independent groups in the case of quantitative variables. On evaluating the differences in the quantitative variables based on different patient characteristics or the treatment received, the factorial analysis of variance model was used, applying Bonferroni or Games-Howell corrections according to the homogeneity of variances, as a control for the error in multiple comparisons. Missing data was described for each variable analysed. The statistical significance level was set at 0.05. The SPSS version 25.0 statistical package was used for the analysis.
Results
A total of 165 patients were included in 14 Spanish provinces and 12 Autonomous Communities, with a mean of 10 patients per site (95% confidence interval: 6 to 13). A total of 69.1% of patients were men (114) and 30.9% (51) were women. Mean patient age was 64.8 years (95% confidence interval: 63.1 to 66.5) with no differences between sexes (p = 0.708).
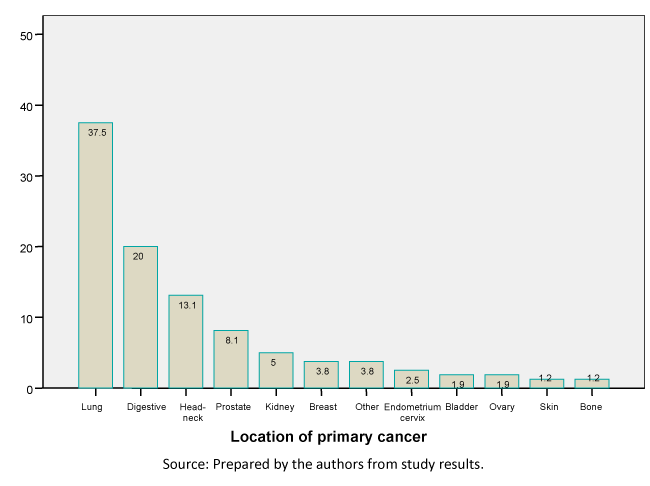
The patient’s socioeconomic level was middle in 71.5% (118), followed by low socioeconomic status in 20% (33), and high in 8.5% (14). The mean body mass index was 25.2 kg/m2 (95% confidence interval: 24.5 to 25.9). Patients were classified into three categories based on their body mass index. A total of 9.7% (16 patients) had a body mass index below 20 kg/m2, defined as a group with cachexia [24]; 46.5% (75) had normal body mass index, greater than 20 and less than 25; 30.3% (50) were overweight; and obesity was identified in 14.5% (24). No statistically significant differences were seen in the distribution of patients by sex in the four groups or by body mass index (p = 0.523). Karnofsky performance status was: 40 in 1.2% (2); 50 in 3.6% (6); 60 in 15.8% (26); 70 in 14.5% (24); 80 in 24.8% (41); 90 in 32.1% (53) and 100% in 7.95 (13). A total of 88.5% (146) of patients had other history of disease in addition to cancer. Figure 1 shows the percentage of patients with each type of primary cancer with a time since diagnosis of 1.8 years (95% confidence interval: 1.4 to 2.2), with a median of 0.7 years.
Table 1 summarizes the characteristics of the breakthrough pain in the patients included in the study. The mean number of breakthrough pain episodes per day was 3.9 (95% confidence interval: 3.7 to 4.2), with a median of four daily episodes, ranging from 0.4 to 10, with no differences between the sexes (p = 0.869). The mean duration of breakthrough pain was 19 minutes (95% confidence interval: 16.3 to 21.8). No significant differences were seen in the duration of the episodes between males and females (p = 0.198). No differences by gender were observed in remaining BTP characteristics.
Statistically significant differences between males and females were seen in the mean ESAS score for tiredness (p = 0.018), which was 1.1 points worse in females (95% confidence interval: 0.2 to 1.9). Mean ESAS symptom scores are shown in Table 2.
Opioid titration for the treatment of breakthrough pain
Information on a total of 200 opioid titration steps was recorded for the 165 patients included in the study. The most commonly titrated active ingredient was transmucosal Fentanyl in 93% of the patients included in the study (186 titrations), followed by Morphine in 4% (eight titrations), Oxycodone in 2% (4 titrations) and Tapentadol and Tramadol, each in one titration (0.5%).
