Open Journal of Parkinson's Disease and Treatment
Restoration of Mitochondrial Dysfunction in 6-Hydroxydopamine Induced Parkinson’s disease: a Complete Review
Sidharth Mehan*, Gurpreet Kaur, Rajesh Dudi, Manju Rajput and Sanjeev Kalra
Cite this as
Mehan S, Kaur G, Dudi R, Rajput M, Kalra S (2017) Restoration of Mitochondrial Dysfunction in 6-Hydroxydopamine Induced Parkinson’s disease: a Complete Review. Open J Parkinsons Dis Treatm 1(1): 001-026. DOI: 10.17352/ojpdt.000001Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by neuronal cell death in the specific brain region like basal ganglia, cerebral cortex and hippocampus. Symptoms associated with PD patients are rigidity, akathesia, tremor, postural imbalance, cognitive and memory dysfunctions. Pathological hallmarks are dopaminergic neuronal degeneration, neuro-inflammation, oxidative stress, free radical generation. In the typical Parkinson’s disease model, 6-Hydroxydopamine (6-OHDA) is delivered unilaterally by stereotactic injection into the SNc (substantia nigra pars-compacta) or the striatum mimics the PD symptoms. In addition, it has been shown that 6-OHDA is toxic to complex I & IV of the mitochondrial respiratory chain, leading to subsequent respiratory inhibition and further processed ATP depletion, oxidative stress and neuro-inflammation. Forskolin (FSK), a diterpene natural plant phytochemical obtained from (Coleus Forskohli), a potent direct activator of adenyl cyclase (AC) enzyme which further activates cAMP/PKA/CREB pathway. FSK mediated activation of AC/cAMP/PKA/CREB pathway is responsible for various neuroprotective mechanisms Based on important and versatile role of FSK, the present study has been designed to investigate the role of cAMP mediated CREB activation in 6-hydroxydopamine induced mitochondrial associated neurotoxicity in rats. Further the studies are extended to understand the disease pathogenesis and to investigate and discuss the various possible central mechanisms involved in the effect of such targets using behavioral paradigm and biochemical markers of neurodegeneration.
Introduction
Parkinson disease (PD) is a neurodegenerative disease characterized by manifestations of motor deficits such as tremors at rest, rigidity in muscles, akinesia and postural imbalance [1,2]. Brain pathology shows loss of neurons in the substantia nigra pars compacta (SNpc), with the presence of eosinophilic protein deposits (Lewy bodies) in the cytoplasm, and dopamine (DA) striatal depletion [3,4]. James Parkinson was first to describe the clinical pathological features of this disease as “shaking palsy” in his classic 1817 monograph as “Involuntary tremulous motion, with lessened muscular power, in parts not in action and even when supported; with a propensity to bend the trunk forwards, and to pass from a walking to a running pace: the senses and intellects being uninjured” [5]. India with one of the world’s lowest incidence of PD (70 out of 100,000) [6]. Motor disabilities of PD associated with dopaminergic neuronal cell loss and resultant dysfunction of the basal ganglia (BG) where a cluster of deep nuclei that participate in the initiation and execution of movements [7]. Non-motor symptoms including impairments of memory and olfaction, disturbance sleep and neuropsychiatric manifestations like depression, hallucinations, and dementia become prominent, and these features are probably due to the spread of Parkinsonian pathology beyond the BG with the continued involvement of inflammation and oxidative stress [8-10]. Several genes that also involved in the progression of PD, are α-synuclein (SNCA), Parkin (PARK2), UCHL-1 (PARK5), DJ-1 (PARK7), PINK1 (PARK6), LRRK2 (PARK8), NR4A2 (NURR1), PARK3, PARK4, PARK9, PARK10 and PARK11 [11].
Neuropathological feature of PD includes degeneration of brain stem nuclei & loss of dopaminergic neurons, abnormalities in mitochondrial complexes I to V cellular protein transport, interaction between SNCA proteins & protein aggregation, excitotoxicity, oxidative stress and depletion of striatal DA levels, cholinergic deficit, presence of intra-neuronal proteinacious cytoplasmic inclusions, termed “lewy bodies”, are the main cause of neuronal cell death in PD [12,13]. The output projections of the striatum have been divided into a direct and an indirect pathway. The direct pathway projects from the striatum to the GPi (globus pallidus internal) and SNpr (substantia nigra pars reticulata) and from there to the thalamus. The indirect pathway projects from the striatum to the GPe (globus pallidus external), which in turn projects to the GPi and the SNpr, further terminates in the thalamus [14]. Decreased levels of DA results in increased activity along the indirect pathway and decreased activity along the direct pathway together result in increased excitation of GPi and SNPr neurons, then to increased inhibition of thalamic neurons, and finally to decreased excitation of the cortex [15-17]. The indirect pathway normally excites the output nuclei which is inhibited in PD [18,19].
In addition, mitochondrial free radical theory also explains the mechanistic basis of aging. Dysfunction of mitochondria is well known to generate reactive oxygen species (ROS), reduce adenosine-triphosphate (ATP) production, increased deoxyribonucleic acid (DNA) mutations as well as induces abnormal cristae structures and impairs intracellular calcium (Ca) levels ultimately affects neurons and accelerates neurodegenerative processes [20,21].
Furthermore, the link between oxidative stress and like dopaminergic neuronal degeneration, inhibition of mitochondrial complex I to V, lewy Bodies (LB) protein aggregation, neuro-inflammation and defect in mitochondrial morphology & membrane potential is further supported by modeling the motor aspects of PD in animals with toxins that cause oxidative stress including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, 1,1’- dimethyl-4,4’-bipyridinium dichloride (paraquat), and 6-hydroxydopamine (6-OHDA) [22-31]. The characterization of the hydroxylated analogue of DA i.e. 6-OHDA, a toxin inducing degeneration of dopaminergic neurons in the nigrostriatal region of brain [32]. The administration of 6-OHDA into the striatum of mice at a dose of 0.5 μl/min into the right striatum (0.9 mm anterior and 1.8 mm lateral from bregma, 3.0 mm ventral from the dura) and in rat 8μg/2μl in 0.1% ascorbic acid-saline (anterior-posterior 0.5 mm, lateral 2.5 mm, 5mm dorso-ventral from dura) mimics PD’s like behavioral and biochemical alterations [33, 34]. 6-OHDA inside dopaminergic neuron destroys the dopaminergic nigrostriatal pathway by inducing oxidative stress, which can lead to the induction of inflammation, where neuro-inflammation lead to over activation of glial cell ultimately cause neuronal cell death [35-37]. The unilateral intra-striatal injection of 6-OHDA in rodents induces pronounced behavioral dysfunctions like aphagia, adipsia, paradoxical kinesia, tremulous jaw movement, epileptic seizures and biochemical damage of glutamatergic, cholinergic, tryptaminergic, GABAergic, noradrenergic and adrenergic alternation in various brain region i.e is similar to same as in PD [38-42].
cAMP (cyclic adenosine monophsphate) system is closely involved in the regulation of brain-derived neurotrophic factor (BDNF) plays an important role in the neuronal survival, neuronal proliferation and differentiation, synaptic plasticity, improvement in learning & memory, reduce excitotoxic damage and prevent amyloid-β (Aβ) toxicity, inhibit apoptotic and necrotic cell death [43-51]. Further elevation of cAMP levels is known to restore the energy levels, enhance biosynthesis & release of neurotransmitters in cholinergic, β-Adrenergic and dopaminergic neurons in specific brain regions like striatum, hypothalamus, nucleus basalis, substantia nigra (SN), locus ceruleus, dorsal raphe nucleus and dorsal vagal nucleus. Moreover, increase level of cAMP involved in the inhibition of apoptotic and necrotic cell death leads to improvement in cognitive functioning [52-61]. Elevation of cAMP is also responsible for both short and long-term enhancement in synaptic transmission and stimulates cholinergic cells to release acetylcholine in the proper initiation of memory formation [62-65].
Further, cAMP dependent CREB (cAMP responsive element binding protein) phosphorylation has too been reported to perform neuro-protective action such as long term memory potentiation (LTP), neuronal cell survival, proliferation, and differentiation in the developing brain, neuronal plasticity, regulate expression of neurotropic factors and anti-apoptotic genes, mitochondrial biogenesis, increase expression of BDNF, regulate levels of bcl-2, polysialylated neuronal cell adhesion molecule (PSA-NCAM), neuronal growth factor (NGF), cyclin D2 and controls the expression of both MeCP2 and miRNA participates in the neuronal morphogenesis, differentiation in response to neurotropic factor NGF, BDNF, FGF (fibroblast growth factor), IGF-1 (insulin like growth factor) and regulates cognitive deficits [66-78]. However, agents that enhance cAMP/PKA/CREB pathways have potential for the prevention of stroke and various neurological disorders like Depression, Schizophrenia, Alzheimer’s and Huntington disease (HD) [79-83]. Forskolin – FSK (Coleus Forskohlii, family Labiatae) used to treat heart and lung disease intestinal spasms, insomnia and convulsions [84-87]. FSK a labdane diterpenoid, is considered the active secondary metabolite because of its ability to directly activate the enzyme adenyl cyclase (AC) [88]. Recent research has shown that FSK has positive effects against a wide range of conditions such as asthma, glaucoma, hypertension, hair loss, cancer and obesity, cardiac remodeling and heart failure prevention, amelioration of mitochondrial dysfunction in cardiomyopathy, anti-platelet aggregation, hydrodynamic alterations in collecting tubule, anti-cystic fibrosis, diabetes, inflammation, glaucoma, smooth muscle relaxation [89-102].
Moreover, FSK can potentiate the absolute inhibition of striatal AC mediated by D-2 dopamine receptors and found that a little change in the percent D-2 inhibition in the presence of FSK, suggesting that its effects on inhibitory guanine nucleotide-binding (cGMP) subunit were minimal [103]. As FSK, a direct activator of AC is responsible for the activation of cAMP-dependent protein kinase (PKA) mediated CREB performed neuro-protective functioning associate with mitochondrial dysfunctioning [104-106]. FSK, cAMP analogs, or neuropeptides effectively alleviated the mitochondrial neuronal impairment through PKA mediated CREB activation [107]. FSK at the dose of 10-20µM in-vitro induces phosphorylation of CREB results in increase in level of CREB, involved in the amelioration of mitochondrial dysfunctioning [108,109]. Indeed, recent studies reported the beneficial effects of natural AC activator Coleus Forskohlii (FSK), against various neurodegenerative abnormalities through the modulation of cAMP CREB, BDNF, Phosphoinositide 3-kinase (PI3K)/Akt and Mitogen-Activated Protein Kinase (ERK1/2) [90,110-113].
FSK has been shown to activate AC in various brain regions like striatum, concentrated in the hippocampus, cerebellum, neocortical areas and peripheral tissues [114-116]. Although the activation of FSK is commonly stated to be mediated primarily by a direct action on the catalytic subunit. A few reports suggest that its effects at the catalytic subunit may potentiate interactions of the catalytic subunit with the cAMP [88,117-118]. In brain, FSK has been reported to elicit the most marked stimulation (-15-fold) of AC activity in the striatum, frontal cortex, hippocampus and in some cases in the middle temporal gyrus, and also localized in pituitary and spinal cord [119,120]. FSK at submaximal doses increases the potency, efficacy, or both of the stimulatory hormones for AC in intact cells [121,122]. However, despite the significantly greater stimulation by FSK in this brain region, little is known concerning the effects of FSK on the stimulation of striatal enzyme activity mediated by cAMP or D-1 3,4 dihydroxyphenylethylamine (DA) receptors[103]. cAMP, mediated signaling of several neurotransmitters including serotonin, acetylcholine, glutamate & DA, plays an important role in the memory & cognitive functioning [123-126]. The activation of the cAMP/PKA/CREB pathway significantly inhibits inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukins (IL-1β, IL-6, and IL-8), i-NOS (inducible nitric oxide synthase), plasminogen activating factor (PAF), human basophils, mast cell degranulation and oxidative stress [127-131]. Mitochondrial dysfunctioning is associated with loss of ATP in the cell further leads to decrease in the level of cAMP which are implicated in various abnormalities [132]. This decrease in the level of cAMP could be overcome by the FSK administration [104]. Therefore, on the basis of above relative information the present review was designed to investigate the neuro-protective role of direct AC activator FSK through activation of cAMP/PKA mediated CREB pathway in 6-OHDA induced PD’s like symptoms in rats.
Parkinson disease (PD)
PD, the most common movement disorder is characterized by a progressive loss of DA releasing neurons in the SNpc, resulting in slowness of movement, rigidity, and tremor as well as the death of neurons in catecholaminergic and cholinergic nucleus [133-135]. PD is typically considered to be a motor disorder ,through the clinical manifestations are highly variable.The etiology behind PD include excessive levels of SNCA, environmental toxicants and genetic factors leading to an atypically low number of dopaminergic neurons at birth and increased susceptibility to PD development [136,137]. The pathophysiology however is characterized by the degeneration of DA neuron in the SN, a region of the degeneration of the midbrain, and axon loss in the striatum, a region of the forebrain. The resulting DA deficiency leads to dysfunction in the BG network [138]. Moreover, serotonergic, noradrenergic, and cholinergic cells are lost [139].
Prevalence of PD
Parkinson’s disease is the second most prevalent neurodegenerative disorder after Alzheimer’s disease and is anticipated to impose an increasing social and economic burden on society as populations continue to age [142-145]. A report by the National Parkinson Foundation (NPF) in the United States (US) suggested that PD affects an estimated four to six million worldwide [146]. In the UK, PD is estimated to affect 100–180 people per 100,000 of the population and has an annual incidence of 4-20 per 100,000 [147]. The incidence of the disease rises with increasing age .One in seven are diagnosed before 50 years of age, with a fivefold increase in diagnosis in those aged over 65 [148,149]. India with one of the world’s lowest incidence of PD (70 out of 100,000) [6].
Genetics in PD
Over 20 loci and 15 disease-causing genes for Parkinsonism have been identified [150]. Mutations in seven genes are robustly associated with autosomal dominant (SNCA, LRRK2, EIF4G1, VPS35) or recessive (parkin/ PARK2, PINK1, DJ1/PARK7) PD.
SNCA
SNCA encodes a 140 amino acid synaptic vesicle-associated protein that regulates synaptic vesicle exocytosis [151]. SNCA was identified as the main component of lewy bodies and lewy neurites in PD patients [152]. SNCA is a natively unfolded soluble protein that can aggregate to form oligomers or protofibrils, and eventually insoluble polymers or fibrils [153]. Oligomeric SNCA may mediate neurodegeneration by disrupting synaptic vesicles [154]. Mitochondrial dysfunction, axonal transport deficits, and SNCA aggregation may participate in a self-perpetuating cycle of neuron damage in PD [155].
Leucine-rich repeat kinase 2 (LRRK2)
LRRK2 is a 2527 amino acid protein that contains functional kinase and guanosine triphosphate (GTP) as domains, and leucine-rich repeat and WD40 protein-interaction domains [156,157]. It is expressed throughout various brain regions, including SN, BG, cortex, hippocampus, and cerebellum [158,159]. Mutations in LRRK2 are the most common cause of familial PD and are linked to both autosomal dominant and sporadic forms [160].
Parkin (PARK2)
PARK2 acts as a regulator of protein breakdown [161]. Mutations in the parkin gene, which encodes for an E3 ubiquitin ligase, are the leading cause of early-onset, autosomal recessive Parkinsonism [162-163]. Parkin levels in neurons are associated with protection from cellular stress and cell-cycle regulation [164]. PARK2 pathological effects on mitochondria were Decrease electron transport chain (ETC) enzyme activities, decreaseprotein levels of several subunits of complexes I and IVdecrease mitochondrial integrity, ubiquitin proteasome system (UPS) & autophagy lysosomal pathway (ALP) dysfunction [165-167].
PTEN-induced putative kinase 1 (PINK1)
PINK1 gene mutations represent the second most common cause of autosomal recessive PD. The gene encodes a 581-amino acid protein with a predicted N-terminal mitochondrial targeting sequence and a conserved serine/threonine kinase domain [168]. More than 40 PINK1 mutations have been identified in PD patients [169].
DJ-1
Point mutations (L166P, D149A) in DJ-1 cause rare autosomal recessive PD with early onset. DJ-1 is a redox-sensitive cytosolic chaperone protein that associates with mitochondria and the nucleus upon oxidation. Mutations cause a loss of function of DJ-1 by inducing instability of the dimeric, functional form of the protein, or lack of expression. Mutations also affect the serine protease activity of DJ-1, another crucial function of this protein [170]. DJ-1 seems to be an important redox-reactive signaling intermediate controlling oxidative stress associated with ischemia, neuroinflammation, and age-related neurodegeneration [171].
