Open Journal of Parkinson's Disease and Treatment
Divergent Effects of Haloperidol on Motor Versus Spatial Functions
Robert Lalonde1* and Catherine Strazielle2
2University of Lorraine, Stress, Immunity, and Pathogens Laboratory, EA7300, 54500 Vandoeuvre-les-Nancy, France
Cite this as
Lalonde R, Strazielle C (2017) Divergent Effects of Haloperidol on Motor Versus Spatial Functions. Open J Parkinsons Dis Treatm 1(1): 032-038. DOI: 10.17352/ojpdt.000004The effects of haloperidol on motor versus cognitive factors were determined in mice. Haloperidol decreased open-field activity and impaired motor coordination in suspended bar and rotorod tests. The drug also augmented escape latencies in swimming towards submerged or visible goals in the Morris water maze without increasing path length or affecting the probe test of spatial memory. These results indicate that the dopamine-2 receptor blocker can reduce brain activation and impair motor control at doses that leave spatial learning unaffected.
Introduction
Parkinson’s disease causes motor and non-motor deficits mitigated at different degrees with dopamine replacement therapy [1-3]. In addition to the cardinal symptoms of muscle rigidity, akinesia, and resting tremor, patients with Parkinson’s disease display postural instabilities. In particular, patients fall more often than controls [4]. More worrisome are the findings that patients’ loss in postural control respond poorly to the primary treatment, l-dopa [5], and that l-dopa even impaired some aspects of it [6].
Motor and non-motor deficits have been reproduced in genetic models of Parkinson’s disease [7], or after injections of neurotoxic agents that deplete dopamine concentrations in striatum [8]. Moreover, parkinsonian symptoms appear in schizophrenic subjects after high doses of dopamine-2 (D2) receptor antagonists such as chlorpromazine (CPZ) and haloperidol, to a lesser extent the more selective D4 receptor antagonist, clozapine [9]. In the present study, motor and non-motor effects of haloperidol were examined in mice for exploratory activity in open-field [10], elevated plus-maze [11,12], and emergence [13] tests, motor coordination in stationary beam, coat-hanger, and rotorod [14] tests, and spatial learning in the Morris water maze [15,16].
Methods
Animals
C57BL/6JIco mice were obtained from Charles River, L’Arbresle, France, kept inside group cages with woodchip bedding under a 12/12 hr light-dark cycle, and tested in a separate room. The mice were controlled for age (range 11-15 months) and gender (males only). The research protocol adhered to guidelines of the National Institutes of Health (USA), the European Council Directive (86/609/EEC), and local animal care regulations.
Procedure
Mice were injected 60 min before the test with haloperidol (Haldol®, 5 mg/ml, Janssen-Cilag), diluted at 0.05 (n=9) or 0.1 mg/kg (n=9) or else saline (n=10) used as the vehicle solution and placebo. One placebo-injected mouse accidently died in the water maze, leaving n=9. The behavioral schedule was presented in randomized orders for different subgroups on weeks 1 and 2, including the following tests: week 1: open-field, elevated plus-maze, emergence, week 2 (after 3 days off): stationary beam, coat-hanger, and rotorod. Week 3 included the Morris water maze (testing days 7-12 for all mice).
The open-field (Letica model LE 8811, Bioseb, France) contained a 45 x 45 cm sized floor made of black perspex and 36 cm high transparent perspex walls. The device distinguished between fast (> 10 cm/s) and slow (< 10 cm/s) ambulatory and stereotyped movements as well as fast and slow rears. Horizontal activity was recorded by infrared photocell detectors with a 2.5 cm intercell distance in the horizontal axis and placed at a height of 1 cm. Vertical activity was recorded by a second row of sensors situated at a height of 5 cm. The mice were placed in the center of the apparatus in a 5-min session.
The elevated plus-maze (Letica model LE 840, Bioseb, France) consisted of 4 cross-shaped arms (length: 45 cm, width: 10 cm, height from floor: 68 cm) and a 10 cm x 10 cm central region with a floor made of black perspex. Two arms were enclosed on three sides by 9-cm high transparent perspex walls while the other two were not. The enclosed arms and the open arms faced each other on opposite sides. Entries (4-paw criterion) and time spent in enclosed and open arms were measured, together with open/total arm entry ratios. An entry occurred whenever the mice crossed from one arm to another with 4 paws, re-entries into the same arm from the central region not being counted. The mice were placed in the center, considered as enclosed time until an open arm was entered. Conversely, an entry into an open arm lasted until the mice entered into an enclosed arm. The mice were evaluated in a single 5-min session.
In the emergence test, the mice were placed inside a small toy object (orange plastic shoe, length: 13 cm, width: 6 cm, height: 7.5 cm) perforated with 3 holes 3 cm in diameter. This object was situated in the middle of a 41 cm x 27 cm enclosure made of white plastic and surrounded by 18-cm high walls. The mice were not pre-exposed to either apparatus. Latencies before emerging with two or four paws with foot contact on the floor of the wider compartment were determined in 2 trials of 5-min separated by 1 hr. Irrespective of whether emergence occurred, the mice were allowed to explore the large enclosure for 10 s. In all three tests of exploratory activity, the apparatus was wiped clean with a damp cloth and dried before evaluating the next mouse. Standard room illumination with white light was used.