A total of 85% of titrations (170) were performed with PecFent® (Fentanyl), 5% (10) with Abstral® (Fentanyl), 2.5% (5) with Instanyl® (Fentanyl), 2% (4) with Morphine, 2% with OxyNorm® (Oxycodone), and 2% with Sevredol® (Morphine), and 0.5% (1) with Avaric® (Fentanyl), Tapentadol and Tramadol, respectively.
The most commonly used drug for the control of breakthrough pain was intranasal fentanyl with pectin in 135 patients of whom 82.2% (111 patients) achieved the titration dose with 100 micrograms.
Table 3 shows the doses used for the titration of each commercial product and the mean dose at which the opioid titration was achieved.
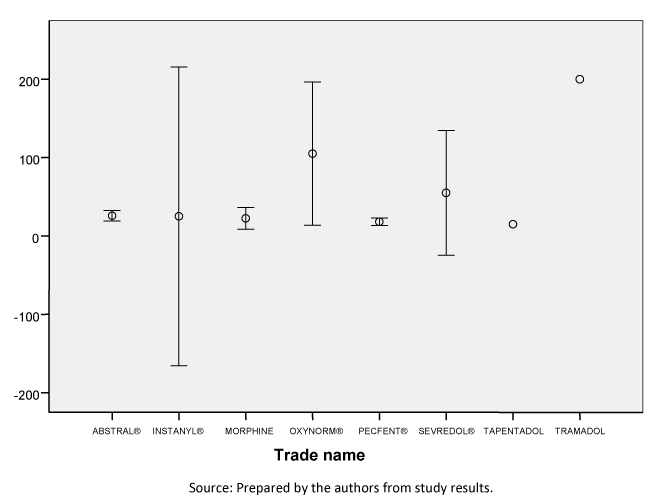
The mean time to the next step in opioid titration was 22.04 minutes (95% confidence interval: 16.96 to 27.13), with a median of 15 minutes. No statistically significant differences were observed between the different commercial products. Figure 2 shows the time required for each commercial product until titration or dose modification.
In the 200 titration steps analysed in the study, the effective dose was achieved in 76% (152), in 21% (42) the dose was increased, in 0.5% (1) the dose was reduced, in 1.5% (3) the opioid was changed due to lack of efficacy, and in 1% (2) the treatment with opioids was discontinued due to toxicity or intolerance.
In all patients for any of the opioids used for the treatment of breakthrough pain that were titrated, only one titration step was required in 86.1% of patients (142), two steps in 9.1% (15), three steps in 4.2% (7), and four steps in 0.6% (1).
No significant differences were seen in opioid type, number of opioid titration steps, nor in the percentage of patients requiring a single step for opioid titration, response time or the final effective dose based on sociodemographic and clinical parameters.
Discussion
As seen in the results of the study, in most patients (86.1%), only one step was required for the titration of the opioid selected for the treatment of breakthrough pain. As this was an observational study on real world data, the patients were treated with different drugs and doses for the background pain and the initial dose of the opioid for the treatment of breakthrough pain was not established for the study. This criterion was justified by results of previous studies of fentanyl products evidencing that the effective dose for the treatment of breakthrough pain was not related to the basal opioid regimen and therefore the doses of fentanyl products should be titrated for each patient starting with the lowest strength [25]. This is in contrast to previous scientific knowledge, given that opioid-tolerant patients receiving high doses of opioids are expected to require a dose proportional to the background opioid regimen to provide a clinical analgesic effect [26,27]. As an example, oral morphine has been safely given for many years in doses proportional to the opioid basal regimen [28].
A pooled analysis of trials of oral transmucosal fentanyl citrate showed a relationship between the breakthrough cancer pain dose and the basal opioid regimen but with relevant interindividual variability [26].