Neuropsychological & neuropsychiatric aspects of PD
Psychiatric syndromes as well as cognitive impairment frequently complicate PD, a neurodegenerative disorder defined by its movement abnormalities.Development of psychopathology in PD is attributed to a number of factors, including underlying disease processes related to PD, medication effects, and psychological reactions to the illness [5].
Motor features
The hallmark clinical signs of PD are its motor triad: a pill-rolling rest tremor, rigidity, bradykinesia/akinesia, internal tremor associated with anxiety, cramps, aches, pains, gait and postural disturbances with a loss of righting reflexes, un-steadiness, imbalance and falls, sialorrhea, dysarthria, visual and genitourinary dysfunction, sleep disturbances, sweating, seborrhea, edema, constipation, paresthesias, fatigue, and a decreased sense of smell [172-175].
Cognitive Deficits
The cognitive features of PD are present to varying degrees early in the course of the disease and are multifactorial in origin, involving subcortical–frontal dopaminergic systems as well as extra striatal systems [174,175]. The various forms of executive dysfunction, visuospatial impairment, memory impairment, and attention deficits that occur in PD .The presence of a mood disorder, which can precede, accompany, or follow cognitive changes, may also confound assessment of cognitive impairment, intensify deficits ,aphasia , apraxia, and memory deficits [176-179] (Table 1).
Psychiatric complications
Common psychiatric disturbances observed in PD subjects include: depression, apathy (i.e., lack of motivation), anxiety, sleep disturbances, psychosis, cognitive impairment, and impulse control disorders, with at least one neuropsychiatric symptom being reported in over 60% of patients [180-182]. Furthermore, several clinical features of PD and depression overlap, altered appetite or sleep, weight change, loss of libido, memory impairment, low energy, lack of facial expression and psychomotor retardation. Sleep disorders, such as insomnia, hypersomnia (excessive daytime sleepiness), restless leg syndrome and rapid eye movement sleep behaviour disorder (RBD), affect the majority of PD patients. Psychosis manifests as hallucinations (visual, auditory or tactile), paranoid delusions or delirium [181,183-186].
Mood disturbances
Up to 90% of PD patients with idiopathic PD experience psychiatric complications, including major mood disorders (major depression, dysthymia, or bipolar disorder); adjustment disorders; disabling anxiety syndromes; drug-induced mood changes; pathological tearfulness; dementia; apathetic states; psychosis; or delirium [187].
Non-motor symptoms of PD
Recent reports in PD patients have confirmed the existence of a variety of non-motor symptoms .These includes hyposmia (reported in 80% of the patients at the time of diagnosis), pain, cognitive deficits (about 50%; deficits in memory, attention, executive function and dementia are reported), sleep disturbances, rapid eye movement (REM) behavior change, constipation and urinary problems, depression (10 – 40 %), changes in aversion, fear and anxiety, compulsive behavior and lack of impulse control [33,188-191].
Pre-motor stages of PD
According to the Hoehn and Yahr scale for PD stages [192], PD patients go through five stages:
Stage I: Very subtle, unilateral existence of one or more of the primary symptoms.
Stage II: Bilateral primary symptoms and additional secondary symptoms.
Stage III: Symptoms from stage two increases in severity, and problems with balance become prominent. At this stage, the person with Parkinson’s is still independent.
Stage IV: Motor symptoms result in disability, leading to the patient needing assistance in most daily activities.
Stage V: Patient is bed or wheelchair bound and needs complete assistance.
Neuropathology of PD
The pathology in PD is known to affect the central, peripheral, and the entric nervous systems [140]. It has been shown to cause substantial cytoskeletal alterations in various brain regions in a slow but persistent manner [42]. In addition to being a disorder of the DA projection system, glutamatergic, cholinergic, tryptaminergic, GABAergic, noradrenergic, and adrenergic damage has also been reported in PD [42,193]. Presence of lewy bodies, lewy neuritis, and dopaminergic degeneration are hallmarks of PD pathology, as was first demonstrated by F. Lewy in 1912 [194]. Lewy bodies are protein aggregates that, among other proteins contain ubiquitin and SNCA. These can proteins be found in cell bodies and neurites of certain neuronal populations including hypothalamus, nucleus basalis, substantia nigra, locus ceruleus, dorsal raphe nucleus and dorsal vagal nucleus [195-199].
Structural alteration in PD
Basal ganglia: Parkinsonism is considered to result primarily from abnormalities of BG function.The BG include the neostriatum (caudate nucleus and putamen), the GPe, GPi, subthalamic nucleus (STN), and the SN with its SNpr and SNpc. They participate in anatomically and functionally segregated loops that involve specific thalamic and cortical areas. These parallel circuits are divided into ‘motor’, ‘associative’ and ‘limbic’ loops, depending on the function of the cortical area involved [200-203]. Patterns of neuronal discharge within the basal ganglia are disturbed in PD [204]. The loss of DA in the SNpc increases the overall excitatory drive in the BG, disrupting voluntary motor control and causing the characteristic features of PD [205].
Striatum: The striatum, a part of the BG, which receives dopaminergic projections from the SN, is also significantly impacted in PD [206,207]. The striatal damage is more severe in the putamen than in the caudate nucleus. The ventral tegmental area is affected to a lesser extent as compared to the SN [208]. The loss of DA in the nucleus accumbens is to a much lesser extent, and the dopaminergic neurons in the hypothalamus appear to be spared in PD [209]. The association of SNCA and mitochondria was especially significant in PD-vulnerable brain regions that are SNpc and striatum [210].
Cerebral cortex: Decreased dopaminergic input to the striatum from the SNpc in PD results in a complex alteration in activity between input and output stations of the BG. Decreased levels of DA result in increased activity along the indirect pathway and decreased activity along the direct pathway, which together result in increased excitation of GPi and SNpr neurons, then to increased inhibition of thalamic neurons, and finally to decreased excitation of the cortex. [16-17].
Mechanism of PD pathogenesis
The first link between mitochondria and PD came with the identification of the deficiency of mitochondrial ETC (electron transport chain) protein complex I activity in SN from patients with PD [211-213]. The role of mitochondria in the pathogenesis of PD has been enhanced by the subsequent identification of mutations in genes encoding mitochondrial proteins like PINK1, DJ1, and parkin, environmental agents, increased intracellular Ca2+, free radical–mediated damage to proteins, lipids, and DNA in SN of PD patients [214-217]. A defect of mitochondrial respiratory chain activity results in impaired oxidative phosphorylation and an increase in free radical generation and thus will affect UPS function by both limiting activity and increasing the substrate load of oxidized protein. (Figure 1) [218].
Oxidative stress & PD
The extensive production of ROS in the brain may provide an explanation for the magnitude of the role that these reactive molecules play in PD. The brain consumes about 20% of the oxygen supply of the body, and a significant portion of that oxygen is converted to ROS [219]. ROS can be generated in the brain from several sources, both in neurons and glia, with the ETC being the major contributor at the mitochondrial level, monoamine oxidase (MAO), NADPH oxidase (NOX) and other flavo-enzymes along with nitric oxide (NO) [219-221].Oxidative stress have been found not only in brain tissues but also in peripheral tissues of individuals affected by PD, mild cognitive impairment (MCI) and other degenerative diseases, including HD, Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and others [222].
Mitochondrial dysfunction & PD
Within the mitochondria, ROS is produced at several sites along the ETC, coupled with a process called oxidative phosphorylation via complex V i.e. ATP synthase. Direct and imprecise interactions of reduced nicotinamide adenosine dineucleotide phosphate (NADH) /reduced flaiv adenosine dineucleotide phosphate (FADH) derived electrons with molecular oxygen or any other electron acceptors lead to generation of ROS [22,223]. Major complexes of ETC indulged in generation of ROS include complex I and complex-III [224]. Figure 1 shows a schematic diagram of the flow of electrons across the ETC and the major sites involved in ROS production. Interestingly, uncoupling proteins (UCP) present in mitochondrial membranes have been found to minimize the generation of excessive ROS by leaking the protons inside mitochondria from the cytoplasm, thereby reducing the overall membrane potential [225]. Though mitochondria have been found to be the major contributors target site of cellular ROS, oxidative stress, inflammatory mediators and Ca2+ overload is the pathological hallmark for mitochondrial dysfunctioning [226]. The amount of ROS present inside a cell has phenomenal physiological significance. Increased levels of ROS lead to cellular damage and reduced levels result in impairment of various signaling pathways essential for cellular proliferation and operation of host defense mechanisms [227-228]. Therefore, a delicate balance between the levels of oxidants and antioxidants is essential to maintain the optimum levels of ROS [229]. An efficient antioxidant defense system which includes enzymes such as superoxide dismutase (SOD), catalase (CAT), total glutathione (GPx) and glutathione reductase (GRd) ensures the optimal level of cellular ROS and any imbalance or impairment in this system results in oxidative stress and its subsequent consequences (Figure 2) [230].
Excitotoxicity and PD
The concept of excitotoxicity has also been applied to PD. various studies have demonstrated that Parkin regulates the function and stability of excitatory glutamatergic synapses [231,232]. Postsynaptic expression of Parkin dampens excitatory synaptic transmission and causes a marked loss of excitatory synapses in hippocampal neurons [233]. Conversely, knockdown of endogenous Parkin or expression of PD-linked Parkin mutants profoundly enhances synaptic efficacy and triggers a proliferation of glutamatergic synapses. This proliferation is associated with increased vulnerability to synaptic excitotoxicity [234]. The resulting excessive glutamatergic drive could be a source of excitotoxicity in the nigra and activation of NMDA receptor increases intracellular Ca2+ levels [12]. A role for elevated intracellular Ca2+ in the events leading to cell death in PD is supported by the observation that dopaminergic neurons expressing the Ca2+-binding protein calbindin may be selectively preserved in PD [235].
Neuro-inflammation and PD
In PD One of the first features of the inflammatory-associated modifications was the unregulated expression of major histocompatibility complex (MHC) molecules [236]. Whether a microglia T-cell dialogue exists and is important for local amplification of the proinflammatory immune response in PD remains to be determined [237]. Another main feature of inflammatory-related processes in PD is a marked increase in cytokine levels in the striatum and cerebrospinal fluid (CSF) of Parkinsonian patients compared with control subjects [238]. These include proinflammatory cytokines (TNF-α, IL-1β, IL-6), T-cell activation–associated cytokine (IL-2), anti-inflammatory cytokine (IL-4), and several growth factors like endothelial growth factor (EGF), transforming growth factor alpha (TGF-α), β-FGF, transforming growth factor beta1 (TGF-β1) [239,240]. Because an increase in the level of cytokines was specific to the nigrostriatal pathway and is not observed in cortical regions, it has been suggested that cytokine production may be strictly confined to the sites of injury [241,242]. Cytokine-induced dopaminergic cell death may involve more direct cytotoxic mechanisms through direct activation of cytokine receptors localized on dopaminergic neurons and coupled to intracellular death–related signaling pathways [240].
Apoptosis/caspases & PD
A pathogenic role for apoptosis in PD is supported by findings of activated caspase-3, 8 and 9 and higher activities of caspases-1 and 3 in dopaminergic SN neurons in PD brain [241,242].
An oxidative mechanism for neuronal apoptosis has been suggested to involve ROS generation, inhibition of mitochondrial complex I activity, and caspase-3 activation, overexpression of mutant SNCA in association with elevated 8-hydroxyguanine, protein carbonyls, lipid peroxidation and 3-nitrotyrosine, consistent with oxidative stress [243-247] (Figure 2).
Neurochemistry of PD
DA in PD
There are two distinct populations of DA receptors, which differentially regulate second messenger pathways in the striatum. D1 receptor activation leads to stimulation of AC, the enzyme responsible for the synthesis of cAMP. D2 receptor activation inhibits AC, which results in decreased synthesis of cAMP. Levels of cAMP regulate the activity of cAMP/PKA, which plays an important role in cell phosphorylation events such as ion channel modulation and regulation of gene expression (Figure 3) [248,249]. DA differentially regulates the direct and indirect pathways. The direct and indirect pathways work together to balance the motor control wielded by the BG. While the direct pathway facilitates movement by means of decreases in tonic inhibition of BG output and disinhibition of thalamocortical and brainstem pathways, the indirect pathway suppresses movement by increasing the inhibitory BG output to the thalamus [58,59]. DA fibers have therefore an inhibitory action on the striatal GABAergic/enkephalinergic cells projecting to the GPe, but an excitatory action on the GABAergic/substance-P (SP) containing neurons projecting directly to the GPi (and the SNPr) [250-252].
GABA in PD
Gamma-aminobutyric acid (GABA), a putative inhibitory neurotransmitter, is distributed throughout the brain and spinal cord [253]. GABA is the main inhibitory neurotransmitter within the central, peripheral and enterinal nervous systems [254,255]. Recent studies indicate a possible diagnostic value of plasma glial cell derived neurotropic factor (GDNF) levels in depression, but whether GDNF and related Ca2+/GABA mechanisms may play prominent role in the development and progression of PD-related depression is unclear at the moment [256]. SNpc DAergic efferents project to GABAergic cells in the striatum these conections can be excitatory & inbihitory mediated by D1 & D2 DA receptors respectively. DAergic neuron loss in the SNpc cause the net inhibition thalmic output to the cortex through both the direct and indirect pathway of the BG, are parrallel pathway from the striatum to the BG output nuclei-GPi & SNpr that mediate thalmocortical activity. The direct pathway normaly provides inhibition to the output nuclei which is disinhibited in PD as well as indirect pathway normally excits the output nuclei also disturbed in PD [18,19].
Glutamate in PD
Glutamate is also the predominant excitatory neurotransmitter in the BG, is the seat of the motor deficits seen in PD [257]. In addition to sending glutamatergic projections to the striatum, the cortex also sends projections to the STN, thalamus, and SNpc, in addition to other nuclei in the brainstem and spinal cord. The SNpc receives further glutamatergic innervation from the STN in the indirect basal ganglia pathway. Evidence has supported that the dopaminergic projection from the SNpc to various nuclei in the BG circuit exerts an important regulatory function on the firing pattern of certain glutamatergic pathways (Figure 4) [258]. Glutamate release can be regulated by GABA receptors located on corticostriatal terminals activation of these receptors exerts a significant inhibitory effect [259]. Glutamate systems are extensively distributed throughout the brain and have been implicated in the central control of many physiological functions. As a consequence, disturbance in glutamatergic activity may underlie many psychological and neurodegenerative disorders including AD, HD, ALS, AIDS dementia complex, and PD [260]. Excessive stimulation of glutamate receptors can have numerous detrimental effects such as Ca2+ homeostasis dysfunction, increased NO production, activation of proteases, an increase in cytotoxic transcription factors, and increased free radicals [261]. Glutamate receptor over-stimulation leads to excessive influx of Ca2+ (and Na+) through glutamate receptor-gated ion channels, followed passively by movements of chloride (Cl-) and water. It causes postsynaptic neurons to be overloaded by extracellular Ca2+ and Na+ as well as intracellular Ca2+ via release from mitochondria. The resulting combination of increased intracellular volume and Ca2+ overload induces various lethal metabolic derangements, internal organelle swelling, and plasma membrane failure leads to necrosis [262].
Acetylcholine in PD
Ach is a prominent neurotransmitter of the peripheral and the central nervous system (CNS). In the CNS, Ach is involved in attention, learning, memory, consciousness, sleep, and control of voluntary movements [263]. In the brain, ACh mediates distant signaling through projection neurons and local signaling via interneurons; the type of message conveyed by Ach depends on a variety of factors, including site of release, the localization of the target neurons, the target receptor subtypes, and the status of the target cells at the time of release [264]. Furthermore, Ach signaling may be circumscribed to the synapse or result from the delocalized diffusion of the neurotransmitter in the extracellular milieu and binding to nonsynaptic sites [265,266]. Among brain structures, the striatum contains some of the highest levels of Ach and DA. The two neurotransmitter systems interact extensively in a bidirectional manner, both at the presynaptic and postsynaptic levels, mediating cognitive mechanisms, the selection of motor responses, and reward [267,268]. In a recent study in 137 PD patients, cholinergic denervation could be related to REM behavior disorder, fall history, gait disorders, and cognitive dysfunction [269].