The stationary beam (length: 110 cm; diameter: 2 cm; height: 70 cm from a padded surface) made of wood was covered with adhesive tape and separated into 11 segments by line drawings. A piece of cardboard was inserted at each end to prevent any mouse from escaping. The mice were placed in the middle of the beam and the number of segments crossed (4-paw criterion), the latencies before falling, and the number of falls were measured in a single 4-trial session with a 1-min cut-off period and a 15-min intertrial interval.
The triangular-shaped coat-hanger consisted of a horizontal steel wire (diameter: 2 mm, length: 41 cm, height: 38 cm from a padded surface) flanked by 2 diagonal side-bars (length: 19 cm; inclination: 35° from the horizontal axis). The mice were placed upside-down in the middle of the horizontal wire and released only when all four paws gripped it. Movement times (MTs) before reaching (snout criterion) the first 10 cm segment and the extremity of the horizontal wire were measured, as well as before climbing with 2, 3, or 4 paws and before reaching the midway and top of the diagonal wire. The latencies before falling and the number of falls were also recorded. A trial ended whenever the mice fell or reached the top of the apparatus, from which it was retrieved with a maximal score of 1 min given for latencies before falling. The test was performed in a single 4-trial session with a 1-min cut-off period and a 15-min intertrial interval.
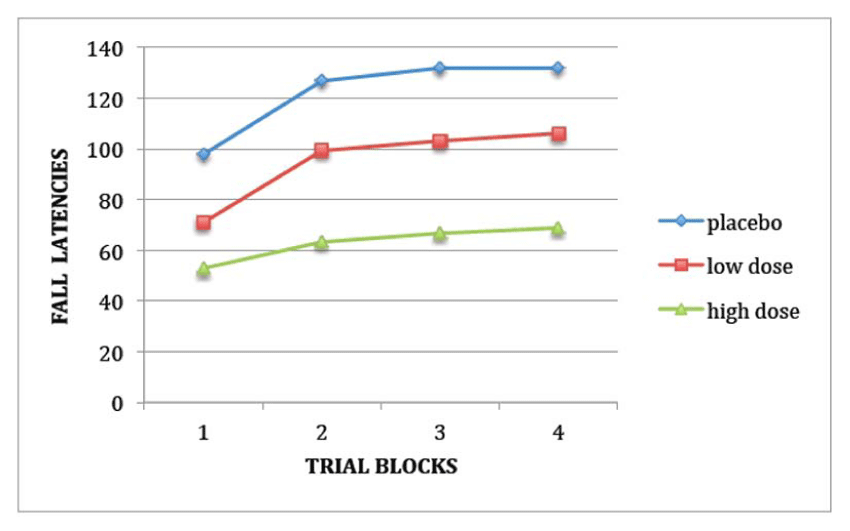
The accelerating rotorod (Letica Rota-Rod/RS, Bioseb, France) was made of striated white perspex (diameter: 3 cm, width: 5 cm; height: 17 cm), providing a firm grip, and flanked at each end by round plates to prevent any mouse from escaping. The mice were placed on top of the already revolving beam at a speed of 4 rpm and facing away from the experimenter’s view in the opposite orientation to beam movement in the longitudinal axis, so that forward locomotion was required for avoiding a fall. The rotorod gradually accelerated from 4 to 40 rpm over the 2-min trial. Latencies before falling were measured in an 8-trial session (4 trial blocks of 2 trials) with a 15-min intertrial interval. When the mice rotated passively for 2 complete turns, they were retrieved and the trials considered as a fall.