Dose titration may make the fentanyl products difficult in daily activities, preferring a traditional oral dosing of morphine (Davies 2008), but the present study demonstrated that titration is only needed in 13,6% of patients.
From the results of this study we can say that titrating a fast-acting opioid is not a difficult process. In the observation of the process in clinical practice, it was shown to be a simple, rapid process adequate for the duration characteristics of the breakthrough pain and that is achieved at the doses recommended in the summaries of product characteristics of the products analysed. Considering that 85% of the titrations analysed were performed with intranasal fentanyl pectin, this affirmation is fully applicable to this product, and as 82.2% of the patients reached the effective dose at the lowest dose of drug available (PecFent 100 micrograms), the specifications detailed in the product information are consistent to the observations in clinical practice. This enhance the practical use of fentanyl products, particularly for outpatients, as 86,1% do not need further steps for titration.
The study conducted has the limitations inherent to an observational study, in which comparative results in titration between different opioids cannot be conclusive also considering that the sample did not contain comparable number of patients for each opioid. We did not complete the analysis of the initial doses for the breakthrough pain in relation to the background treatment due to high variability of drugs and doses. So, we could not know if higher background pain were related to higher initial doses for breakthrough pain treatment in clinical practice. The relationship between the breakthrough pain characteristics and the response to titration was not analysed. The satisfaction of the patients with the titration process was not examined, and of course this might be relevant for the conclusions of the analysis.
However, the joint analysis of the process in clinical practice provides a picture of the situation in real life. The titration of the treatment for breakthrough pain was performed in cancer patients, so the results, in principle, could only be extrapolated to this population. However, the nature of breakthrough pain has not been reported to be different in cancer patients compared to non-cancer patients, so the populations may, therefore, be similar. In turn, patients had different cancers and performance status that could influence their response to treatment. However, the study has the advantage of its general application, since if the selection of patients for the study had been limited to one type of cancer or type of breakthrough pain, the conclusions could only have been generalized to similar groups of patients.
Regarding the treatment of chronic pain, some international agencies [29-31] warned about patients with serious adverse effects arising from the inappropriate use of fentanyl patches in relation to factors such as: start of titration with fentanyl at excessive doses or with too-fast increases; combination with other central nervous system depressants; drug interaction with antivirals (lopinavir, ritonavir); patch breakages resulting from applying pressure or bending and relative overdosing in elderly and thin patients. None of these situations were observed in the study.
We have found no literature references analysing the opioid titration process for breakthrough pain in clinical practice, so the results cannot be compared to other studies. Only changes in opioid and dose equivalences in the case of change have been analysed [32-34].
Conclusion
As conclusion, the decision algorithm in the dose adjustment for the treatment of breakthrough pain should be like that used for opioid titration for the treatment of chronic pain, though in general, opioid titration for breakthrough pain is much faster and simpler [30]. The study results may serve as a starting point and reference for the opioid titration process for breakthrough pain in real life. It would be relevant to design future randomized comparative studies comparing the titration processes and their clinical response among the different opioids recommended for the treatment of breakthrough pain, together with the assessment of their safety and efficacy.
Funding
This study was sponsored by Kyowa Kirin Farmacéutica S. L. U., Madrid, Spain. The sponsor was not involved in the study design, data collection, or analysis. The decision to publish this work was the sole initiative of the authors. Also, there was no influence on the preparation, review or approval of the manuscript.
The participation of the following researchers in the study is appreciated: Investigators Study TITULAR: Francisco Javier Afonso Afonso; Jonathan Aires Machado; Teresa Bonfill Abella; Pablo Borrega García; María Socorro Cabello Díaz; Nuria Calvo Verges; Raúl Carrillo Vicente; Luis Enrique Chara Velarde; Diego Díaz Cárdenas; Manuel Carlos Fernández Fernández; José Luis Firvida Pérez; Sebastián Gallardo Quesada; Almudena García Castaño; María Paola García Coves; Lara Iglesias Docampo; Julio José Lambea Sorrosal; Miguel Angel Lara Álvarez; Iker López Calderero; Almudena Martín Marino; Javier Munarriz Ferrandis; Juan Antonio Núñez Sobrino; Paola Pimentel Cáceres; Antonio Andrés Rueda Ramos; José Luis Sánchez Sánchez; Rocío Vilchez Simo; Pablo Villace Gallego.