Adenosine receptors in PD
Adenosine receptors have a unique cellular and regional distribution in the BG and are particularly concentrated in caudate, putamen and the GP areas, which are richly innervated by DA [270]. In the BG, A2A receptors are prevalently and selectively localised in dendrites, dendritic spines and axons of GABAergic neurons of the indirect pathway projecting from the caudate putamen to the GPe [271,272]. Adenosine influences striatal output pathways known to be involved in motor symptoms and the onset of dyskinesia in PD. Therefore, inhibition of A2A receptors seems to be a potential target for neuroprotection in PD [273,274]. Indeed, in rodent models of PD, A2A antagonism exerts Anti-parkinsonian actions [272,275,276]. The highest expression of adenosine A2A receptors is found in the BG, particularly in the corpus striatum, which is involved in controlling complex motor activities by specific motivational stimuli as well as in habit formation [277]. Evidence for presynaptic localization of A1 receptors on DA axons is indirect, with confirmed absence of A2A receptors [271,278-281]. Nevertheless, both regulate striatal DA release, likely indirectly, with inhibition by A1-receptor activation and enhancement by A2A receptors activation [282-284].
Cannabinoid receptors in PD
Cannabinoids mainly act through two types of receptors, CB1 (present in CNS and to a lesser extent in the peripheral nervous system) and CB2 (present outside the CNS, preferentially in the immune system). The hypokinetic effect associated with the activation of CB1 receptors located in different neuronal subpopulations within the BG [285]. It is reasonable to expect that the cannabinoid signaling system, and particularly this receptor type, would experience an up regulatory response in PD or other hypokinetic disorders [286]. A therapy with cannabinoids in PD would imply the use of CB1 receptor antagonists for the alleviation of motor inhibition but also the use of different types of cannabinoids, preferably antioxidant cannabinoids, for the control of disease progression [285,287-289].
Neuropeptides in PD
Neuropeptides are found in many mammalian CNS neurons where they play key roles in modulating neuronal activity [290]. Other peptides such as neuropeptide Y (NPY) are synthesized throughout the brain, and neurons that synthesize the peptide in one region have no anatomical or functional connection with NPY neurons in other brain regions [291]. Neuropeptides are thought to modulate the excitability of dopaminergic neurons in the extrapyramidal system [206,292]. NPY-medulla; neuronal loss was found in PD patient. Damage to multiple neuronal systems causing complex biochemical changes and pathophysiological disturbances may represent the basis for the variable clinical picture of PD including motor, vegetative, behavioral, and cognitive dysfunctions, depression, pharmacotoxic psychoses, and other symptoms that usually increase with progressive stages of the disease [293,294].
SP and Enkephalin (Enk) in PD
SP that is highly concentrated in SN and the inner pallidum and neurons of the ponto mesencephalic tegmentum have an excitatory effect on dopaminergic neurons [58]. The PD brains shows a 30-40% decrease in SP in SN and pallidum without essential changes in cortex, hippocampus, striatum, and hypothalamus [295]. There is reduction of SP-immonoreactivity in SN or pallidum was observed in PD, AD, and Guam-PDC but significant reduction of SP was observed immunoreactivity in GPi, in SNpr and SNpc, and in the NBM in PD [206,290,296,297].
Animal models for PD
Mitochondrial complex I and IV toxin PD models (6-OHDA)
The characterization of the hydroxylated analogue of DA, 6-OHDA, as a toxin-inducing degeneration of dopaminergic neurons in the nigro-striatal tract has led to it being a widely used tool to induce Parkinsonism in rodents [32,34, 35, 298]. 6-OHDA is injected into the nigro-striatal tract at one of three locations: into the SNpc where the A9 dopaminergic cell bodies are located; into the median forebrain bundle (mfb), through which the dopaminergic nigro-striatal tract ascends; or into the terminal region, the striatum [299-301]. Current understanding behind 6-OHDA mechanism is that, once inside dopaminergic neurons, 6-OHDA initiates degeneration through a combination of oxidative stress and mitochondrial respiratory dysfunction [32,302]. Certainly, 6-OHDA readily oxidizes to form ROS, to reduce striatal levels of antioxidant enzymes, elevate levels of iron in the SN and to interact directly with complexes I and IV of the mitochondrial respiratory chain, leading to subsequent respiratory inhibition [35,303-306] (Figure 5). The 6-OHDA model also mimics many of the biochemical features of PD, including reduced levels of striatal DA and tyrosine hydroxylase (TH; rate-limiting step of DA biosynthesis), increased firing of the STN parallely increase in glutamate levels and firing within the BG output regions (entopeduncular nucleus and SNpr) , elevated striatal Enk levels or depressed striatal SP and dynorphin levels , increased firing in the GPi in PD patients and Parkin-containing aggregate formation in 6-OHDA-lesioned rat (Figure 6). [307-316].
(Complex I: NADH dehydrogenase; Complex III: Cytochrome bc1 or cytochrome c reductase; Complex IV: Cytochrome c oxidase; Complex V: ATP synthase)
6-OHDA activates inflammatory features including NF-κB-mediated responses accompanied by inhibition of antioxidant systems regulated by Nrf2, TNF-α, complement component 1q subcomponent- binding protein, increases the expression levels of neuroinflammation markers such as TNF-α, IL-1b, and IL-6 and in astrocytes increases pro-inflammatory cytokine TNF-α, nitric oxide synthease (iNOS) and NO, cyclo-oxygenase-2 (COX-2), and PGE2 [244,317-319].
(That exhibit nigro-striatal tract degeneration. *Indicates those agents that are administered directly into the brain; all other agents are delivered systemically. Maneb is believed to inhibit complex III of the mitochondrial respiratory chain, whilst the other mitochondrial toxins mainly inhibit complex I. This activity leads to the generation of ROS or reduced ATP production, which lead to apoptosis and the cells demise. 6-OHDA and MPP+ may also induce the production of ROS directly within the cytoplasm. LPS-activates microglial cells to stimulate the release of inflammatory mediators, which in turn produce reactive nitrogen species (RNS). Inhibition of proteasome activity allows a build up damaged proteins that through DNA damage (not shown for clarity), and other processes can lead to cell death. Cell death is most likely apoptotic in nature, though this remains controversial for some agents)
Cyclic Nucleotides
The cyclic nucleotides 3-5-cAMP and 3-5-cyclic GMP are diffusible intracellular second messengers that act as critical modulators of neuronal function [320]. cAMP is generated in response to binding of a number of neurotransmitters to G-protein coupled receptors (GPCRs) and subsequent activation of AC [321]. Serotonin, adrenergic, dopaminergic, adenosine, vasoactive intestinal peptide, muscarinic, GABA, and opioid receptors, among others, signal through the cAMP cascade via specific heterotrimeric G proteins [322]. Through these effectors cAMP controls a bewildering number of neuronal functions, ranging from regulation of ion channel activity and, consequently, neuronal excitability, to cell volume control and axon guidance; from metabolism and transcription to neurotransmitter release and learning and memory formation [323].
Cyclic adenosine monophosphate (cAMP)
cAMP is synthesized from ATP by AC located on the inner side of the plasma membrane and anchored at various locations in the interior of the cell [324]. The brain contains a large number of different GPCRs. Each individual neuron can express at its plasma membrane a number of these receptors, each of which will generate a cAMP signal upon binding to its ligand [322]. In addition, glutamatergic stimulation can generate both cAMP and cGMP signals in response to Ca2+ influx [325]. The second messenger concept of signaling was developed with the finding of cAMP and its ability to influence metabolism, cell shape and gene transcription (via reversible protein phosphorylations) [326]. cAMP exerts an important role as second messenger molecule controlling multiple cellular processes in the brain [327]. Elevation of cAMP causes both short and long-term increase in synaptic strength and stimulates cholinergic cells to release Ach [63-65,328]. However, the levels of cAMP are reported to be decreased in neuropath logical conditions [50,329]. Further, cAMP dependent CREB phosphorylation has too been reported to induce LTP by the action of an enzyme AC in response to a variety of extracellular signals such as hormones, growth factors and neurotransmitters [66,67, 330]. cAMP controls a bewildering number of neuronal functions ranging from regulation of ion channel activity , neuronal excitability, control cell volume and axon guidance ; from metabolism and transcription to neurotransmitter release, regulate BDNF , improve learning and memory, restore energy levels, reduce excitotoxic damage, prevent Aβ toxicity ,inhibit apoptotic and necrotic cell death, regulate synaptic efficacy [43,48-51,63,328,331-334]. Furthermore, cAMP/PKA signaling pathway seems to play a role in the final phases of memory consolidation, which requires protein synthesis [66,335].
cAMP response element binding protein (CREB)
CREB activation
CREB is one of the most well-characterized transcription factors in learning and memory [66]. It was originally described due to its association with the cAMP response element (CRE) DNA sequence, and widely expressed throughout the brain, playing a critical role in plasticity in many brain regions, including hippocampus [336,337]. CREB increases the synaptic availability of DA and induces many DA dependent adaptive responses culminating in the transcription of striatal CRE-1 dependent genes, such as the immediate early gene c-and the neuropeptides dynorphin, SP, and Enk’s. CREB phosphorylation, initially thought to be mediated exclusively by the cAMP/PKA pathway, is also induced by Ca2+ -dependent signal transduction pathways [338]. Two members of the Ca2+ /calmodulin-dependent kinase family (CaMK), CaMKII and CaMKIV, are activated by Ca2+ entry through an L-type voltage-sensitive Ca2+ channel or glutamate N-methyl-D-aspartic acid (NMDA) receptors and induce CREB phosphorylation [339-342]. There are a number of signaling cascades upstream of CREB phosphorylation, such as the cAMP/PKA, CaMKII and IV) [68,336].
Cyclic nucleotide mediated CREB activation. GDP: Guanosine diphosphate, GTP : Guanosine Triphosphate, AC : Adenylyl Cyclase, GC: Guanylyl cyclase, ATP : Adenosine Triphosphate, cAMP : Cyclic Adenosine monophosphate, 5 AMP :5 Adenosine monophosphate, PDE : Phosphodiesterase, cGMP : Cyclic Guanosine monophosphate, 5 GMP : 5 Guanosine monophosphate, EPAC: Exchange protein activated cAMP, ERK : Extracellular signal- regulated Kinase, PKA : Protein Kinase A, PKG : Protein Kinase G, CREB : Cyclic AMP Response element binding Protein, BDNF : Brain Derived Neurotrophic Factor, NGF : Nerve Growth Factor.
These cascades are activated by stress, inflammatory cytokine MAPK or PI3)/Akt pathways, growth factor/receptor tyrosine kinase activity (Ras/Erk/RSK2 pathway), and phospholipase C (PLC)-PKC signaling [68,343]. All these cascades have been shown to induce CREB phosphorylation [331,344] (Figure 3). CREB phosphorylation is facilitated by membrane-bound NMDA receptors, the non-receptor tyrosine protein kinase c-Src, fibroblast growth factor receptor1 (FGFR1), and estrogen receptors, all of which are part of distinct intracellular cascades [345-348]. The molecules involved in the modulation of CREB phosphorylation include several neurotransmitters DA, glutamate, serotonin, GABA, growth factors like IGF-1; vascular endothelial growth factor, VEGF), and neurotrophins BDNF [68]. However, CREB also regulated by multiple signaling cascades downstream of neuronal activity, including cAMP and Ca2+ (Figure 3) [349].
Role of CREB in neuronal functioning
CREB regulates a wide range of neuroprotective processes, including the expression of trophic factors, antiapoptotic genes, detoxifying enzymes, mitochondrial biogenesis, as a regulator of cell survival, proliferation, and differentiation in the developing brain, whereas its roles in the adult brain include learning, memory, neuronal plasticity [68-70,350,351]. Among the molecules related to adult neurogenesis that are also affected by CREB signaling, BDNF expression, prolactin release, bcl-2 activation, PSA-NCAM, NGF, cyclin D2, MeCP2 and miRNA-132, participates in neuronal morphogenesis, and regulates cognitive capacity [71-78,319,352]. A number of more recent studies have demonstrated that CREB is the main element underlying the conversion of short term memory (STM) to long term memory (LTM) [353-355]. In addition, some neuropsychiatric or neurodegenerative diseases such as depression, schizophrenia, HD and AD are associated with memory loss. In this regard, CREB has been postulated to change the sensitivity of the nucleus accumbens to rewarding and aversive drugs [80-83,356,357]. Moreover CREB modulates adult neurogenesis through the control of other adult neurogenesis regulators such as neurotransmitters, steroid hormones, or cytokines (Figure 6) [358].
Forskolin
Biological source
FSK, a labdane diterpene, is a major active compound isolated from tuberous roots of Coleus Forskohlii Briq. (Labiatae) (Figure 5) [359]. C. forskohlii has been used as an important folk medicine in India. Futher, FSK has been found to be a potent activator of AC, leading to an increase in levels of c-AMP dependent PKA mediated CREB activation [360]. The presence of yellowish to reddish brown cytoplasmic vesicles in cork cells of C. forskohlii tubers is unique character of this plant and these vesicles store contains secondary metabolites i.e. FSK [361]. It grows wild in arid and semi-arid regions of India, Nepal and Thailand and the plant is found mostly on the dry and barren hills [362].
Ethnopharmacological profile of FSK
The genus Coleus of the family Lamiaceae (Labiatae) comprises a number of herbaceous medicinal plants which are particularly employed in home remedies for various ailments. Three species are most popular and commonly cultivated. They are Coleus aromaticus, C. vettiveroides and C. forkoshlii.long slender raceme [363]. Fruits are orbicular or ovoid nutlets. The leaves are useful cephalgia, otalgia, anorexia, dyspepsia, flatulence, colic, diarrhoea and cholera especially in children, halitosis, convulsions, epilepsy, cough, chronic asthma,high cough, bochitis, renal and vesical calculi, strangury, hepatopathy, malarial fever, antispasmodic and cathartic [87,364,365]. The whole plant is useful in hyperpiesia, vitiated conditions of pitta, burning sensation, strangury, leprosy, skin diseases, leucoderma, fever, vomiting, diarrhoea, and ulcers and as hair tonic [302,366,367]. FSK is also used as a condiment in India and the tubers are prepared as pickle and eaten [368]. The roots are used in treatment of worms, festering boils, and eczema and skin infections [369,370,371]. The leaves are bitter, acrid, thermogenic, aromatic, anodyne, appetizing, digestive, carminative, stomachic, anthelmintic, constipating, deodorant, expectorant, lithontriptic, diuretic and liver tonic [363,372,373].Coleus Forskohlii has been used to treat hypertension, congestive heart failure, eczema, colic, respiratory disorders, painful urination, insomnia, and convulsions. Clinical studies of the plant and the FSK constituent support these traditional uses, but also indicate that it may have therapeutic benefit in asthma, angina, psoriasis, and prevention of cancer metastases [87,302,363]. In traditional Indian systems of medicine, the roots of Coleus Forskohlii are used as a tonic as well as used for veterinary purposes [374]. FSK is also used in the preparation of medicines preventing hair greying and restoring grey hair to its normal colour [375] Forskolin is also a potent vasodilatory, hypotensive and inotropic agent [117].
Pharmacokinetic profile
FSK ability to inhibit platelet aggregation is of additional benefit in cardiovascular disease [376,377]. FSK also demonstrates a direct effect on cerebrovascular vasodilatation via cAMP activation [378]. Asthma and other allergic conditions are characterized by decreased cAMP levels in bronchial smooth muscle, as well as high levels of plasminogen activating factor. In response to allergenic stimuli, mast cells degranulate, histamine is released, and bronchial smooth muscle contracts. FSK activation of cAMP inhibits human basophil and mast cell degranulation, resulting in subsequent bronchodilation [131]. Research has demonstrated aerosolized dry FSK powder results in significant relaxation of bronchial muscles and relief of asthma symptoms [379,380]. In one randomized, double-blind, placebo-controlled trial, 16 asthma patients were given a single inhaled (aerosolized) 10-mg dose of dry FSK powder, an asthma medication (0.4 mg fenoterol), or placebo [380]. The ability of FSK to regulate cAMP levels in skin cells has been shown to have therapeutic benefit for sufferers of psoriasis [87]. A significant decrease in intraocular pressure (IOP) in rabbits, monkeys, and humans administered a topical FSK suspension (1% FSK). This effect was present at one hour post application and remained significant for at least five hours [381]. In vitro and animal studies demonstrate lipolysis in fat cells is stimulated by FSK via activation of AC and increased levels of cAMP antioxidant status of different parts of Coleus Forskohlii including roots, stem, leaves and tubers shows the activities of SOD, peroxidase, polyphenol oxidase and CAT were significantly higher (P < 0.05) in tubers than in the leaves, roots and stem [369,382]
Pharmacological action of FSK (Table 2)
FSK and Brain
FSK Binding sites
There is increasing evidence of neurotropic effects of FSK. Specific binding sites for FSK have been shown in rat brain membranes and visualized by quantitative in vitro autoradiography in rat CNS [397,398]. FSK exhibits marked stimulatory effects on striatal AC activity [88]. Direct binding studies with a suitable, labelled derivative of FSK may be useful in determining the site of its action. Accordingly [3H] 14,15-dihydroforskolin as a ligand of binding sites in membranes of rat liver and rat brain [399]. 14,15-Dihydroforskolin has been reported to activate AC, FSK binds in frontal cortex as well as in hippocampus and in some cases in the middle temporal gyrus, striatum and also localized in pituitary and spinal cord [400]. High densities of binding sites were observed, particularly in the limbic system and the basal ganglia [119,120]. The highest density of binding was in the corpus striatum, in which AC is particularly sensitive to stimulation by FSK [103].