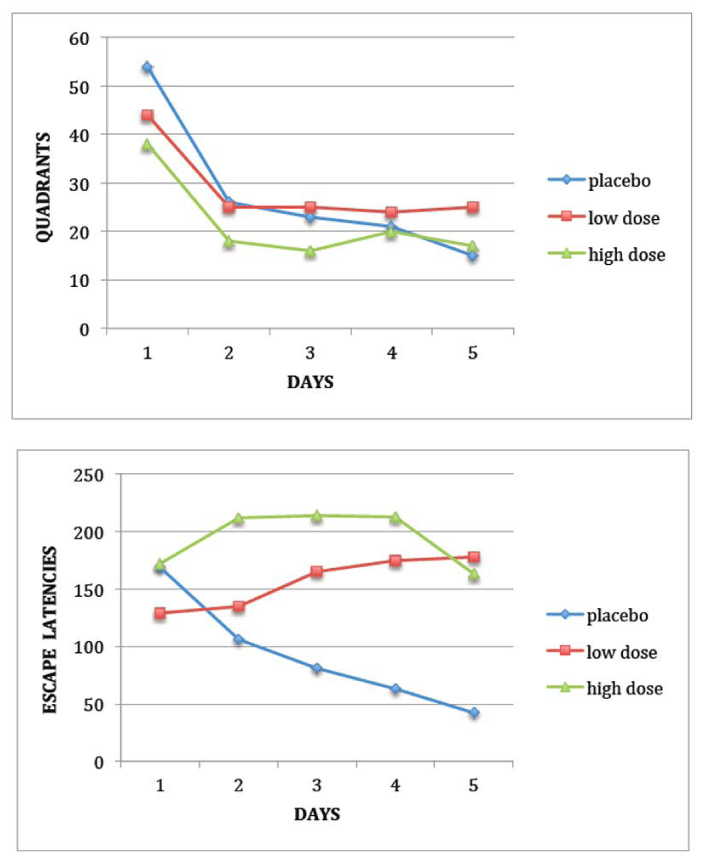
The Morris water maze consisted of a basin (diameter: 86 cm, wall height: 30 cm) made of white plastic and filled with water (22°C) at a height of 21 cm. Yellow plastic beads were evenly spread over the water surface to camouflage the escape platform (diameter: 8 cm) made of white plastic and covered with a yellow wire mesh grid to ensure a firm grip. The pool was contained in a room with several extramaze visual cues, such as light fixtures and laboratory instruments. The mice were placed next to and facing the wall successively in north (N), east (E), south (S), and west (W) positions, with the escape platform hidden 1 cm beneath water level in the middle of the NW quadrant. The experimenter followed their swimming trajectories on a videomonitor, on which the image of the pool was separated into 4 equally spaced quadrants. The quadrant entries (4-paw criterion) and escape latencies were measured in 4-trial sessions for 5 days with a 15-min intertrial interval. The mice remained on the escape platform for at least 5 s. Whenever the mice failed to reach the platform within the 1 min cut-off period, they were retrieved from the pool and placed on it for 5 s. After their swim, the mice were kept dry in a plastic holding cage filled with paper towels. The day after the acquisition phase (testing day 6), a probe trial was conducted by removing the platform and placing the mouse next to and facing the N side. The time spent in the previously correct quadrant was measured in a single 1-min trial. One hour later, the visible platform version was evaluated, with the escape platform lifted 1 cm above water level and shifted to the SE quadrant. A 13-cm high pole was inserted on top of the escape platform as a viewing aid. In an identical manner to the place learning task, quadrant entries and escape latencies were measured for 4 trials per session, the animals stayed on the platform for 5 s, and a 1-min cut-off period was imposed with a 15-min intertrial interval, except that the test was conducted in a single day.
Data analyses
The three groups were compared with analyses of variance (ANOVAs) with or without repeated measures followed by pairwise comparisons with PLSD Fisher. Data are presented in mean values ± SEM and statistical significance level set at P=0.05.
Results
Exploratory activity
In the open-field, haloperidol decreased all six facets of motor activity (F2,25>7.6, P < 0.01) except fast rears (F2,25=1.14, P > 0.05), reflecting in part escape attempts. The 0.1 mg/kg dose reduced the five measures, whereas the 0.05 mg/kg dose reduced two of the five (Table 1). In the elevated plus-maze (Table 1), haloperidol decreased open (F2,25=13.84, P < 0.001) and enclosed (F2,25=22.47, P < 0.001) arm entries without affecting open/total arm entries and duration. The groups did not differ in emergence from the small chamber (Table 1) despite higher latencies for haloperidol, possibly because of high intragroup variances.
Motor coordination
Haloperidol exerted detrimental actions on stationary beam, coat-hanger, and rotorod tests (Table 2 and Figure 1). On the stationary beam, haloperidol decreased segments crossed (F2,25=11.97, P < 0.001) without affecting fall latencies or number of falls (P > 0.05). On the coat-hanger, haloperidol increased all MTs (F2,25>3.89, P < 0.05) as well as number of falls (F2,25=4.54, P < 0.05), except MTs to the midway point or top of the diagonal bar (P > 0.05), probably because of a ceiling effect. It also diminished fall latencies (F2,25=5.83, P < 0.01). The haloperidol-induced decrease in fall latencies appeared even worse on the rotorod (F2,25=10.15, P < 0.001), the trial effect being also significant due to rising values as a result of practice (F3,75=11.78, P < 0.001). All these effects were more prominent at the high relative to the low dose.
Spatial learning
Haloperidol increased escape latencies (Figure 2, F2,24=30.4, P < 0.001) but decreased quadrant entries (F2,24=3.8, P < 0.05) in the submerged platform subtask. There was a drug x day interaction on escape latencies (F8,96=8.81, P < 0.001) as a result of the faster placebo group as training proceeded, but only a day effect on quadrant entries as a result of learning (F4,96=30.88, P < 0.001). There was no effect in the probe test (P > 0.05, maximal: 60 s, placebo: 29.7 ± 2.1 s; low-dose haloperidol: 30.8 ± 5.9 s; high-dose haloperidol: 26.3 ± 5.8 s). In the visible platform subtask, haloperidol augmented escape latencies (F2,24=22.7, P < 0.001; summed over 4 trials placebo: 32.0 ± 7.1 s, low-dose haloperidol: 91.3 ± 9.7 s, high-dose haloperidol: 115.9 ± 10.1 s) without affecting quadrant entries (placebo: 12.9 ± 1.8, low-dose haloperidol: 13.8 ± 1.2, high-dose haloperidol: 15.6 ± 1.5). Higher escape latencies occurred at both doses.