Authors contributions
LID: Conceptualization, Methodology, Investigation, Writing (review and editing), Visualization, Supervision, Project administration, Funding acquisition. PPC, PGC, JFP, JLS, ARR, ILC and JNS: Conceptualization, Methodology, Investigation, Writing (review and editing), Visualization. AJL and ACA: Conceptualization, Methodology, Validation, Resources, Visualization, Project administration, Funding acquisition. BSL: Conceptualization, Methodology, Software, Validation, Formal analysis, Data curation, Writing (original draft preparation), Writing (review and editing), Visualization, Supervision, Project administration, Funding acquisition.
- Van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten E, Tjan-Heijnen VC, Janssen DJ (2016) Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J Pain Symptom Manage 51: 1070-1090. Link: https://tinyurl.com/yymzsflw
- Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E et al. (2014) Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 32: 4149-4154. Link: https://tinyurl.com/y2g9d3o6
- Van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, et al. (2007) Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol 18: 1437-1449. Link: https://tinyurl.com/y3ojc8qd
- World Health Organization (1996) Cancer pain relief and palliative care. World Health Organization. Link: https://tinyurl.com/yyrhtxs3
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C et al. (2012) Use of opioids in the treatment of cancer pain: Evidence based recommendations from the EAPC. Lancet Oncol 13: e58-e68. Link: https://tinyurl.com/yys37fjw
- Ripamonti CI, Bandieri E, Roila F, ESMO Guidelines Working Group (2011) Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol 22: vi69-vi77. Link: https://tinyurl.com/y4u9qe8e
- Portenoy RK, Hagen NA (1990) Breakthrough pain: definition, prevalence and characteristics. Pain 41: 273–281. Link: https://tinyurl.com/y2qyf8lw
- Portenoy RK, Payne D, Jacobsen P (1999) Breakthrough pain: characteristics and impact in patients with cancer pain. Pain 81: 129-134. Link: https://tinyurl.com/y2tkgz3h
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G, et al. (2009) The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 13: 331-338. Link: https://tinyurl.com/y56fkvza
- Bennett D, Burton AW, Fishman S, Fortner B, McCarberg BC et al. (2005) Consensus panel recommendations for the assessment and management of breakthrough pain. Part I Assessment P&T 30: 296-301. Link: https://tinyurl.com/y4gg5za4
- Bennett D, Burton AW, Fishman S, Fortner B, McCarberg B et al. (2005) Consensus panel recommendations for the assessment and management of breakthrough pain. Part 2 Management P&T 30: 354-361. Link: https://tinyurl.com/y6c9wjqy
- Deandrea S, Corli O, Consonni D, Villani W, Greco MT, et al. (2014) Prevalence of breakthrough pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage 47: 57-76. Link: https://tinyurl.com/y24vpgsv
- Gómez-Batiste X, Madrid F, Moreno F, Gracia A, Trelis J et al. (2002) Breakthrough Cancer Pain: Prevalence and Characteristics in Patients in Catalonia, Spain. J Pain Symptom Manage 24: 45–52. Link: https://tinyurl.com/y58eqxos
- Porta-sales J, Garzón C, Juliá J, Casals M (2010) Dolor irruptivo en cáncer. Med Clin 135: 280-285. Link: https://tinyurl.com/y5kdqc8l
- Muriel Villoria C. Dolor irruptivo (2002) Documento de Consenso. SEOM/ SECPAL/ SE/ Meditex. Madrid 27-28.