Role of FSK in brain
Several co-activators that influenced the DAergic differentiation have been reported. These co-activators are DA, cAMP, phorbol 12-myristate 13-acetate (TPA), isobutylmethylxanthine (IBMX) and FSK [401,402]. The percentage of DAergic neurons in the present conditions indeed needs to be modulated to further increase the DAergic neuron population, however FSK plays the co-activator role on growth factors and these extrinsic cues may be crucially effective in promoting the differentiation of a DAergic phenotype in human-derived NPCs [403].
GPCR and FSK
DA signaling is mediated by two major classes of DA receptors. D1 type receptors activate AC through coupling to Gs/Golf G-proteins whereas D2 type receptors inhibit AC through Gi/Go G-proteins [404-406]. AC, the mutual target of both D1 and D2 like receptor signaling pathways, is a membrane bound protein which catalyzes the conversion of ATP to cAMP. The patterns of alteration of AC and the regional selectivity are both of particular interest. In the midbrain plus brainstem, nicotine initially enhanced basal AC activity; inferences about the underlying mechanism for this alteration can be drawn from the fact that FSK stimulation of AC was also increased. FSK also suggests that neuroprotective effects through the activation of AC mediated cAMP activation are obtained in the presence of Gs [407].
Acetylcholine and FSK
The increase in the level of acetylcholinesterase (AChE) in FSK treated cells could be also an indirect effect of cAMP. cAMP is a second messenger and modulates a plethora of other factors within the cell [20,408-410]. The release of Ach from the presynaptic nerve terminal of nicotinic synapses and subsequent binding to recognition sites located on the subunits of the Ach receptor-ion channel complex (AchR) results in conformational changes of the AchR which yield channel opening [411]. A widely accepted hypothesis is that subunit phosphorylation, in particular that induced by cAMP-dependent PKA, desensitizes nicotinic acetylcholinesterase (nAChR) [412-414]. The direct action of FSK on nAChRs was demonstrated by exposing patch-membrane nAChRs to FSK, which inhibits channel activity possibly through non-competitive mechanisms [415]. The indirect action of FSK on nAChR was proven by applying FSK to the non-patch membrane, after the gigaseal were formed, and by observing a decrease in the activity of nAChR-channels. This indirect action was most likely mediated by cytosolic mechanisms, namely the cAMP/PKA pathway and can be attributed to nAChR phosphorylation [88,414].
Neuroprotective action of FSK
FSK against mitochondrial dysfunctioning
Mitochondrial dysfunctioning is associated with loss of ATP in the cell, further leads to decrease in the level of cAMP. This decrease in the level of cAMP could be overcome by FSK induction [232]. As FSK is a direct activator of AC is responsible for activation of cAMP leads to PKA activation further leads to CREB activation and performs neuroprotective functioning associate with mitochondrial dysfunctioning [104,416].
FSK against Neuro-inflammation
FSK has other properties as well, including inhibition of the pro-inflammatory substance known as platelet-activating factor (PAF) [417]. Inflammatory related processes in PD are a marked increase in cytokine levels in the striatum and CSF of parkinsonian patients [238]. Because an increase in the level of cytokines was specific to the nigrostriatal pathway and is not observed in cortical regions, it has been suggested that cytokine production may be strictly confined to the sites of injury. In support of this idea, the density of glial cells expressing proinflammatory cytokines, including TNF-α, IL-1β and IFN-λ, also was elevated in the SNpc of PD patients [241,242]. Administered a water soluble derivative of FSK (colforsin; 0.5μg/kg/min), they showed a reduced inflammatory response [100]. Recent studies have shown that FSK had an antagonistic effect on TNF-α, and it reduced the levels of IL-1β, 6, and 8 [418].
FSK against Neuro-oxidation
ROS can be generated in the brain from several sources, both in neurons and glial cell with in the ETC, major contributor at the mitochondrial level [220,221]. Other ROS sources include MAO, NOX and other flavo-enzymes along with NO, is abundant in the brain due to the presence of NOS [219]. FSK which is responsible for activation of cAMP mediated CREB, is when pretreated at a dose of 5µM, in-vitro and is able to inhibit expression of inducible nitric oxide synthase via inhibiting the mitogen activated protein kinase (MAPK) in C6 cells [419]. Chronic administration of FSK may induce a change in the balance of cAMP in response to glucose, which could enhance insulin release. This may be another mechanism for reducing glucotoxicity, in addition to the reduction of oxidative stress [420].
FSK and Cognitive dysfunctions
The various forms of executive dysfunction, visuospatial impairment, memory impairment, and attention deficits that occur in PD can render patients less able to accomplish familiar tasks or make them feel overwhelmed in situations that were not previously challenging [421,422]. The presence of a mood disorder which can precede, accompany or follow cognitive changes may also confound assessment of cognitive impairment and intensify deficits [142,177]. Like most mental disorders, cognitive disorders are caused by a variety of factors. Some are due to hormonal imbalances in the womb, others to genetic predisposition and still others to environmental factors [423]. FSK 5 mg/kg, i.p. is able to activating cAMP/CREB in the hippocampal region responsible for memory improvement [424]. Moreover FSK 50 µM direct potentiates synaptic response and induced LTP [425,426].
Possible involvement of FSK in 6-OHDA induced PD
Summarizing the whole information given above, FSK confirmed a versatile role in PD where it activates the AC/cAMP mediated PKA/CREB activation (Figure 7) Moreover, on other side FSK act as a coactivator in brain that follows the GS pathway through the activation of D1 receptor. There is least availability of selective AC activation and so far only limited reports suggest beneficial effect of FSK in neurodegeneration animal model.
Thus, combined with all information given above on the basis of review and research papers, it was first effort by us to explore the restorative and symptomatic profile of FSK through the activation of cAMP/PKA/CREB pathway in 6-OHDA induced neurotoxic PD’s like behavioral and biochemical parameters in rats.
Conclusion
Thus in conclusion neuroprotective and neurorestoration effects of FSK may be due to favorable modulation of CREB mediated signaling and direct D1 activation in the striatum. The involvement of cAMP/PKA/CREB pathway, anti-oxidant, anti-inflammatory and neuro-modulatory effect of test drug FSK may be the possible mechanisms at least in part underlying the observed effects.
Based on important and versatile role of cAMP/PKA/CREB signaling in regulation of neuronal functioning, essential to investigate the role of cAMP mediated CREB activation in 6-OHDA induced experimental PD in rats and to find out if cAMP mediated CREB pathway is equally implicated in the disease pathogenesis or progression. It may perhaps be safe to further conclude that the beneficial effects of the investigated test drug FSK may be due to its combined improved motor functions, pro-cognitive and to restore the energy levels as well as antioxidant and anti-inflammatory defense system in 6-OHDA model.
- Donaldson I, Marsden CD, Schneider S, Bhatia K (2012) Marsden’s book of movement disorders. Oxford University Press: Oxford Link: https://goo.gl/KmHXks
- Goldman Jennifer G, and Postuma Ron (2014) Premotor and non-motor features of Parkinson’s disease. Curr Opin Neurol 27: 434–441. Link: https://goo.gl/N2rnVZ
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, et al. (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136: 2419–2431 Link: https://goo.gl/JEmeE9
- Fearnley JM, Lees AJ (1991) Ageing Parkinson’s disease: Substantia nigra regional selectivity. Brain 114: 2283–2301 Link: https://goo.gl/AhwxHX
- Parkinson J (2002) An essay on the shaking palsy. 1817. J Neuropsychiatry. Clin Neurosci 14: 223-236. Link: https://goo.gl/8U8X3T
- Tags Chris (2012) Who gets Parkinson’s disease? Link: https://goo.gl/4ifKNL
- Shuman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6: 193-222. Link: https://goo.gl/yKr4mL
- Schenck CH, Boeve BF, Mahowald MW (2013) Delayed emergence of a parkinsonian disorder or dementia in 81% of older males initially diagnosed with idiopathic REM sleep behavior disorder (RBD): 16 year update on a previously reported series. Sleep Med 14: 744-748. Link: https://goo.gl/1ziNYM
- Assal F, Jeffrey L. Cummings, Jeffrey L (2002) Neuropsychiatric symptoms in the dementias. Curr Opin Neurol 15: 445–450. Link: https://goo.gl/NrfmJh
- Chaudhuri KR, Martinez-Martin P (2008) Quantitation of non-motor symptoms in Parkinson's disease. Eur J Neurol 2: 2-7. Link: https://goo.gl/zsaHDz
- De Lau LM, Breteler MM (2006) Epidemiology of Parkinson's disease. Lancet Neurol England 5: 525-535. Link: https://goo.gl/xIK03N
- Ciobica A, Paduraria M, Hritcu L (2012) The effect of short term nicotine administration on Behavioural & oxidative stress deficiencies induced in rat model of Parkinsons Disease. Psychiatria Danubina 24: 194-205. Link: https://goo.gl/n6HlE4
- Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, et al. (2011) Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin induced diabetes. Behav Brain 220: 30-41. Link: https://goo.gl/blmDK1
- Mathai A, Smith Y (2011) The corticostriatal and corticosubthalamic pathways: two entries, one target.So what ? Front Syst Neurosci 5: 64. Link: https://goo.gl/sA7PhE
- Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, et al. (2001) Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J Neurosci 21: RC128. Link: https://goo.gl/w4s7Mr
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, et al. (2001) Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci 21: 1033–1038. Link: https://goo.gl/fuOsv2
- Foffani G, Priori A, Egidi M, et al. (2003) 300-Hz subthalamic oscillations in Parkinson’s disease. Brain 126: 2153–2163. Link: https://goo.gl/EsE9yb
- Vila M, Herrero MT, Levy R, Faucheux B, Ruberg M, et al. (1996) Consequences of nigrostriatal denervation on the gamma-aminobutyric acidic neurons of substantia nigra pars reticulata and superior colliculus in parkinsonian syndromes. Neurology 46: 802-809 Link: https://goo.gl/OF4Kqb
- Levy R, Herrero MT, Ruberg M, Villares J, Faucheux B, et al. (1995) Effects of nigrostriatal denervation and L-dopa therapy on the GABAergic neuron in the striatum in MPTP-treated monkeys and Parkinson disease: an in situ hybridization study of GAD67 mRNA .Eur J Neuro Sci 7: 1199-1209 Link: https://goo.gl/blNiIh
- Reddy PH, Beal MF (2005) Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Rev 49: 618–632. Link: https://goo.gl/8OS0B5
- Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J (2005) Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal 7: 1117–1139. Link: https://goo.gl/oIiJ0z
- Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120: 483-495. Link: https://goo.gl/wDTSR5
- Henry J, Smeyne RJ, Jang H, et al. (2010) Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord 16: 566–571 Link: https://goo.gl/efmDer
- Irrcher I, Aleyasin H, Seifert EL, et al. (2010) Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet 19: 3734–3746. Link: https://goo.gl/mZdLdt
- Shim JH, Yoon SH, Kim KH, Han JY, Ha JY, et al. (2011) The antioxidant Trolox helps recovery from the familial Parkinson's disease-specific mitochondrial deficits caused by PINK1- and DJ-1- deficiency in dopaminergic neuronal cells. Mitochondrion 11:707-715. Link: https://goo.gl/Oe8XZY
- Heo JY, Park JH, Kim SJ, Seo KS, Han JS, et al. (2012) DJ-1 null dopaminergic neuronal cells exhibit defects in mitochondrial function and structure: involvement of mitochondrial complex I assembly 7: e32629. Link: https://goo.gl/wYrirQ
- Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW (2005) Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci 88:193–201. Link: https://goo.gl/cR5R3R
- Callio J, Oury T, Chu C (2005) Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J Biol Chem 280: 18356–18542. Link: https://goo.gl/cFzw2t
- Vila M, Przedborski S (2003) Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 4: 365–375. Link: https://goo.gl/5hf8NZ
- Perier C, Bovè J, Vila M, Przedborski S (2003) The rotenone model of Parkinson’s disease. Trends Neurosci 26: 345–346. Link: https://goo.gl/3LSvsd
- Fukushima T, Gao T, Tawara T, Hojo N, Isobe A, et al. (1997) Inhibitory effect of nicotinamide to paraquat toxicity and the reaction site on complex I. Arch Toxicol 71: 633–637. Link: https://goo.gl/uDAx89
- Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. British Journal of Pharmacology 164 1357–1391 Link: https://goo.gl/UmWDwX
- Chen M, Gu H, Ye Y, Lin B, Sun L, et al. (2010) Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem Toxicol 48: 2980-2987. Link: https://goo.gl/iWp3f1
- Priya N, Vijayalakshmi K, Khadira S (2014) Investigation on the neuroprotective effects of hesperidin on behavioural activities in 6-ohda induced parkinson Model. Int J Pharm Bio Sci 5: 570 - 577 Link: https://goo.gl/RNbF5R
- Oestreicher E, Sengstock GJ, Riederer P, Olanow CW, Dunn AJ, et al. (1994) Degeneration of nigrostriatal dopaminergic neurons increases iron within the substantia nigra: a histochemical and neurochemical study. Brain Res 660: 8–18. Link: https://goo.gl/nLvdSN
- Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, et al. (2009) Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem 110: 966–975 Link: https://goo.gl/LO1ues
- Lev N, Barhum Y, Ben-Zur T, Melamed E, Steiner I, et al. (2013) Knocking Out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci 50: 542-550 Link: https://goo.gl/wZHGMx
- Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, et al. (1998) Tremulous jaw movements in rats: a model of parkinsonian tremor. Prog Neurobiol 56: 591–611. Link: https://goo.gl/MaS3oM
- Finn M, Mayorga AJ, Conlan A, Salamone JD (1997) Involvement of pallidal and nigral GABA mechanisms in the generation of tremulous jaw movements in the rats. Neuroscience 80: 535–544. Link: https://goo.gl/RCXTxa
- Jicha GA, Salamone JD (1991) Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletion: possible relation to Parkinsonian symptoms. J Neurosci 11: 3822–3829. Link: https://goo.gl/3qXmn5
- Braak H, Muller CN, Rub U, Ackermann H, Bratzke H, et al. (2006) Pathology associated with sporadic Parkinson’s disease – where does it end?. J Neural Transm Suppl 70: 89–97. Link: https://goo.gl/BGYi1Z
- Brettschneider J, Tredici KD, Lee VM, Trojanowski JQ (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16: 109–120. Link: https://goo.gl/QJ2x8b
- Ji Y, Pang PT, Feng L, Lu B (2005) Cyclic AMP controls BDNF- induced TrkB phosphorylation and dentritic spine formation in mature hippocampal neurons. Nat Neurosci 8: 165-172. Link: https://goo.gl/gjy82z
- Wang Q, Zheng JQ (1998) cAMP-Mediated Regulation of Neurotrophin-Induced Collapse of Nerve Growth Cones. The Journal of Neuroscience 18: 4973–4984. Link: https://goo.gl/Ajmuce
- Pizzorusso T, Ratto GM, Putignano E, Maffei L (2000) Brain-Derived Neurotrophic Factor Causes cAMP Response Element-Binding Protein Phosphorylation in Absence of Calcium Increases in Slices and Cultured Neurons from Rat Visual Cortex. J Neurosci 20: 2809–2816. Link: https://goo.gl/4tueqb
- Zhang J, Wang Q, Zhu N, Yu M, Shen B, et al. (2008) Cyclic AMP inhibits JNK activation by CREB-mediated induction of c-FLIPL and MKP-1, thereby antagonizing UV-induced apoptosis. Cell Death Differ 15: 1654–1662. Link: https://goo.gl/Mw6mcI
- Zheng F, Zhou X, Moon C, Wang H (2012) Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol 4: 188-200. Link: https://goo.gl/ysWDJg
- Blaylock RL (2004) Excitotoxicity: A possible central mechanism in fluoride neurotoxicity. Fluoride 37: 301–314. Link: https://goo.gl/1vlfh7
- Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K (2002) The neuropeptide PACAP attenuates -amyloid (1–42)-induced toxicity in PC12 cells. Peptides 23: 1471–1478. Link: https://goo.gl/H0d9EB
- Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, et al. (2005) Amyloid-Peptide Inhibits Activation of the Nitric Oxide/cGMP/cAMP-Responsive Element-Binding Protein Pathway during Hippocampal Synaptic Plasticity. The Journal of Neuroscience 25: 6887–6897. Link: https://goo.gl/x8qDPR
- Nishihara H, Kondoh SK, Insel PI, Eckmann L (2003) Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through nduction of inhibitor of apoptosis protein (IAP)-2. PNAS 100: 8921–8926. Link: https://goo.gl/od6gXH
- Zou J, Crews F (2006) CREB and NF-κB Transcription Factors Regulate Sensitivity to Excitotoxic and Oxidative Stress Induced Neuronal Cell Death. Cellular and Molecular Neurobiology 26: 385–405. Link: https://goo.gl/p3Zko7
- Hampson AJ, Grimaldi M (2001) Cannabinoid receptor activation and elevated cyclic AMP reduce glutamate neurotoxicity. Eur J Neurosci 13: 1529-1536. Link: https://goo.gl/rG5qWG
- Leenders AG, Sheng ZH (2005) Modulation of neurotransmitter release by second messenger activated protein kinases: Implications for presynaptic plasticity. Implications for presynaptic plasticity. Pharmacol Ther 105: 69–84. Link: https://goo.gl/ajhQDa
- Duman RS, Nestler EJ (1999) Functional Roles for cAMP and cGMP. American Society for Neurochemistry
- Ferrero JJ, Alvarez AM, Ramı´rez-Franco J, Godino MC, Bartolomé-Martín D et al. (2013) β-Adrenergic receptors activate exchange protein directly activated by cAMP (Epac), translocate Munc13-1, and enhance the Rab3A-RIM1a interaction to potentiate glutamate release at cerebrocortical nerve terminals. J Biol Chem 288: 31370–31385. Link: https://goo.gl/Dw8Sln
- Mink JW, Thach WT (1991) Basal ganglia motor control. I. Nonexclusive relation of pallidal dis-charge to five movement modes. J. Neurophysiol 65: 273–300. Link: https://goo.gl/yWro8c
- Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381-425. Link: https://goo.gl/FnXf41
- Wenger KK, Musch KL, Mink JW (1999) Impaired reaching and grasping after focal inactivation of globus pallidus pars interna in the monkey. J Neurophysiol 82: 2049 –2060. Link: https://goo.gl/0Lv2gV
- Nishihara H, Kondoh SK, Insel PI, Eckmann L (2003) Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through nduction of inhibitor of apoptosis protein (IAP)-2. PNAS 100: 8921–8926. Link: https://goo.gl/93SfwB
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, et al. (2004) Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. The Journal of Clinical Investigation 114: 1624-1634. Link: https://goo.gl/pIz4ep
- Kendel ER (2012) The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 5: 14. Link: https://goo.gl/otxtP3
- Kandel ER (2001) The Molecular Biology of Memory Storage: A Dialogue Between Genes and Synapses. science 294: 1030-1038. Link: https://goo.gl/1faudw
- Vogelsberg V, Neff NH, Hadjiconstantinou M (1997) Cyclic AMP Mediated enhancement of high affinity choline Transport and Achetylcholine synthesis in brain. J. Neurochein 68: 1062-1070. Link: https://goo.gl/rgevK4
- Vijayaraghavan S, Schmid HA, Halvorsen SW, Berg DK (1990) Cyclic AMP-Dependent Phosphorylation of a Neuronal Acetylcholine Receptor a-Type Subunit. J Neurosci 10: 3255-3262. Link: https://goo.gl/mKcM7r
- Carlezon WA, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends in Neurosciences 28: 436-445. Link: https://goo.gl/olCTtl
- Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu. Rev Neurosci 21: 127–148. Link: https://goo.gl/is5S1L
- Yamashima T (2012) ‘PUFA-GPR40-CREB signaling’ hypothesis for the adult primate neurogenesis. Prog Lipid Res 51: 221–231. Link: https://goo.gl/woMTWe
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709–726. Link: https://goo.gl/das20i
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, et al. (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408. Link: https://goo.gl/gu4Yab
- Sable P, Kale A, Joshi A, Joshi S (2014) Maternal micronutrient imbalance alters gene expression of BDNF ,NGF ,TrkB and CREB in the offspring brain at an adult age. Int J Dev Neurosci 34: 24–32. Link: https://goo.gl/ncTe5N
- Fujii M, Sherchan P, Soejima Y, Hasegawa Y, Flores J, et al. (2014) Cannabinoid receptor type 2 agonist attenuates apoptosis by activation of phosphorylated CREB-Bcl-2 pathway after subarachnoid hemorrhage in rats. Exp Neurol 261: 396–403. Link: https://goo.gl/mFnalg
- Park C, Shin KS, Ryu JH, Kang K, Kim J, et al. (2004) The inhibition of nitric oxide synthase enhances PSA-NCAM expression and CREB phosphorylation in the rat hippocampus. Neuroreport 15: 231–234. Link: https://goo.gl/YaflDr
- Lim S, Moon M, Oh H, Kim HG, Kim SY, et al. (2014) Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J Nutr Biochem 25: 1058–1065. Link: https://goo.gl/qBfBxV
- Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, et al. (2004) The critical role of cyclin D2 in adult neurogenesis. J Cell Biol 167: 209–213. Link: https://goo.gl/sLbsBH
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, et al. (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229. Link: https://goo.gl/s2HKv9
- François F, Godinho MJ, Grimes ML (2000) CREB is cleaved by caspases during neural cell apoptosis. FEBS Lett 486: 281-284. Link: https://goo.gl/tSJYhO
- Hansen C, Bjorklund T, Petit GH, Lundblad M, Murmu RP, et al. (2013) A novel a-synuclein-GFP mouse model displays progressive motor impairment, olfactory dysfunction and accumulation of a-synuclein-GFP. Neurobiol Dis 56: 145–155. Link: https://goo.gl/Zdme9t
- Mehan S, Nain VS, Meena H, Sharma D, Sankhla R, et al. (2010) Comparative effect of phosphodiesterase inhibitors on intracerebroventricular cochicine model of memory impairement in rats. International Journal of Pharma Professional’s Research 1: 122-136
- Zaninotto L, Guglielmo R, Calati R, Ioime L, Camardese G, et al. (2015) Cognitive markers of psychotic unipolar depression: a meta-analytic study. J Affect Disord 174: 580–588. Link: https://goo.gl/3h9Xwp
- Burton CZ, Twamley EW (2015) Neurocognitive insight, treatment utilization and cognitive training outcomes in schizophrenia. Schizophr Res 161: 399–402. Link: https://goo.gl/wVdN7o
- Takeda S, Sato N, Morishita R (2014) Systemic inflammation, blood- brain barrier vulnerability and cognitive/non-cognitive symptoms in alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 6: 171. Link: https://goo.gl/qC0uNZ
- Shaba P, Sidharth M, Gurpreet K, Umesh K (2015) Therapeutic potential of nature molecule “forskolin” mediated cyclic AMP/CREB activation for the amelioration of neurodegenerative disorder like huntington’s. IJRAPR 5: 296-301
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC (2004) Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487-493 Link: https://goo.gl/tQ3eLU
- Patel MB (2010) Forskolin: A Successful Therapeutic Phytomolecule; East and Central African. Journal of Pharmaceutical Sciences 13: 25- 32 Link: https://goo.gl/94H0OG
- Souza NJ, De Dohadwalla AN, Reden J (1983) Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev 3: 201-219. Link: https://goo.gl/WucMhL
- Dubey MP, Srimal RC, Nityanand S, Dhawan BN (1981) Pharmacological studies on coleonol, a hypotensive diterpene from Coleus forskohlii. J Ethnopharmacol 1–13. Link: https://goo.gl/jK5mBl
- Seamon KB, Daly JW (1981) Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res 7: 201–224. Link: https://goo.gl/UB8ndw
- Rao BN, Rao BS (2010) Antagonistic effects of zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis 25: 577–587 Link: https://goo.gl/7t3fr9
- Russo R, Adornetto A, Cavaliere F, Varano GP, Rusciano D, et al. (2015) Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Molecular Vision 21:718-729 Link: https://goo.gl/7xZb1b
- Simplicio JA, Simão MR, Ambrosio SR, Tirapelli CR (2016) The Labdane Ent-3-Acetoxy-Labda-8(17), 13-Dien-15-Oic decreases blood pressure in hypertensive rats. Arq Bras Cardiol 106: 481-490. Link: https://goo.gl/0u8ORs
- Muchekehu RW, Quinton PM (2010) A new role for bicarbonate secretion in cervico-uterine mucus release. The Journal of Physiology 588: 2329-2342. Link: https://goo.gl/ztrNRi
- Godard MP, Johnson BA, Richmond SR (2005) Body Composition and Hormonal Adaptations Associated with Forskolin Consumption in Overweight and Obese Men. Obes Res 13: 1335-1343 Link: https://goo.gl/vVUfCR
- Hohendanner F, Ljubojevic S, MacQuaide N, MacQuaide N, Sacherer M, et al. (2013) Intracellular Dyssynchrony of Diastolic Cytosolic Ca2+ Decay in Ventricular Cardiomyocytes in Cardiac Remodeling and Human Heart Failure. Circ Res 113: 527-538. Link: https://goo.gl/SoKEKJ
- Liu M, Gua L, Sulkin MS (2013) Mitochondrial dysfunction causing cardiac sodium channel downregulation in cardiomyopathy. J Mol Cell Cardiol 54: 25–34. Link: https://goo.gl/m48Inl
- Adnot S, Desmier M, Ferry N, Hanoune J, Sevenet T (1982) Forskolin (a powerful inhibitor of human platelet aggregation). Biochem Pharmacol 31: 4071-4074. Link: https://goo.gl/y8ehBE
- Dillingham MA, Kim JK, Horster MF, Anderson RJ (1984) Forskolin Increases Osmotic Water Permeability of Rabbit Cortical Collecting Tubule. J Membrane Biol 80: 243-248. Link: https://goo.gl/WJVKZ8
- Li H, Yang W, Mendes F, Amaral MD, Sheppard DN (2012) Impact of the cystic fibrosis mutation F508del-CFTR on renal cyst formation and growth. Am J Physiol Renal Physiol 303: 1176–1186. Link: https://goo.gl/108tLW
- Rios-Silva M, Trujillo X, Trujillo-Hernandez B, Sánchez-Pastor E, Urzúa Z, et al. (2014) Effect of Chronic Administration of Forskolin on Glycemia and Oxidative Stress in Rats with and without Experimental Diabetes. Int J Med Sci 11: 448-452. Link: https://goo.gl/oQ4t2K
- Hayashida N, Chihara S, Tayama E, Takaseya T, Enomoto N, et al. (2001) Antiinflammatory Effects of Colforsin Daropate Hydrochloride, a Novel Water-Soluble Forskolin Derivative. Ann Thorac Surg 71: 1931–1938. Link: https://goo.gl/Pwbl2p
- Majeed M, Nagabhushanam K, Natarajan S, Vaidyanathan P, Karri SK (2014) A Double-blind, Randomized Clinical Trial to Evaluate the Efficacy and Safety of Forskolin Eye Drops 1% in the Treatment of Open Angle Glaucoma – A Comparative Study. J Clin Trials 4: 5. Link: https://goo.gl/4szVDK
- Neto MD, Lunardi CN, Rodrigues GJ, Bendhack LM (2011) Vasodilatation induced by forskolin involves cyclic GMP production. Journal of Biophysical Chemistry 2: 373-379. Link: https://goo.gl/ASbMAO
- Battaglia G, Norman AB, Hess EJ (1985) Creese I. D-2 dopamine receptor inhibition of forskolin-stimulated adenylate cyclase activity in rat striatum. Newusci Left 59: 177-182. Link: https://goo.gl/PjO8Cm
- Anwar Z, Albert JL, Gubby SE, Boyle JP, Roberts JA, et al. (1999) Regulation of cyclic AMP by extracellular ATP in cultured brain capillary endothelial cells. British Journal of Pharmacology 128: 465- 471. Link: https://goo.gl/pR6uxu
- Liu M, Gua L, Sulkin MS, Liu H, Jeong Em, et al. (2013) Mitochondrial dysfunction causing cardiac sodium channel downregulation in cardiomyopathy. J Mol Cell Cardiol 54: 25–34. Link: https://goo.gl/MQE7Pq
- Chiong M, Saavedra BC, Soto IN, Ruff DM, Morales PE, et al. (2014) Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Cell and developmental biology 2: 1-9. Link: https://goo.gl/bXgE9x
- Rui Y, Tiwari P, Xie Z, Zheng JQ (2006) Acute Impairment of Mitochondrial Trafficking by -Amyloid Peptides in Hippocampal Neurons. J Neurosci 26: 10480 –10487. Link: https://goo.gl/dP9ggj
- Arnould T, Vankoningsloo S, Renard P, Houbion A, Ninane N, et al. (2002) CREB activation induced by mitochondrial dysfunctions is anew signaling pathway that impairs cell proliferation. The EMBO Journal 21: 53-63. Link: https://goo.gl/Pol6AY
- Cribbs JT, Strack S (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939-944. Link: https://goo.gl/POYNFy
- Corral SR, Tan, DX, Manchester L, Reiter RJ (2015) Diabetes and Alzheimer Disease, Two Overlapping Pathologies with the Same Background: Oxidative Stress. Oxid Med Cell Longev. 1-14. Link: https://goo.gl/OhcJtP
- Heo H, Yoo M, Han D, Cho Y, Joung I, et al. (2013) Upregulation of TrkB by forskolin facilitated survival of MSC and functional recovery of memory deficient model rats. Biochem Biophys Res Commun 431: 796–801. Link: https://goo.gl/echvyd
- Chen Y, Huang X, Zhang YW, Rockenstein E, Bu G, et al. (2012) Alzheimer’s β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid. J Neurosci 32: 11390–11395. Link: https://goo.gl/PW1Z9J
- Troadec JD, Marien M, Mourlevat S, Debeir T, Ruberg M, et al. (2002) Activation of the Mitogen-Activated Protein Kinase (ERK1/2) Signaling Pathway by Cyclic AMP Potentiates the Neuroprotective Effect of the Neurotransmitter Noradrenaline on Dopaminergic Neurons. Mol Pharmacol 62: 1043–1052. Link: https://goo.gl/buOAGX
- Xia Z, Refdal CD, Mercant KM, Dorsa DM, and Storm DR (1991) Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron 6: 431–443. Link: https://goo.gl/RDJLy0
- Mons N, Cooper DMF (1995) Adenylate cyclases: critical foci in neuronal signaling. Trends Neurosci 18: 536–542. Link: https://goo.gl/9NblVz
- Mons N, Decorte L, Jaffard R, Cooper DM (1998) Ca2+-sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sci 62: 1647–1652. Link: https://goo.gl/1PzDqu
- Seamon KB, Vaillancourt R, Edwwards M, Daly JW (1984) Binding of 3H forskolin to rat brain membranes. cad Sci USA 81: 5081-5085. Link: https://goo.gl/YGkH4R
- Darfler FJ, Mahon LC, Koachman AM, lnsel PA (1982) Stimulation by forskolin of intact S49 lymphoma cells involves the guanine nucleotide regulatory protein of adenylate cyclase. J B Chem 257: 11901-11907. Link: https://goo.gl/yVLYV8
- Poat JA, Cripps HE, Iversen LL (1988) Differences between high-affinity forskolin binding sites in dopamine-rich and other regions of rat brain. Proc Natl Acad Sci USA 85: 3216-3220. Link: https://goo.gl/TuPeHq
- Gehlert DR, Dawson TM, Yamamura HI, Wamsley JK (1985) Quantitative autoradiography of 3H Forskolin binding sites in brain. Brain Res 361: 351-360. Link: https://goo.gl/rJovYF
- Daly JW, Padgett W, Seamon KB (1982) Activation of cyclic AMP-generating systems in brain membranes and slices by the diterpene forskolin: augmentation of receptor-mediated responses. J Neurochem 38: 532-544. Link: https://goo.gl/hjFktA
- Siege AM, Daly JW, Smith JB (1982) Inhibition of aggregation and stimulation of cyclic AMP generation in intact human platelets by the diterpene forskolin. Mol Pharmacol 21: 680-687. Link: https://goo.gl/73pgqx
- Ciranna L (2006) Serotonin as a Modulator of Glutamate and GABA-Mediated Neurotransmission: Implications in Physiological Functions and in Pathology. Current Neuropharmacology 4: 101-114. Link: https://goo.gl/BCtHD2
- Gloerich M, Bos JL (2010) Epac: Defining a New Mechanism for cAMP Action. Annu Rev Pharmacol Toxicol 50: 355–375. Link: https://goo.gl/KH5AtW
- Yambolieva VN, Smyth L, Bobalova J (2003) Involvement of cyclic AMP-mediated pathway in neural release of noradrenaline in canine isolated mesenteric artery and vein. Cardiovasc Res 57: 217–224. Link: https://goo.gl/D7iM26
- Hearst SM, Lopez ME, Shao Q, Liu Y, Vig PJS (2010) Dopamine D2 Receptor Signaling Modulates Mutant Ataxin-1 S776 Phosphorylation and Aggregation. J Neurochem 114: 706–716. Link: https://goo.gl/8TwMcX
- Chong YH, Shin YJ, Suh YH (2003) Cyclic AMP inhibition of Tumor necrosis factor α production induced by Amyloidogenic C –terminal peptide of Alzheimer’s Amyloid precursor protein in macrophages: involvement of multiple intracellular pathways and Cyclic AMP response element binding protein. Mol Pharmacol 63: 690-698. Link: https://goo.gl/BHB2LV
- Hichami A, Boichot HE, Germain N, Berdyshev E, Coqueret O, Lagente V (1996) Phosphodiesterase 4 inhibitors and db-cAMP inhibit TNF- release from human mononuclear cells. Effects of cAMP and cGMP-dependent protein kinase inhibitors. Mediators of Inflammation 5: 425-428. Link: https://goo.gl/xuh16x
- Saha RN, Pahan K (2006) Regulation of Inducible Nitric Oxide Synthase Gene in Glial Cells. Antioxid Redox Signal 8: 929–947. Link: https://goo.gl/PxgXn9
- Pahan K, Namboodiri AM, Sheikh FG, Smith BT, Singh I (1997) Increasing cAMP Attenuates Induction of Inducible Nitric-oxide Synthase in Rat Primary Astrocytes. The Journal of biological chemistry 272: 7786–7791. Link: https://goo.gl/DTpZVO
- Wysham DG, Brotherton AF, Heistad DD (1986) Effects of forskolin on cerebral blood flow: implications for a role of adenylate cyclase. Stroke 17: 1299-1303. Link: https://goo.gl/bf4E97