Discussion
Exploratory activity
Haloperidol reduced horizontal and vertical open-field activity in C57BL/6JIco mice, for fast ambulation at 0.1 mg/kg, for slow ambulation with at a dose as little as 0.05 mg/kg IP. Likewise, at 0.3 mg/kg SC, haloperidol reduced motor activity in Swiss–Kunming mice [17], and at 0.1 mg/kg IP in rats [18], as when the drug was injected into the nucleus accumbens [19]. Such effects are likely mediated by the D2 receptor, perhaps also the D4 receptor, since a more specific D4 receptor antagonist, L-745,870, also decreased mouse open-field activity [20]. Higher haloperidol doses are required to cause catalepsy, as for example 2 mg/kg IP in rats [21].
Motor coordination
Haloperidol at 0.1 mg/kg IP slowed down rotorod acquisition in C57BL/6JIco mice, the same deficit found after acute haloperidol administration in Swiss [22,23], and FVB/N [24,25], mice as well as rats [26,27], or rats chronically administered with the drug over a 3-week period [28,29]. Effective acute doses were 0.03, 0.1, and 0.3 mg/kg IP [26], 0.8 mg/kg IP [22], 1 mg/kg IP [23,25], but not 0.3 mg/kg IP in ddY mice [30]. The rotorod deficit appeared even when combined with apomorphine [31]. A rotorod impairment was also reported in Drd2 null mutant mice deficient for the D2 receptor but rather linked to the genetic background [32]. The deficit appears with other D2 receptor blockers such as CPZ [33,34], but the influence of D4 receptors must be considered, since selective antagonists, clozapine [35], and L-745,870 [20], also caused rotorod deficits in mice.
In addition to the rotorod, haloperidol diminished distance travelled on the stationary beam, but in view of open-field decrements, this is likely due to hypoactivity. Haloperidol increased latencies before crossing the stationary beam and footslips in rats [28]. Haloperidol-induced defects extended to longer MTs and shorter fall latencies in the coat-hanger test.
Spatial learning
Relative to controls, haloperidol in the 0.05-0.1 mg/kg IP range increased escape latencies before reaching the submerged platform while decreasing quadrant entries. Likewise, escape latencies to the submerged platform, not distance swum, rose after haloperidol administration at 0.15 mg/kg PO for 14 days [36], 1.0 or 2.0 mg/kg IP for 6 weeks [37], or after being dissolved in drinking water for 90 days [38]. In these studies, haloperidol-induced slowing was not accompanied by spatial disorientation as would be the case if path lengths were longer. In our study, the probe test was unaffected, indicating accurate memory of platform location. As in our study, escape latencies before reaching the submerged platform increased with as low a dose as 0.1 mg/kg SC in Swiss–Kunming mice [17]. At even lower doses of 0.04 and 0.07 mg/kg IP, haloperidol increased escape latencies in the submerged platform subtask in rats without affecting the visible platform subtask, while 0.1 mg/kg affected both [18], the same effect as in our study at that dose and mimicked when the drug was infused in the nucleus accumbens [19]. In addition to normal mice and rats, haloperidol deteriorated maze learning in rats with traumatic brain injury [39,40]. A different conclusion may be reached in regard to cognitive versus motor effects on considering that haloperidol at 0.04 mg/kg SC increased the number of rats unable to reach the submerged platform within a minute without slowing down swim speed, while at 0.08 mg/kg both variables were impaired [41]. However, path length was not measured in that study. Neuroleptic effects on other spatial paradigms are variable. On one hand, CPZ impaired delayed spatial discrimination performance [42], on the other, haloperidol did not affect recognition of familiar versus unfamiliar environments [43].
Non-spatial learning
Extensive experiments have shown the detrimental impact of neuroleptics on shock avoidance learning. In particular, CPZ impaired the acquisition and performance of active avoidance responses to a conditioned stimulus in rodents [44-46], and humans [47], without affecting shock escape responses. This defect did not extend to brightness discrimination performance motivated by shock [48], or the conditioned emotional response [49]. Yet CPZ delayed acquisition and performance of a light-dark discrimination motivated by water escape [50,51]. The deleterious impact included pre- and post-trial administration, indicating interference with memory consolidation.