- Narayana A, Katz N, Shillington AC, Stephenson JJ, Harshaw Q et al. (2015) National breakthrough pain study: prevalence, characteristics and associations with health outcomes. Pain 156: 252-259. Link: https://tinyurl.com/y35em9e6
- Portenoy RK, Payne R, Coluzzi P, Raschko JW, Lyss A, et al. (1999) Oral transmucosal fentanyl citrate (OTFC) for the treatment of breakthrough pain in cancer patients: a controlled dose titration study. Pain 79: 303-312. Link: https://tinyurl.com/y2pnmlzq
- Olarte JM (2017) Breakthrough cancer pain and rational drug use. Support Care Cancer 25: 11-17. Link: https://tinyurl.com/y4cwx8wh
- Mercadante S (2015) Breakthrough pain in cancer patients: prevalence, mechanisms and treatment options. Curr Opin Anesthesiol 28: 559-564. Link: https://tinyurl.com/y3d33ljb
- Zeppetella G, Ribeiro MD (2006) Opioids for the management of breakthrough (episodic) pain in cancer patients. Cochrane Database Syst Rev CD004311. Link: https://tinyurl.com/y24c6v5d
- Karnofsky D, Burchenal J (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C, Ed. Evaluation of Chemotherapeutic Agents. New York, Columbia University Press: 191–205. Link: https://tinyurl.com/y3hz3hb4
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7: 6-9. Link: https://tinyurl.com/y3q5qtjt
- Carvajal A, Hribernik N, Duarte E, Sanz-Rubiales A, Centeno C (2013) The Spanish version of the Edmonton Symptom Assessment System-revised (ESAS-r): first psychometric analysis involving patients with advanced cancer. J Pain Symptom Manage 45: 129-136. Link: https://tinyurl.com/y2hu5gs7
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489-495. Link: https://tinyurl.com/y3lkgvb3
- Mercadante S, Valle A, Porzio G, Aielli F, Adile C et al. (2013) Relationship between background cancer pain, breakthrough pain, and analgesic treatment: a preliminary study for a better interpretation of epidemiological and clinical studies. Curr Med Res Opin 29: 667-671. Link: https://tinyurl.com/y6hlnulj
- Hagen NA, Fisher K, Victorino C, Farrar JT (2007) A titration strategy is needed to manage breakthrough pain effectively: observations from pooled from three clinical trials. J Palliat Med 10: 47-55. Link: https://tinyurl.com/y3srbsw8
- Mercadante S, Villari P, Ferrera P, Bianchi M, Casuccio A (2004) Safety and effectiveness of intravenous morphine for episodic (breakthrough) pain using a fixed ratio with the oral daily morphine dose. J Pain Symptom Manage 27: 352-359. Link: https://tinyurl.com/yxfelyyl
- Hanks GW, Conno F, Cherny N, Hanna M, Kalso E et al. (2001) Expert working group of the research network of the European association for palliative care. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 84: 587-593. Link: https://tinyurl.com/yxl5s5a4
- (2008) RAPID RESPONSE REPORT: NPSA/2008/RRR05: Reducing Dosing Errors with Opioid Medicines, National Patient Safety Agenc 1-11. Link: https://tinyurl.com/yy7keztg
- Health Canada (2008) Fentanyl transdermal patch and fatal adverse reactions. Canadian Adverse Reaction Newsletter 18: 1-2. Link: https://tinyurl.com/yyhtre89
- Ueberall MA, Lorenzl S, Lux EA, Voltz R, Perelman M (2016) Efficacy, safety and tolerability of fentanyl pectin nasal spray in patients with breakthrough pain. J Pain Res 9: 571-585. Link: https://tinyurl.com/yxepwkht
- Quigley C (2004) Opioid switching to improve pain relief and drug tolerability. Cochrane Database Syst Rev CD004847. Link: https://tinyurl.com/y3vt5la6
- Mercadante S, Bruera E (2006) Opioid switching: a systematic and critical review. Cancer Treat Rev 32: 304-315. Link: https://tinyurl.com/y5h9ycja
- Fallon M, Hanks G, Cherny N (2006) Principles of control of cancer pain. BMJ 332: 1022-1024. Link: https://tinyurl.com/y433e6sf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