- Boulanger L, Poo MM (1999) Gating of BDNF- Induced Synaptic Potentiation by cAMP. Science 284: 1982-1984. Link: https://goo.gl/kfV0Q9
- Lee JK, Tran T, Tansey MG (2009) Neuroinflammation in Parkinson’s disease. J Neuroimmun Pharmacol 4: 419–429. Link: https://goo.gl/0wGx8w
- Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79: 368–376. Link: https://goo.gl/Kiyliy
- Sulzer D, Surmeier DJ (2013) Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord 28: 715–724. Link: https://goo.gl/83XBHh
- Kasten M, Klein C (2013) The many faces of alpha-synuclein mutations. Mov Disord 28: 697–701. Link: https://goo.gl/xn04JM
- Singleton AB, Farrer MJ, Bonifati V (2013) The genetics of Parkinson’s disease: progress and therapeutic implications. Mov Disord 28: 14–23. Link: https://goo.gl/cMCnBN
- Huot P, Fox SH, Brotchie JM (2011) The serotonergic system in Parkinson’s disease. Prog Neurobiol 95: 163-212. Link: https://goo.gl/Q4kUdf
- Vijverman AC, Fox SH (2014) New treatments for the motor symptoms of Parkinson’s disease. Expert Rev. Clin. Pharmacol 7: 761–777. Link: https://goo.gl/iaOTVQ
- Hawkes CH, Del Tredici K, Braak H (2010) A timeline for Parkinson's disease. Parkinsonism Relat Disord 16: 79-84. Link: https://goo.gl/UeoCnX
- Gaig C, Tolosa E (2009) When does Parkinson's disease begin? Mov Disord 24: S656-664. Link: https://goo.gl/avw2bQ
- Mayeux R, Marder K, Cote L, Denaro J, hemenegildo N, et al. (1995) The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan. American journal of epidemiology 142: 820-827. Link: https://goo.gl/mZ2cTY
- Bower JH, Maraganore DM, Mcdonnell SK, Rocca WA (2011) Incidence and distribution of parkinsonism in Olmsted County, Minnesota. Neurology 52: 1214-1220. Link: https://goo.gl/YHT5UH
- Nussbaum RL, Ellis CE (2003) Alzheimer's disease and Parkinson's disease. New England Journal of Medicine 348: 1356-1364. Link: https://goo.gl/mIORY4
- De Lau, LML, Breteler MMB (2006) Epidemiology of Parkinson's disease. The Lancet Neurology 5: 525-535. Link: https://goo.gl/vzh0tf
- Oberdorf J, Schmidt P (2010) Improving care for people with Parkinson's disease. National Parkinson Foundation Website Link: https://goo.gl/BmQXlP
- Nice (2006) The National Collaborating Centre for Chronic Conditions. Parkinson's disease: National clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians. Link: https://goo.gl/86gnf9
- Schrag A., Ben-Shlomo Y, Quinn N (2000) Cross sectional prevalence survey of idiopathic Parkinson's disease and parkinsonism in London. Bmj 321: 21-22. Link: https://goo.gl/7gUx4p
- Findley L, Aujla M, Bain PG, Barker M, Beach C, et al. (2003) Direct economic impact of Parkinson's disease: A research survey in the United Kingdom. Movement Disord18: 1139-1145. Link: https://goo.gl/L2tg9i
- Deng H, Yuan L (2014) Genetic variants and animal models in SNCA and Parkinson disease. Ageing Res Rev 161-176. Link: https://goo.gl/p14IdG
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, et al. (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65: 66–79. Link: https://goo.gl/MxEdJv
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. (1997) Alpha-synuclein in Lewy bodies. Nature 388: 839–840. Link: https://goo.gl/gYr4vZ
- Conway KA, Harper JD, Lansbury PT Jr (2000) Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry 39: 2552–2563. Link: https://goo.gl/Uu1uxg
- Rockenstein E, Nuber S, Overk CR, Ubhi K, Mante M, et al. (2014) Accumulation of oligomer-prone α-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 137: 1493-1513 Link: https://goo.gl/uZa9Yh
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, et al. (2013) Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154: 103–117. Link: https://goo.gl/dgU1kl
- Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simon J, et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44: 595–600. Link: https://goo.gl/dQWv6z
- Zimprich A, Biskup S, Leitner P, Farrer M, Lincoln S, et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44: 601–607. Link: https://goo.gl/Vp1W2F
- Biskup S, Moore DJ, Celsi F, Hiqashi S, West AB, et al. (2006) Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol 60: 557–569. Link: https://goo.gl/7M9DFJ
- Healy DG, Falchi M, O’Sullivan SS, Binifati V, Durr A, et al. (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol 7: 583–590. Link: https://goo.gl/3hGLZX
- Correia Guedes L, Ferreira JJ, Rosa MM, Coetho M, Bonifati V, et al. (2010) Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 16: 237–242. Link: https://goo.gl/8P3PwZ
- Miklya I, Goltl P, Hafenscher F, Pencz N (2014) The role of parkin in Parkinson’s disease. Neuropsychopharmacol Hung 16: 67–76. Link: https://goo.gl/8Ah3Ss
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608. Link: https://goo.gl/ww3RX6
- Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, et al. (2000) Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med 342: 1560–1567. Link: https://goo.gl/wRi03H
- Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee JK, et al. (2011) Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B PLoS One 6: e23660. Link: https://goo.gl/zjy5wE
- Fernández-Moriano Carlos, González-Burgos Elena, Gómez-Serranillos M (2015) Pilar ; Mitochondria-Targeted Protective Compounds in Parkinson’s and Alzheimer’s Diseases. Oxidative Medicine and Cellular Longevity: 1-30. Link: https://goo.gl/1LiXkK
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, et al. (2011) Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 20: 1726–1737. Link: https://goo.gl/ZeFUS9
- Cookson MR, Dauer W, Dawson T, Fon EA, Guo M, et al. (2007) The Roles of Kinases in Familial Parkinson’s Disease; The Journal of Neuroscience 27: 11865–11868. Link: https://goo.gl/JgmC33
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, et al. (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304: 1158–1160. Link: https://goo.gl/eLYsK7
- Corti O, Brice A (2013) Mitochondrial quality control turns out to be the principal suspect in parkin and PINK1-related autosomal recessive Parkinson’s disease. Curr Opin Neurobiol 23: 100–108. Link: https://goo.gl/QJwzEc
- Alberio T, Lopiano L, Fasano M (2012) Cellular models to investigate biochemical pathways in Parkinson’s disease. FEBS J 279: 1146-1155 Link: https://goo.gl/cFTT0o
- Kahle PJ, Waak J, Gasser T (2009) DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med 47: 1354–1361. Link: https://goo.gl/F89I4a
- Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56: 33–39. Link: https://goo.gl/i7cW3n
- Gonera EG, van’t Hof M, Berger JC, Weel CV, Horstink M (1997) Symptoms and duration of the prodromal phase in Parkinson’s disease. Mov Disord 12: 871–876. Link: https://goo.gl/1AHI9z
- Levin BE, Katzen HL (1995) Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. Adv Neurol 65: 85–95. Link: https://goo.gl/Y49Mv2
- Dubois B, Pillon B (1996) Cognitive deficits in Parkinson’s disease. J Neurol 244: 2–8. Link: https://goo.gl/clE0VM
- Mayeux R (1992) The mental state in Parkinson’s disease, in Handbook of Parkinson’s Disease, 2nd Edition. Edited by Koller WC. New York, Marcel Dekker 159–184.
- Sano M, Stern Y, Williams J, Cote L, Rosenstein R, et al. (1989) Coexisting dementia and depression in Parkinson’s disease. Arch Neurol 46: 1284–1286. Link: https://goo.gl/Nwqv11
- Mohr E, Litvan I, Williams J, et al. (1990) Selective deficits in Alzheimer and parkinsonian dementia: visuospatial function. Can J Neurol Sci 17: 292–297. Link: https://goo.gl/MfesXd
- Mohr E, Mendis T, Grimes JD (1995) Late cognitive changes in Parkinson’s disease with an emphasis on dementia. Adv Neurol 65: 97–113. Link: https://goo.gl/qJcn9r
- Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5: 235-245. Link: https://goo.gl/KHNhP8
- Aarsland D, Marsh L, Schrag A (2009) Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 24: 2175-2186. Link: https://goo.gl/SI34R0
- Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, et al. (1999) Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 67: 492-496. Link: https://goo.gl/Sq4bPi
- Poewe W, Luginger E (1999) Depression in Parkinson’s disease: impediments to recognition and treatment options. Neurology 52: S2-6. Link: https://goo.gl/ImQFOZ
- Rickards H (2005) Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J Neurol Neurosurg Psychiatry 76: 48-52. Link: https://goo.gl/ECDSrz
- Ferreri F, Agbokou C, Gauthier S (2006) Recognition and management of neuropsychiatric complications in Parkinson’s disease. CMAJ 175: 1545-1552. Link: https://goo.gl/v1dIFl
- Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M (2012) Nonmotor symptoms in Parkinson’s disease in 2012: relevant clinical aspects. Parkinsons Dis 198316. Link: https://goo.gl/UIFOKY
- Starkstein SE, Mayberg HS (1993) Depression in Parkinson’s disease, in Depression in Neurologic Disease, edited by Starkstein SE, Robinson RG. Baltimore, MD, Johns Hopkins University Press 97–116.
- Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, et al. (2006) Startling facts about emotion in Parkinson's disease: blunted reactivity to aversive stimuli. Brain 129: 3356-3365. Link: https://goo.gl/SxRtDi
- Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Mechanisms of cognitive set flexibility in Parkinson's disease. Brain 124: 2503-2512. Link: https://goo.gl/olsgBU
- Hawkes CH (2008) The prodromal phase of sporadic Parkinson's disease: does it exist and if so how long is it? Mov Disord 23: 1799-1807. Link: https://goo.gl/xRNCWQ
- Hawkes CH, Shephard BC, Daniel SE (1997) Olfactory dysfunction in Parkinson's disease. J Neurol Neurosurg Psychiatry 62: 436-446. Link: https://goo.gl/SCMDy7
- Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17: 427-442 Link: https://goo.gl/E5XlMW
- Braak H, Braak E (2000) Pathoanatomy of Parkinson's disease. J Neurol 247: II3-10. Link: https://goo.gl/1szNEA
- Wakabayashi K, Mori F, Takahashi H (2006) Progression patterns of neuronal loss and Lewy body pathology in the substantia nigra in Parkinson's disease. Parkinsonism & Related Disorders 12: S92-S98. Link: https://goo.gl/F244zP
- Lotharius J, Brundin P (2002) Pathogenesis of parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3: 932-942. Link: https://goo.gl/Cz5dJJ
- Jager WA, Bethlem J (1960) The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. Journal of Neurology, Neurosurgery & Psychiatry 23: 283-290. Link: https://goo.gl/8lwRtm
- Kakita A, Takahashi H, Homma Y, Ikuta F (1994) Lewy bodies in the cerebellar dentate nucleus of a patient with Parkinson's disease. Pathology international 44: 878-880. Link: https://goo.gl/jsLQW5
- Oyanagi K, Wakabayashi K, Ohama E, Takeda S, Horikawa Y, et al. (1990) Lewy bodies in the lower sacral parasympathetic neurons of a patient with Parkinson's disease. Acta Neuropathological 80: 558-559. Link: https://goo.gl/q4Mlgz
- Wakabayashi K, Takahashi H (1997) The intermediolateral nucleus and Clarke‘s column in Parkinson‘s disease. Acta Neuropathological 94: 287-289. Link: https://goo.gl/4J2bG4
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357-381. Link: https://goo.gl/AvWX8z
- Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, `prefrontal’ and `limbic’ functions. Prog Brain Res 85: 119-146. Link: https://goo.gl/OwUq5z
- Kelly RM, Strick PL (2004) Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res 143: 449-459. Link: https://goo.gl/Yh2OsL
- Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31: 236-250. Link: https://goo.gl/ej7q7P
- Widnell Katherine (2005) Pathophysiology of Motor Fluctuations in Parkinson’s disease. Movement Disorders 11: 17-22. Link: https://goo.gl/Ni7wq3
- Gasparini F, Di Paolo T, Gomez-Mancilla B (2013) Metabotropic glutamate receptors for Parkinson's disease therapy. Parkinson's disease 196028. Link: https://goo.gl/iZ9inP
- Graybiel AM, Hirsch EC, Agid Y (1990) The nigrostriatal system in Parkinson's disease. Adv Neurol 53: 17-29. Link: https://goo.gl/luBprH
- Kish SJ, Shannak K, Hornykiewicz 0 (1988) Uneven patterns of dopamine loss in the striatum of patients with Parkinson's disease. N Engl I Med 318: 876-880. Link: https://goo.gl/kWvz18
- Gibb WR, Lees A (1991) Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry 54: 388-396. Link: https://goo.gl/p7vozA
- Matzuk MM, Saper CB (1985) Preservation of hypothalamic dopaminergic neurons in Parkinson's disease. Ann Neurol 18: 552-555. Link: https://goo.gl/Ph45fL
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283: 9089-9100. Link: https://goo.gl/zljAtJ
- Schapira AHV, Cooper JM, Dexter D, CLARK JB, JENNER, et al. (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Ann Neurol 26: 122-123. Link: https://goo.gl/VZVJzO
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, et al. (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1: 1269. Link: https://goo.gl/Dql8cI
- Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, et al. (1994) Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol 36: 876-881. Link: https://goo.gl/nFQlT3
- Owen AD, Schapira AH, Jenner P, Marsden CD (1996) Oxidative stress and Parkinson’s disease. Ann N Y Acad Sci 786: 217-223 Link: https://goo.gl/vQbnqC
- Smith PR, Cooper JM, Govan GG, Harding AE, Schapira AH (1993) Smoking and mitochondrial function: a model for environmental toxins. Q J Med 86: 657-660. Link: https://goo.gl/xGoc7B
- McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW (2003) Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol 179: 38–46. Link: https://goo.gl/0VKdco
- Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, et al. (1998) Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci 158: 24-29. Link: https://goo.gl/32StkZ
- Hoglinger GU, Carrard G, Michel PP, Medja F, Lombès A, et al. (2003) Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J Neurochem 86: 1297-1307. Link: https://goo.gl/yUoEEj
- Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4: 1399-1440. Link: https://goo.gl/jTnG8E
- Dumont M, Beal MF (2011) Neuroprotective strategies involving ROS in Alzheimer’s disease. Free Radic Biol Med 51: 1014-1026. Link: https://goo.gl/GahS0D
- Yan MH, Wang X, Zhu X (2011) Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 62: 90–101. Link: https://goo.gl/zIFcre
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, et.al. (2014) Oxidative Stress and Its Significant Roles in Neurodegenerative Diseases and Cancer. Int J Mol Sci 16: 193-217. Link: https://goo.gl/LJe54y
- Schütt Claes WH (2008) Cadmium mediated changes in mitochondrial metabolic function of a renal epithelial cell line (A6), evaluated with the MTT-assay; Master project in Molecular Biology Institute for Nature, Systems and Models Ro ‘skilde University (RUC). Link: https://goo.gl/425DY7
- Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ (2004) Ageing, oxidative stress and mitochondrial uncoupling. Acta Physiol Scand 182: 321-331. Link: https://goo.gl/M5AJPO
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, et al. (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96-99. Link: https://goo.gl/ptHKlk
- Hernandez-Aguilera A, Rull A, Rodríguez-Gallego E, Marta Riera-Borrull, Fedra Luciano-Mateo, et al. (2013) Mitochondrial Dysfunction: A Basic Mechanism in Inflammation-Related Non-Communicable Diseases and Therapeutic Opportunities. Mediators of Inflammation 2013: 1-13. Link: https://goo.gl/kDZpXl
- Yadav Renu, Chanu S Idiyasan, Raj Kritika, Sarkar Surajit (2013) Rise and Fall of Reactive Oxygen Species (ROS): Implications in Aging and Neurodegenerative Disorders. Cell Dev Biol 2: 4. Link: https://goo.gl/oCuWBq
- Starkov Anatoly A (2008) The Role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147: 37–52. Link: https://goo.gl/HMyjym
- Poljsak Borut, Šuput Dušan and Milisav Irina (2013) Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Medicine and Cellular Longevity. Link: https://goo.gl/tpFOhK
- Finkel T, Holbrook NJ (2000) Oxidants oxidative stress and the biology of ageing. Nature 408: 239-247. Link: https://goo.gl/Tfvjpk
- Dong Xiao-xia, wang yan, zheng-hong qin (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta pharmacol 30: 379–387. Link: https://goo.gl/SDdGUW
- Kaur Gurpreet, Kukkar Umesh, Mehan Sidharth, Kalra Sanjeev (2015) Mitochondrial ETC Complex I & IV: Novel Destination Sites for Prevention of Neurodegenerative Disorders. IJRAPR 5: 77-86.