The influence of cognitive factors underlying neuroleptic action can best be ascertained in appetitive as opposed to shock avoidance or water escape paradigms where negative results predominate when injected pre-trial [52]. CPZ had no effect in rats acquiring simultaneous discriminations for stimulus brightness [53], or shape despite longer choice latencies at two levels of task difficulty [54]. Moreover, CPZ had no effect on the acquisition of a simultaneous light-dark discrimination in rat offspring when given to dams from gestation day 17 to 21, though it impaired reversal training [55,56]. Another potent neuroleptic, pimozide, had no effect on the acquisition or perfomance of simultaneous light-dark discrimination learning in food-deprived rats [57,58], and performance in food-deprived pigeons [59]. However, CPZ impaired visual discrimination performance in pigeons trained with error-filled though not errorless trials [60], and haloperidol impaired the performance of concurrent object discriminations with 4-hr [61], or 24-hr [62], intertrial intervals in monkeys, the former being dependent and the latter independent, respectively, on the limbic system. The drug-induced vulnerability in the monkey task is likely due to added complexity relative to those required in rodents. Yet the influence of the limbic system is further indicated by poorer discrimination learning after post-trial intrahippocampal injection of haloperidol in rats [63]. Moreover, post-trial IP injections of CPZ slowed down acquisition of a discrimination task in water-deprived rats [64]. Pre-trial neuroleptic administration can also impair limbic-dependent recognition memory, as shown by deteriorated matching-to-sample performance between seven geometric symbols in monkeys [65], and two color-matching in pigeons [66,67], though not two color-matching [42], or clip art and digitized photo non-matching [62], in monkeys.
Although neuroleptics mitigate the impact of reinforcement in operant paradigms requiring lever-presses, response accuracy is mainly unaffected. Primozide was without effect on successive discriminated operant responses distinguishing reinforced from non-reinforced trials for food reward in rats at doses which reduced arousal and the effects of reinforcement [68]. Likewise, accuracy in discriminative operant responding of lever choice was normal after administration of haloperidol or CPZ, although clozapine decreased it [69]. Pimozide also declined response rates without affecting choice accuracy in a self-stimulation discrimination paradigm [70]. Moreover, acquisition of a discriminated operant task requiring the adjustment of response rates according to changes in reinforcement schedule was normal in PPP1R1B null mutant mice deficient in dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32), though reversal training was impaired [71]. Thus, dopamine blockade prevents reward mechanisms at doses that leave cognition unaffected except when cognitive shifting is required.
In a parallel manner, concept learning was intact in hyperactive children chronically treated with CPZ [72]. In normal young adult subjects, CPZ impaired the continuous performance task dependent on arousal more than the digit symbol substitution task dependent on executive control [73], although neuroleptics impaired the latter relative to placebo in young [74,75], and old [75], subjects and prevented an intersession practice effect in the young [76].
- Spiegel J, Uhrig I, Krick C, Behnke S, Fassbender K, et al. (2014) Performance of repetitive alternating elbow movements in Parkinson's disease. Eur Neurol 71: 84-88. Link: https://goo.gl/g726wY
- Aarsland D, Brønnick K, Fladby T (2011) Mild cognitive impairment in Parkinson's disease. Curr Neurol Neurosci Rep 11: 371-378. Link: https://goo.gl/VmTFvB
- Leroi I (2010) Cognitive and behavioural complications of Parkinson's disease. Adv Clin Neurosci 10: 10-14. Link: https://goo.gl/kYdbo2
- Allen NE, Schwarzel AK, Canning CG (2013) Recurrent falls in Parkinson's disease: a systematic review. Parkinson's Dis 2013: 906274. Link: https://goo.gl/4squCc
- Kataoka H, Ueno S (2017) Can postural abnormality really respond to levodopa in Parkinson's disease? J Neurol Sci 377: 179-184. Link: https://goo.gl/srHMA5
- Bonnet CT, Delval A, Szaffarczyk S, Defebvre L (2017) Levodopa has primarily negative influences on postural control in patients with Parkinson's disease. Behav Brain Res 331: 67-75. Link: https://goo.gl/3UUhVK
- Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson's disease. Neuron 66: 646-661. Link: https://goo.gl/i66Hw6
- Mehan S, Kaur G, Dudi R, Manju Rajput M, Kalra S (2017) Restoration of mitochondrial dysfunction in 6-hydroxydopamine induced Parkinson's disease: a complete review. Open J Parkinsons Dis Treatm 1: 001-026. Link: https://goo.gl/JPx2NH
- Shin HW, Chung SJ (2012) Drug-induced parkinsonism. J Clin Neurol 8: 15-21. Link: https://goo.gl/dkJohv
- Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83: 482-504. Link: https://goo.gl/i97ZoT
- Cole JC, Rodgers RJ (1993) An ethological analysis of the effects of chlordiazepoxide and bretazenil (Ro 16-6028) in the murine elevated plus-maze. Behav Pharmacol 4: 573-580. Link: https://goo.gl/wWxefY
- Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149-167. Link: https://goo.gl/XmHZEA
- Paré WP, Tejani-Butt S, Kluczynski J (2001) The emergence test: effects of psychotropic drugs on neophobic disposition in Wistar Kyoto (WKY) and Sprague Dawley rats. Prog Neuropsychopharmacol Biol Psychiatry 25: 1615-1628. Link: https://goo.gl/eePqWT
- Lalonde R, Strazielle C (2013) Motor coordination in normal mouse strains and the underlying role of the cerebellar system. In Handbook of Behavioral Genetics of the Mouse, Volume 1. Edited by Crusio WE, Sluyter F, Gerlai R, Pietropaolo S, Cambridge University Press 81-87. Link: https://goo.gl/2yS2PF
- Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297: 681-683. Link: https://goo.gl/fDYp3i
- McNamara RK, Skelton RW (1993) The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev 18: 33-49.
- Hou Y, Wu CF, Yang JY, Guo T (2006) Differential effects of haloperidol, clozapine and olanzapine on learning and memory functions in mice. Prog Neuropsychopharmacol Biol Psychiatry 30: 1486-1495. Link: https://goo.gl/zQ2CMP
- Ploeger GE, Spruijt BM, Cools AR (1992) Effects of haloperidol on the acquisition of a spatial learning task. Physiol Behav 52: 979-983. Link: https://goo.gl/MuYhkc
- Ploeger GE, Spruijt BM, Cools AR (1994) Spatial localization in the Morris water maze in rats: acquisition is affected by intra-accumbens injections of the dopaminergic antagonist haloperidol. Behav Neurosci 108: 927-934. Link: https://goo.gl/kM7mG4
- Bristow LJ, Collinson N, Cook GP, Curtis N, Freedman SB, et al. (1997) L-745,870, a subtype selective dopamine D4 receptor antagonist, does not exhibit a neuroleptic-like profile in rodent behavioral tests. J Pharmacol Exp Ther 283: 1256-1263. Link: https://goo.gl/7JaEWB
- Depoortere R, Boulay D, Perrault G, Bergis O, Decobert M, et al. (2003) SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist. II: Behavioral profile predictive of an atypical antipsychotic activity. Neuropsychopharmacology 28: 1889-1902. Link: https://goo.gl/TBzW72
- Mabrouk OS, Marti M, Morari M (2010) Endogenous nociceptin/orphanin FQ (N/OFQ) contributes to haloperidol-induced changes of nigral amino acid transmission and parkinsonism: a combined microdialysis and behavioral study in naïve and nociceptin/orphanin FQ receptor knockout mice. Neuroscience 166: 40-48. Link: https://goo.gl/hNzuJv
- Mohajjel Nayebi A, Sheidaei H (2010) Buspirone improves haloperidol-induced Parkinson disease in mice through 5-HT(1A) receptors. Daru 18: 41-45. Link: https://goo.gl/v14YKz
- Kirschbaum KM, Hiemke C, Schmitt U (2009) Rotarod impairment: catalepsy-like screening test for antipsychotic side effects. Int J Neurosci 119: 1509-1522. Link: https://goo.gl/wz6KR5
- Kirschbaum KM, Henken S, Hiemke C, Schmitt U (2008) Pharmacodynamic consequences of P-glycoprotein-dependent pharmacokinetics of risperidone and haloperidol in mice. Behav Brain Res 188: 298-303. Link: https://goo.gl/So4kZi
- Steinpreis RE, Anders KA, Branda EM, Kruschel CK (1999) The effects of atypical antipsychotics and phencyclidine (PCP) on rotorod performance. Pharmacol Biochem Behav 63: 387-394. Link: https://goo.gl/Eo5Qqq
- Johnson SK, Wagner GC, Fischer H (1992) Neurochemical and motor effects of high dose haloperidol treatment: exacerbation by tryptophan supplementation. Proc Soc Exp Biol Med 200: 571-575. Link: https://goo.gl/FGHE3v
- Datta S, Jamwal S, Deshmukh R, Kumar P (2016) Beneficial effects of lycopene against haloperidol induced orofacial dyskinesia in rats: possible neurotransmitters and neuroinflammation modulation. Eur J Pharmacol 771: 229-235. Link: https://goo.gl/ATvm8G
- Samad N, Haleem DJ (2014) Haloperidol-induced extra pyramidal symptoms attenuated by imipramine in rats. Pak J Pharm Sci 27: 1497-1501. Link: https://goo.gl/U1YXpW
- Nagai H, Egashira N, Sano K, Ogata A, Mizuki A, et al. (2006) Antipsychotics improve Delta9-tetrahydrocannabinol-induced impairment of the prepulse inhibition of the startle reflex in mice. Pharmacol Biochem Behav 84: 330-336. Link: https://goo.gl/k8kqq2
- Fenton HM, Leszczak E, Gerhardt S, Liebman JM (1984) Evidence for heterogeneous rotational responsiveness to apomorphine, 3-PPP and SKF 38393 in 6-hydroxydopamine-denervated rats. Eur J Pharmacol 106: 363-372. Link: https://goo.gl/RW7tX1
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, et al. (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18: 3470-3479. Link: https://goo.gl/RvZquY
- Gomita Y, Ichimaru Y, Moriyama M, Furuno K, Suemaru K, et al. (1990) Behavioral effects of HR-592, a new derivative of indole. Jpn J Pharmacol 52: 609-619. Link: https://goo.gl/PDD3zH
- Perez-Raya MD, Risco S, Navarro C, Jimenez J, Cabo J (1989) Changes in chlorpromazine induced CNS effects when associated with other neuroleptics and anxiolytics. Pharm Acta Helv 64: 146-150. Link: https://goo.gl/V4kq5L
- Park WK, Jeong D, Yun CW, Lee S, Cho H, et al. (2003) Pharmacological actions of a novel and selective dopamine D3 receptor antagonist, KCH-1110. Pharmacol Res 48: 615-622. Link: https://goo.gl/Tur3WJ