- Blaylocka Russell l (2004) Excitotoxicity: A possible central mechanism in Fluoride neurotoxicity. Fluoride 37: 301–314. Link: https://goo.gl/rhe3XK
- Helton TD, Otsuka T, Lee MC, Mu YY, Ehlers MD (2008) Pruning and loss of excitatory synapses by the Parkin ubiquitin ligase. Proc Natl Acad Sci USA 105: 19492–19497. Link: https://goo.gl/lpSZAy
- Lang AE, Lozano AM (1998) Parkinson’s disease-first of two parts. Med Prog 339: 1044–1053. Link: https://goo.gl/09AqF5
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38: 1285–1291. Link: https://goo.gl/7YIXIf
- McGeer PL, McGeer EG, Itagaki S, Mizukawa K (1987) Anatomy and pathology of the basal ganglia. Can J Neurol Sci 14: 363–372. Link: https://goo.gl/BxqSBR
- Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm 60: 277–290. Link: https://goo.gl/ecks8Z
- Hunot Stephane, Hirsch EC (2003) Neuroinflammatory Processes in Parkinson’s disease. Ann Neurol 53: S49–S60. Link: https://goo.gl/XFH3jD
- Tansey Malú G, Frank-Cannon Tamy C, McCoy Melissa K, Lee Jae Kyung, Martinez Terina N, et al. (2008) Neuroinflammation in Parkinson’s disease: Is there sufficient evidence for mechanism-based interventional therapy?. Frontiers in Bioscience 13: 709-717. Link: https://goo.gl/gvFaZq
- Boka GP, Anglade D, Wallach F, Javoy-Agid Y, Agid EC, et al. (1994) Hirsch: Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett 172: 151-154. Link: https://goo.gl/yTNb3A
- Hunot SN, Dugas B, Faucheux A, Hartmann M, Tardieu P, et al. (1991) FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci 19: 3440-3447. Link: https://goo.gl/N84XZg
- Hartmann A, Troadec JD, Hunot S, Kikly K, Faucheux BA, et al. (2001) Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson's disease, but pathway inhibition results in neuronal necrosis. J Neurosci 21: 2247- 2255. Link: https://goo.gl/j5ceST
- Mogi MA, Togari T, Kondo Y, Mizuno O, Komure S, et al. (2001) Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm 107: 335-341. Link: https://goo.gl/s3c1wH
- Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, et al. (2000) Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson's disease. J Neurochem 74: 1384-1392. Link: https://goo.gl/sLhc6s
- Zhou W, Schaack J, Zawada WM, Freed CR (2002) Overexpression of human alpha-synuclein cause’s dopamine neuron death in primary human mesencephalic culture. Brain Res 926: 42-50. Link: https://goo.gl/538yPb
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, et al. (1997) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem 69: 1196-1203. Link: https://goo.gl/DPk5Zo
- Insel PA, Ostrom RS (2003) Forskolin as a Tool for Examining Adenylyl Cyclase Expression, Regulation, and G Protein Signaling. Cellular and Molecular Neurobiology 23: 305-314. Link: https://goo.gl/ujRtuu
- Widnell Katherine (2005) Pathophysiology of Motor Fluctuations in Parkinson’s disease. Movement Disorders. 20: S17–S22. Link: https://goo.gl/1RgnZC
- Freeman TB, Sanberg PR, Isacson O (1995) Development of the human striatum: implications for fetal striatal transplantation in the treatment of Huntington’sdisease. Cell Transplant 4: 539–545. Link: https://goo.gl/h4Jm5Y
- Durieux PF, Schiffmann SN, de Kerchove d’ EA (2011) Targeting neuronal populations of the striatum. Front.Neuro anat 5: 40. Link: https://goo.gl/jVQvYr
- Feng Q, Ma Y, Mu S, Wu J, Chen S, et al. (2014) Specific reactions of different striatal neuron types in morphology induced by quinolinic acid in rats. PloS One 9: e91512. Link: https://goo.gl/UtrfPz
- Choii Gayoung, Jaewon Ko Gephyrin (2015) A central GABAergic synapse organizer. Experimental & Molecular Medicine 47. Link: https://goo.gl/WIfyiU
- Petroff OA (2002) Book Review: GABA and glutamate in the human brain. Neuroscientist 8: 562–573. Link: https://goo.gl/l9jbtT
- Watanabe W, Maemura K, Kanbera K, Tamayama T, Hayasaki H (2002) GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol 213: 1–47. Link: https://goo.gl/sTaC3r
- Ibanez CF, Andressoo JO (2016) Biology of GDNF and its receptors-Relevance for disorders of the central nervous system. Neurobiol Epub 97. Link: https://goo.gl/WkcdpG
- Greenamyre JT (1993) Glutamate–dopamine interactions in the basal ganglia: relationship to Parkinson’s disease. J Neural Transm Gen Sect 91: 255–269. Link: https://goo.gl/wUFIVP
- Obeso JA, Rodriguez-Orez MC, Benitez-Temino B, Blesa FJ, Guridi J, et al. (2008) Functional organization of the basal ganglia: therapeutic implications for the treatment of Parkinson’s disease. Mov Disord 23: S548–S559. Link: https://goo.gl/wh0MvY
- Logie Christopher, Bagetta Vincenza, Bracci Enrico (2013) Presynaptic Control of Corticostriatal Synapses by Endogenous GABA. The Journal of Neuroscience 33: 15425–15431. Link: https://goo.gl/HTWycf
- Mattson MP (2003) Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolec Med 3: 65-94. Link: https://goo.gl/Nx0I1q
- Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15: 1382-1402. Link: https://goo.gl/gbLF1T
- Mody I, Mac Donald JF (1995) NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends in Pharmacological Sciences 16: 356–359. Link: https://goo.gl/kRud5k
- Bundel D, Sarre S, Eeckhaut A, Smolders I, Michotte Y (2008) Critical Evaluation of Acetylcholine Determination in Rat Brain Microdialysates using Ion-Pair Liquid Chromatography with Amperometric Detection. Sensors 8: 5171-5185. Link: https://goo.gl/0i4I96
- Picciotto Marina R, Higley Michael J, Mineur Yann S (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76: 116–129. Link: https://goo.gl/AR8E0W
- Descarries L, Gisiger V, Steriade M (1997) Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 53: 603–625. Link: https://goo.gl/82wjse
- Descarries L, Parent M (2014) The Synapse. Academic Press, Boston 447–466.
- Threlfell S, Cragg SJ (2011) Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci 5: 11. Link: https://goo.gl/SO3Plk
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, et al. (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75: 58–64. Link: https://goo.gl/ut2Ddu
- Muller ML, Nicolaas I Bohnen, Robert A Koeppe, Vikas Kotagal, Peter JH Scott, et al. (2015) Clinical markers for identifying cholinergic deficits in Parkinson's disease. Mov Disord 30: 269–273. Link: https://goo.gl/DhjaHX
- Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, et al. (2007) Role of adenosine A2A receptors in Parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol 83: 293-309. Link: https://goo.gl/Ey7TqL
- Hettinger BD, Lee A, Linden J, Rosin DL (2001) Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol 431: 331-346. Link: https://goo.gl/2sShBL
- Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, et al. (2009) Adenosine, adenosine A2A antagonists, and Parkinson's disease. Parkinsonism Relat Disord 15: 406-413. Link: https://goo.gl/l4G0SX
- Dal-Cim T, Molz S, Egea J, Parada E, Romero A, et al. (2012) Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase- 1 via PI3 K/Akt/GSK-3b pathway. Neurochem Int 61: 397–404. Link: https://goo.gl/7DH5ZZ
- Schwarzschild MA, Xu K, Oztas E, Petzer JP, Castagnoli K, et al. (2003) Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson’s disease. Neurology 61: S55–61. Link: https://goo.gl/3jA9z2
- Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, et al. (1999) Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology (Berl) 147: 90-95. Link: https://goo.gl/HigdPa
- Hauber W, Neuscheler P, Nagel J, Muller CE (2001) Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A(2A) receptors in the caudate-putamen of rats. Eur J Neurosci 14: 1287- 1293. Link: https://goo.gl/64Nb9T
- Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit Formation. Reviews 7: 464-476. Link: https://goo.gl/OVhsT2
- Glass M, Faull RL, Dragunow M (1996) Localisation of the adenosine uptake site in the human brain: a comparison with the distribution of adenosine A1 receptors. Brain Res 710: 79-91. Link: https://goo.gl/kqlYh3
- Mahan LC, Mcvittie LD, Smyk-Randall EM, Nakata H, Monsma FJ, et al. (1991) Sibley. Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol 40: 1-7. Link: https://goo.gl/xXCYo1
- Rivkees SA, Price SL, Zhou FC (1995) Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Res 677: 193-203. Link: https://goo.gl/mMvs3c
- Rosin DL, Hettinger BD, Lee A, Linden J (2003) Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology 61: S12-18. Link: https://goo.gl/jLLRAb
- Jin S, Johansson B, Fredholm BB (1993) Effects of adenosine A1 and A2 receptor activation on electrically evoked dopamine and acetylcholine release from rat striatal slices. J Pharmacol Exp Ther 267: 801-808. Link: https://goo.gl/JpkFUu
- Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, et al. (2004) Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem 91: 873-880. Link: https://goo.gl/fPfxYh
- Golembiowska K, Zylewska A (1998) Agonists of A1 and A2A adenosine receptors attenuate methamphetamine-induced overflow of dopamine in rat striatum. Brain Res 806: 202- 209. Link: https://goo.gl/mx7yVt
- Gerdeman GL, Fernández-Ruiz J (2008) Cannabinoids and the Brain Kofalvi A Ed. Springer-Verlag. Link: https://goo.gl/wj8yfP
- García-Arencibia, Moisés, García, Concepcion, Fernandez-Ruiz, et al. (2009) Concepción and Fernández-Ruiz Javier; Cannabinoids and Parkinson’s disease. CNS & Neurological Disorders - Drug Targets 8: 432-439. Link: https://goo.gl/djGZQf
- Fernández-Ruiz J, González S (2005) Cannabinoids. Handbook of Experimental Pharmacology Pertwee RG Ed. Springer- Verlag 168: 479-507. Link: https://goo.gl/ulreo7
- Gerdeman GL, Fernández-Ruiz J (2008) Cannabinoids and the Brain. Kofalvi A Ed, Springer-Verlag. Link: https://goo.gl/6jhRmJ
- Sagredo O, García-Arencibia M, de Lago E, Finetti S, Decio A, et al. (2007) Cannabinoids and neuroprotection in basal ganglia disorders. Mol Neurobiol 36: 82-91. Link: https://goo.gl/Vrx0vy
- Halliday CM, Blumbergs PC, Cotton RGH, Howe PRC, Blessing WW, et al. (1990b) Neuropathology of immunohistochemically identified brainstem neurons in Parkinson's disease. Ann Neural 27: 373-385. Link: https://goo.gl/tLuSIl
- Pol AN (2012) Neuropeptide transmission in brain circuits. Neuron 76: 98–115. Link: https://goo.gl/J63SVn
- McGeer EC, McGeer PL (1989) Biochemical neuroanatomy of the basal ganglia, Drugs for the Treatment of Parkinson's Disease. Handbook of Experimental Pharmacology, Springer. Link: https://goo.gl/YwjWCL
- Przuntek H, Miiller T (1999) Diagnosis and Treatment of Parkinson's disease - State of the Art. Department of Neurology 3: 1-211. Link: https://goo.gl/u0fTNe
- Jellinger KA (1991) Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14: 153-197. Link: https://goo.gl/fBs1fg
- Fearnley JM, Lees AJ (1991) Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114: 2283-2301. Link: https://goo.gl/s3h7jE
- Cheng Hsiao-Chun, Ulane Christina M, Burke Robert E (2010) Clinical Progression in Parkinson's disease and the Neurobiology of Axons. Ann Neurol 67: 715–725. Link: https://goo.gl/bvy1tR
- Grafe MR, Forno LS, Eng LF (1985) Immunocytochemical studies of substance P and Met-enkephalin in the basal ganglia and substantia nigra in Huntington's, Parkinson's and Alzheimer's diseases. J Neuropathol Exp Neurol 44: 47-59. Link: https://goo.gl/d8dD1r
- Zech M, Bogerts B (1985) Methionine enkephalin and substance P in the basal ganglia of normals, Parkinson patients. Huntington patients and schizophrenics: a qualitative immunohistochemical study. Acta Neuropathologica (Berl) 68: 32-38. Link: https://goo.gl/cQ3vZJ
- Ungerstedt U (1968) 6-Hydroxydopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5: 107-110. Link: https://goo.gl/w4nseK
- Vernon AC, Croucher MJ, Dexter DT (2008) Additive neuroprotection by metabotropic glutamate receptor subtypeselective ligands in a rat Parkinson’s model. Neuroreport 19: 475–478. Link: https://goo.gl/wacyo7
- Austin PJ, Betts MJ, Broadstock M, O’Neill MJ, Mitchell SN, et al. (2010) Symptomatic and neuroprotective effects following activation of nigral group III metabotropic glutamate receptors in rodent models of Parkinson’s disease. Br J Pharmacol 160: 1741–1753. Link: https://goo.gl/nBVvzP
- Iczkiewicz J, Broom L, Cooper JD, Wong AM, Rose S, et al. (2010) The RGD-containing peptide fragment of osteopontin protects tyrosine hydroxylase positive cells against toxic insult in primary ventral mesencephalic cultures and in the rat substantia nigra. J Neurochem 114: 1792–1804. Link: https://goo.gl/pk4DJP
- kaur Gurpreet, Kukkar Umesh, Mehan Sidharth (2016) Ameliorative strategies of natural adenyl cyclase activator to restore the mitochondrial dysfunction against 6-OHDA induced parkinson’s type neurodegenerative disorders. IJRAPR 6: 21-30. Link: https://goo.gl/xEx5Qv
- Mazzio EA, Reams RR, Soliman KF (2004) The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res 1004: 29–44. Link: https://goo.gl/baVpAk
- Perumal AS, Gopal VB, Tordzro WK, Cooper TB, Cadet JL (1992) Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull 29: 699–701. Link: https://goo.gl/MYP8sL
- Kunikowska G, Jenner P (2001) 6-Hydroxydopamine-lesioning of the nigrostriatal pathway in rats alters basal ganglia mRNA for copper, zinc- and manganese-superoxide dismutase, but not glutathione peroxidase. Brain Res 922: 51–64. Link: https://goo.gl/m6UmxO
- Glinka Y, Gassen M, Youdim MB (1997) Mechanism of 6- hydroxydopamine neurotoxicity. J Neural Transm Suppl 50: 55–66. Link: https://goo.gl/OsVo6R
- Hassani OK, Mouroux M, Feger J (1996) Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience 72: 105–115. Link: https://goo.gl/J1O1iS
- Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, et al. (1998) Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol 44: 622–628. Link: https://goo.gl/k9XkCq
- Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, et al. (2002) Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord 17: S145–S149. Link: https://goo.gl/Bonf1Z
- Breit S, Lessmann L, Unterbrink D, Popa RC, Gasser T, et al. (2006) Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur J Neurosci 24: 2275–2282. Link: https://goo.gl/CHKawg