- Nowakowska E, Kus K, Czubak A, Glowacka D, Matschay A (2007) Some behavioural effects of carbamazepine - comparison with haloperidol. J Physiol Pharmacol 58: 253-264. Link: https://goo.gl/QJw67Y
- Xu H, Yang HJ, Rose GM (2012) Chronic haloperidol-induced spatial memory deficits accompany the upregulation of D(1) and D(2) receptors in the caudate putamen of C57BL/6 mouse. Life Sci 91: 322-328. Link: https://goo.gl/ocYSNc
- Terry AV Jr, Hill WD, Parikh V, Evans DR, Waller JL, et al. (2002) Differential effects of chronic haloperidol and olanzapine exposure on brain cholinergic markers and spatial learning in rats. Psychopharmacology 164: 360-368. Link: https://goo.gl/nydfhk
- Phelps T, Bondi CO, Ahmed RH, Olugbade YT, Kline AE (2015) Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J Neurotrauma 32: 590-597. Link: https://goo.gl/yeixD5
- Wilson MS, Gibson CJ, Hamm RJ (2003) Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am J Phys Med Rehabil 82: 871-879. Link: https://goo.gl/27f5Sw
- Skarsfeldt T (1996) Differential effect of antipsychotics on place navigation of rats in the Morris water maze. A comparative study between novel and reference antipsychotics. Psychopharmacology (Berl) 124: 126-133. Link: https://goo.gl/2omdTU
- Hironaka N, Miyata H, Ando K (1992) Effects of psychoactive drugs on short-term memory in rats and rhesus monkeys. Jpn J Pharmacol 59: 113-120. Link: https://goo.gl/bGBoLH
- Orsetti M, Colella L, Dellarole A, Canonico PL, Ghi P (2007) Modification of spatial recognition memory and object discrimination after chronic administration of haloperidol, amitriptyline, sodium valproate or olanzapine in normal and anhedonic rats. Int J Neuropsychopharmacol 10: 345-357. Link: https://goo.gl/xD3vZX
- Ader R, Clink D (1957) Effects of chlorprozamine on the acquisition and extinction of an avoidance response in the rat. J Pharmacol Exp Ther 121: 144-148. Link: https://goo.gl/vmyvHf
- Crismon C (1967) Chlorpromazine and imipramine: parallel studies in animals. Psychopharmacol Bull 4: 1-151. Link: https://goo.gl/o3KPDj
- Doty LA, Doty BA (1964) Effects of age and chlorpromazine on animal memory consolidation. J Comp Physiol Psychol 57: 331-334. Link: https://goo.gl/kwFVAv
- Fischman MW, Smith RC, Schuster CR (1976) Effects of chlorpromazine on avoidance and escape responding in humans. Pharmacol Biochem Behav 4: 111-114. Link: https://goo.gl/4YL9U4
- Tang AH, Franklin SR (1983) Disruption of brightness discrimination in a shock avoidance task by phencyclidine and its antagonism in rats. J Pharmacol Exp Ther 225: 503-508. Link: https://goo.gl/VXsHZc
- Lepore F, Ptito M, Freibergs V, Guillemot JP (1974) Effects of low doses of chlorpromazine on a conditioned emotional response in the rat. Psychol Rep 34: 231-237. Link: https://goo.gl/SDQXmj
- Castellano C (1971) Lysergic acid diethylamide, amphetamine and chlorpromazine on water maze discrimination in mice. Psychopharmacologia 19: 16-25. Link: https://goo.gl/Jnj8CR
- Castellano C (1977) Effects of chlorpromazine and imipramine on discrimination learning, consolidation, and learned behavior in two inbred strains of mice. Psychopharmacology (Berl) 53: 27-31. Link: https://goo.gl/e46t5A
- Salamone JD (1994) The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res 61: 117-133. Link: https://goo.gl/Mk2SVX
- Telner JI, Vikis-Freibergs V, Lepore F (1976) Effects of low and moderate doses of chlorpromazine on discrimination learning in the rat. Psychopharmacologia 47: 205-208. Link: https://goo.gl/XizUky
- Lalonde R, Vikis-Freibergs V (1982) The effects of chlorpromazine and lithium on appetitive discrimination learning in the rat. Psychopharmacology (Berl) 76: 218-221. Link: https://goo.gl/YZqHWw
- Hironaka N, Umemura T (1988) Influence of maternal chlorpromazine on discrimination learning in rat offspring. Neurotoxicol Teratol 10: 199-205. Link: https://goo.gl/eB7fst
- Umemura T, Hironaka N, Takada K, Sasa H, Yanagita T (1983) Influence of chlorpromazine administered to rat dams in the peripartum and nursing periods on the learning behavior of the second generation. J Toxicol Sci 8: 105-118. Link: https://goo.gl/BWVxBa
- Tombaugh TN, Ritch MA, Shepherd DT (1980) Effects of pimozide on accuracy of performance and distribution of correct responding on a simultaneous discrimination task in the rat. Pharmacol Biochem Behav 13: 859-862. Link: https://goo.gl/pB51N5
- Tombaugh TN, Szostak C, Mills P (1983) Failure of pimozide to disrupt the acquisition of light-dark and spatial discrimination problems. Psychopharmacology (Berl) 79: 161-168. Link: https://goo.gl/UkKNZq
- Tombaugh TN (1981) Effects of pimozide on nordiscriminated and discriminated performance in the pigeon. Psychopharmacology (Berl) 73: 137-141. Link: https://goo.gl/4LDqz6
- Terrace HS (1963) Errorless discrimination learning inthe pigeon: effects of chlorpromazine and impiramine. Science 140: 318-319. Link: https://goo.gl/RkxWy4
- Turchi J, Devan B, Yin P, Sigrist E, Mishkin M (2010) Pharmacological evidence that both cognitive memory and habit formation contribute to within-session learning of concurrent visual discriminations. Neuropsychologia 48: 2245-2250. Link: https://goo.gl/HJHfjH
- Turchi J, Buffalari D, Mishkin M (2008) Double dissociation of pharmacologically induced deficits in visual recognition and visual discrimination learning. Learn Mem 15: 565-568. Link: https://goo.gl/bxCDF2
- Grecksch G, Matties H (1981) The role of dopaminergic mechanisms in the rat hippocampus for the consolidation in a brightness discrimination. Psychopharmacology (Berl) 75: 165-168. Link: https://goo.gl/qYwq7L
- Ishikawa K, Saito S (1976) Differences in the effects of post-trial chlorpromazine, reserpine, and amphetamine on discrimination learning in rats. Psychopharmacology (Berl) 48: 45-51. Link: https://goo.gl/4EAaHo
- Ferguson SA, Paule MG (1992) Acute effects of chlorpromazine in a monkey operant behavioral test battery. Pharmacol Biochem Behav 42: 333-341. Link: https://goo.gl/45kMMS
- Newland MC, Marr MJ (1985) The effects of chlorpromazine and imipramine on rate and stimulus control of matching to sample. J Exp Anal Behav 44: 49-68. Link: https://goo.gl/HiEir9
- Watson JE, Blampied NM (1989) Quantification of the effects of chlorpromazine on performance under delayed matching to sample in pigeons. J Exp Anal Behav 51: 317-328. Link: https://goo.gl/MhjF5i
- Beninger RJ (1982) A comparison of the effects of pimozide and nonreinforcement on discriminated operant responding in rats. Pharmacol Biochem Behav 16: 667-669. Link: https://goo.gl/88KYQ4
- Picker M (1988) Effects of clozapine on fixed-consecutive-number responding in rats: a comparison to other neuroleptic drugs. Pharmacol Biochem Behav 30: 603-612. Link: https://goo.gl/QxupRj
- Bowers W, Hamilton M, Zacharko RM, Anisman H (1985) Differential effects of pimozide on response-rate and choice accuracy in a self-stimulation paradigm in mice. Pharmacol Biochem Behav 22: 521-526. Link: https://goo.gl/WdWXwf
- Heyser CJ, Fienberg AA, Greengard P, Gold LH (2000) DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res 867: 122-130. Link: https://goo.gl/DgRNb1
- Freibergs V, Douglas VI, Weiss G (1968) The effect of chlorpromazine on concept learning in hyperactive children under two conditions of reinforcement. Psychopharmacologia 13: 299-310. Link: https://goo.gl/JBdwJU
- Mirsky AF, Kornetsky C (1964) On the dissimilar effects of drugs on the digit symbol substitution and continuous performance tests. Psychopharmacologia 5: 161- 177. Link: https://goo.gl/ehyhe6
- Lynch G, King DJ, Green JF, Byth W, Wilson-Davis K (1997) The effects of haloperidol on visual search, eye movements and psychomotor performance. Psychopharmacology (Berl) 133: 233-239. Link: https://goo.gl/62nAth
- Allain H, Tessier C, Bentué-Ferrer D, Tarral A, Le Breton S, et al. (2003) Effects of risperidone on psychometric and cognitive functions in healthy elderly volunteers. Psychopharmacology (Berl) 165: 419-429. Link: https://goo.gl/yF5gHt
- Veselinovic T, Schorn H, Vernaleken IB, Hiemke C, Zernig G, et al. (2013) Effects of antipsychotic treatment on cognition in healthy subjects. J Psychopharmacol 27: 374-385. Link: https://goo.gl/1viWy4
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