- Goto S, Hirano A, Matsumoto S (1990) Met-enkephalin immunoreactivity in the basal ganglia in Parkinson’s disease and striatonigral degeneration. Neurology 40: 1051–1056. Link: https://goo.gl/YxIZlc
- Nisbet AP, Foster OJ, Kingsbury A, Eve DJ, Daniel SE, et al. (1995) Preproenkephalin and preprotachykinin messenger RNA expression in normal human basal ganglia and Parkinson’s disease. Neuroscience 66: 361–376. Link: https://goo.gl/XOegLZ
- Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM (2003) Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson’s disease. Exp Neurol 183: 458–468. Link: https://goo.gl/dd2F8E
- Fernandez A, De Ceballos ML, Jenner P, Marsden CD (1992) Striatal neuropeptide levels in Parkinson’s disease patients. Neurosci Lett 145: 171–174. Link: https://goo.gl/T7Pd6M
- Hutchison WD, Lozano AM, Davis KD, Saint-Cyr JA, Lang AE, et al. (1994) Differential neuronal activity in segments of globus pallidus in Parkinson’s disease patients. Neuroreport 5: 1533–1537. Link: https://goo.gl/pqzBth
- Um JW, Park HJ, Song J, Jeon I, Lee G, et al. (2010) Formation of parkin aggregates and enhanced PINK1 accumulation during the pathogenesis of Parkinson’s disease. Biochem Biophys Res Commun 393: 824–828. Link: https://goo.gl/OBi6HE
- Tobón-Velasco JC, Limón-Pacheco JH, Orozco-Ibarra M, Macías-Silva M, et al. (2013) 6-OHDAinduced apoptosis and mitochondrial dysfunction are mediated by early modulation of intracellular signals and interaction of Nrf2 and NF-κB factors. Toxicology 8: 109–119. Link: https://goo.gl/dQkUl0
- Yan J, Sun J, Huang L, Fu Q, Du G (2014) Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J Neurosci Res 92: 634–640. Link: https://goo.gl/i7GCLa
- Wang HM, Zhang T, Li Q, Huang JK, Chen RF, et al. (2013a) Inhibition of glycogen synthase kinase-3b by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem Int 63: 345–353. Link: https://goo.gl/6LCxzV
- Threlfell Sarah and West Anthony R (2013) Review: Modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition. Basal Ganglia 3: 137–146. Link: https://goo.gl/k2voui
- Zaccolo Manuela (2009) cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. British Journal of Pharmacology 158: 50–60. Link: https://goo.gl/6JORd9
- Alberini Cristina M (2009) Transcription Factors in Long-Term Memory and Synaptic Plasticity. Physiological Reviews 89: 121-145. Link: https://goo.gl/enXAMn
- Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science. 296: 1636–1639. Link: https://goo.gl/TpCCFR
- Rahman N, Buck J, Levin LR (2013) pH sensing via bicarbonateregulated “soluble” adenylate cyclase (sAC). Front Physiol 4: 343. Link: https://goo.gl/JWgjyR
- Tasken, K, Aandahl EM (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84: 137–167. Link: https://goo.gl/4wZmB1
- Fajardo AM, Piazza GA, Tinsley HN (2014) The Role of Cyclic Nucleotide Signaling Pathways in Cancer: Targets for Prevention and Treatment. Cancers 6: 436-458 Link: https://goo.gl/ZmgNBK
- Hofer AM, Lefkimmiatis K (2007) Extracellular Calcium and cAMP: Second Messengers as “Third Messengers”? Physiology 22: 320–327. Link: https://goo.gl/2LftVo
- Kendel ER (2012) The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Kandel Molecular Brain 5: 14. Link: https://goo.gl/hToQkE
- Nagakura A, Niimura M, Takeo S (2002) Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. British Journal of Pharmacology 135: 1783-1793. Link: https://goo.gl/LjXmfN
- Schenk PW, B. Snaar-Jagalska E (1999) Signal perception and transduction: the role of protein kinases. Biochimica et Biophysica Acta 1449: 1-24. Link: https://goo.gl/EPXwnh
- Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605– 623. Link: https://goo.gl/XjAXtm
- Poser S, Impey S, Xia Z, Storm DR (2003) Brain-Derived Neurotrophic Factor Protection of Cortical Neurons from Serum Withdrawal-Induced Apoptosis Is Inhibited by cAMP. J Neurosci 23: 4420–4427. Link: https://goo.gl/P0l9cb
- Walton MR, Dragunow M (2000) Is CREB a key to neuronal survival? Trends Neurosci 23: 48–53. Link: https://goo.gl/QbRxCv
- Nordeen SK, Young DA (1977) Separation of Effects of Adenosine on Energy Metabolism from Those on Cyclic AMP in Rat Thymic Lymphocytes. J Biol Chem 252: 5324-5331. Link: https://goo.gl/gmjHEa
- Quevedo J, Vianna MR, Roesler R, Martins MR, de-Paris F, et al. (2013) Pretraining but not preexposure to the task apparatus prevents the memory impairment induced by blockade of protein synthesis, PKA or MAP kinase in rats. Neurochemical Research 30: 61–67. Link: https://goo.gl/mtFEuW
- Montminy MR, Bilezikjian LM (1987) Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 328: 175–178. Link: https://goo.gl/6sZXK4
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, et al. (2003) Non-steroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 60: 1059–1064. Link: https://goo.gl/jLclpF
- Gonzales GA, Montminy MR (1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675–680. Link: https://goo.gl/XmNXMD
- Sheng M, Mc Fadden G, Greenberg ME (1990) Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 4: 571–582. Link: https://goo.gl/CRkWQd
- Bito H, Deisseroth K, Tsien RW (1996) CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87: 1203–1214. Link: https://goo.gl/zUhmlL
- Deisseroth K, Heist EK, Tsien RW (1998) Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392: 198–202. Link: https://goo.gl/shDo4m
- Das S, Grunert M, Williams L, Vincent SR (1997) NMDA and D1 Receptors Regulate the Phosphorylation of CREB and the Induction of c-fos in Striatal Neurons in Primary Culture. Synapse 25: 227–233. Link: https://goo.gl/odsciP
- Ghosh A, Ginty DD, Bading H, Greenberg ME (1994) Calcium regulation of gene expression in neuronal cells. J Neurobiol 25: 294–303. Link: https://goo.gl/ijh35h
- Du K, Montminy M (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273: 32377–32379. Link: https://goo.gl/2oaorG
- Ghiani CA, Beltran-Parrazal L, Sforza DM, Malvar JS, Seksenyan A, et al. (2007) Genetic program of neuronal differentiation and growth induced by specific activation of NMDA receptors. Neurochem Res 32: 363-376. Link: https://goo.gl/jeSwSi
- Zhao WQ, Alkon DL, Ma W (2003a) c-Src protein tyrosine kinase activity is required for muscarinic receptor-mediated DNA synthesis and neurogenesis via ERK1/2 and c-AMP-responsive element-binding protein signaling in neural precursor cells. J Neurosci Res 72: 334–342. Link: https://goo.gl/7FWzed
- Hu Y, Fang X, Dunham SM, Prada C, Stachowiak EK, (2004) 90-kDa ribosomal S6 kinase is a direct target for the nuclear fibroblast growth factor receptor1 (FGFR1): role in FGFR1 signaling. J Biol Chem 279: 29325–29335. Link: https://goo.gl/Iq5XfB
- Sharma K, Mehra RD, Dhar P, Vij U (2007) Chronic exposure to estrogen and tamoxifen regulates synaptophysin and phosphorylated cAMP response element-binding (CREB) protein expression in CA1 of ovary ectomized rat hippocampus. Brain Res 1132: 10–19. Link: https://goo.gl/ehXSya
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, et al. (2012) Ca (V)1 and Ca (V)2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149: 1112–1124. Link: https://goo.gl/lMKhr8
- Shieh PB, et al. (1998) Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20: 727–740. Link: https://goo.gl/25toIW
- Wilson BE, E Mochon, and L M Boxer (1996) Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol 16: 5546–5556. Link: https://goo.gl/AWZp4y
- Kida S (2012) A functional role for CREB as a positive regulator of memory formation and LTP. Exp Neurobiol 21: 136–140. Link: https://goo.gl/SC3LTz
- Barco A, Pittenger C, Kandel ER (2003) CREB, memory enhancement and the treatment of memory disorders: promises, pitfall sand prospects. Expert Opin Ther Targets 7: 101–114. Link: https://goo.gl/KZG3wT
- Kim J, Kwon JT, Kim HS, Han JH (2013) CREB and neuronal selection for memory trace. Front. Neural Circuits 7: 44. Link: https://goo.gl/Jia2ZI
- Vogt MA, Inta D, Luoni A, Elkin H, Pfeiffer N, et al. (2014) Inducible forebrain-specific ablation of the transcription factor creb during adult hood induces anxiety but no spatial/contextual learning deficits. Front Behav Neurosci 8: 407. Link: https://goo.gl/3bcFQt
- Bilbao A, Rieker C, Cannella N, Parlato R, Golda S, et al. (2014a) CREB activity in dopamine D1 receptor expressing neurons regulates cocaine-induced behavioral effects. Front Behav Neurosci 8: 212. Link: https://goo.gl/AS60lo
- Bilbao A, Rieker C, Cannella N, Parlato R, Golda S, et al. (2014a) Corrigendum: CREB activity in dopamine D1 receptor expressing neurons regulates cocaine-induced behavioral effects. Front. Behav.Neurosci 8: 239. Link: https://goo.gl/PlWk4E
- Ortega - Martínez Sylvia (2015) A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Frontiers in Molecular Neuroscience 8: Article 46. Link: https://goo.gl/18vmp0
- Bhat SV, Bajqwa BS, dornauer H, de Scousa NJ, Fehlhabar HW (1977) Structures and stereochemistry of new labdane diterpenoids from Coleus forskohlii Briq. Tetrahedron Lett 18: 1669-1672. Link: https://goo.gl/rQdD8M
- Metzger H, Lindner E (1981) The positive inotropic-acting forskolin, a potent adenylate cyclase activator. Drug Res 31: 1248-1250. Link: https://goo.gl/85aBVc
- Anbuselvan S, Muralikrishnan V (2013) Antimicrobial activity of coleus forskohlii root extract against human pathogens. International Journal of Phytopharmacology 4: 42-49. Link: https://goo.gl/tg2Tqm
- Kamini K, Ashashri S, Lalit P (2013) A Comprehensive Review: Coleus Forskohlii. Int J ayurvedic and herbal medicine 3: 1106-1113. Link: https://goo.gl/YGw13B
- Himesh Soni, Kumar Singhai Akhlesh (2012) Recent updates on the genus coleus: a review. Asian J Pharm Clin Res 5: 12-17. Link: https://goo.gl/cKM87Y
- Warier PK, Nambier VP (1996) Indian Medicinal Plants: A compendium of 500 species. Published by Orient Longman Limited, Chennai. Link: https://goo.gl/EzholZ
- Pullaiah T. Encyclopaedia of world medicinal plants. (Edn reprint Regency Publication New Delhi).
- Sharma PV (1997) Dravyaguna Vigyan, Chowkambha Sanskrit Sansthan.
- Rupp RH, de Souza NJ, Dohadwalla AN (1986) Forskolin: Its chemical biological and medical potential. Proc of the International Symposium, Hoechst India Ltd, Bombay. Link: https://goo.gl/ui72tZ
- Lakshmanan GM, Manikandan S, Panneerselvam R (2013) Plectranthus forskohlii (Wild) Briq. (Syn: Coleus forskohlii) – A Compendium on its Botany and Medicinal uses. International Journal of Research in Plant Science 3: 72-80. Link: https://goo.gl/ry1kck
- Ammon HP, Muller AB (1985) Forskolin: from an ayurvedic remedy to a modern agent. Planta Med 51: 473–477. Link: https://goo.gl/VY4avy
- De Souza NJ, Shah V (1988) Forskolin-an adenylate cyclase activating drug from an Indian herb. Econ Med Plant Res 2: 1–16. Link: https://goo.gl/JlbxZT
- Saleem AM (2013) Methods of Isolation and Analysis of Forskolin from Coleus forskohlii. Department of Pharmaceutical Biology, Faculty of Pharmaceutical Sciences, UCSI University 3325-3343. Link: https://goo.gl/RkWM6Y
- Valdes L, Mislankar S, Paul A (1987) Coleus barbatus (C. forskohlii) (Lamiaceae) and the potential new drug forskolin (Coleonol). Econ Bot 41: 474–483. Link: https://goo.gl/LMzYmA
- Puranam Kaushik, Rani H Surekha (2014) Forskolin: Its Therapeutic Applications. Int J Pharm Bio Sci 5: 68-73. Link: https://goo.gl/vbS7cM
- Rao SN (2014) Integrated nutrient management for growth and high yield in coleus forskohlii. IJABPT 5: 291-295. Link: https://goo.gl/eixmP6
- Singh R, Saklani S, Kumar A, Singh R (2011) Chemical Composition of the Essential Oil from Coleus Forskohlii. International Journal of Natural Products Research 1: 38-43. Link: https://goo.gl/icCima
- Palani S, Raja S, Naresh R, Kumar BS (2010) Evaluation of nephroprotective, diuretic and antioxidant activities of Plectranthus amboinicus on acetaminophen-induced nephrotoxic rats. Toxicol Mech Methods 20: 213-21. Link: https://goo.gl/3wKM66
- Agarwal KC, Parks RE Jr (1982) Synergistic inhibition of platelet aggregation by forskolin plus PGE1 or 2- fluoroadenosine: effects of 2’,5’-dideoxyadenosine and 5’-methylthioadenosine. Biochem Pharmacol 31: 3713-3716. Link: https://goo.gl/tw2vll
- Siegl AM, Daly JW, Smith JB (1982) Inhibition of aggregation and stimulation of cyclic AMP Generation in intact human platelets by the diterpene forskolin. Mol Pharmacol 21: 680-687. Link: https://goo.gl/0GwwJw
- Rao DA, Rao VP, Rao KR (2010) Pharmacological effects of forskolin isolated from coleus aromaticus on the lung damage rats. An International Journal of Advances in Pharmaceutical Sciences 1: 18-21. Link: https://goo.gl/RaXMsc
- Lichey R, Friedrich T, Priesnitz M, Biamino G, Usinger P, et al. (1984) Effect of forskolin on methacholine-induced bronchoconstriction in extrinsic asthmatics. The Lancet 167. Link: https://goo.gl/OoQ9EK
- Caprioli J, Sears M, Bausher L, Gregory D, Mead A (1984a) Forskolin lowers intraocular pressure by reducing aqueous inflow. Invest Ophthalmol Vis Sci 25: 268-277. Link: https://goo.gl/WxLjkx
- Seto C, Eguchi S, Araie M, et al. (1986) Acute effects of topical forskolin on aqueous humor dynamics in man. Jpn J Ophthalmol 30: 238-244. Link: https://goo.gl/eQKcME
- Kariya T, Morito F, Sakai T, Takahata K, Yamanaka M (1985) Effect of forskolin on platelet deaggregation and cyclic AMP generation. Arch Pharmacol 331: 119-121. Link: https://goo.gl/P56c8S
- Nourshargh S, Hoult JR (1987) Divergent effects of co-carcinogenic phorbol esters and a synthetic diacylglycerol on human neutrophil chemokinesis and granular enzyme secretion. Br J Pharmac 91: 557-568. Link: https://goo.gl/wytQnO
- Vazin T, K. Ball KA, Luc H, et al. (2014) Efficient derivation of cortical glutamatergic neurons from human pluripotent stem cells: A model system to study neurotoxicity in Alzheimer's disease. Neurobiology of Disease 62: 62–72. Link: https://goo.gl/ybGSvK
- Maeda H, Ozawa H, Saito T, Irie T, Takahata N (2013) Potential antidepressant properties of forskolin and a novel water-soluble forskolin (nkh477) in the forced swimming test. Life Sciences. 1997; 61(25):2435-2442. Link: https://goo.gl/XfPORI
- Morinobu S, Fujimaki K, Okuyama N, Takahashi M, Duman RS (1999) Stimulation of Adenylyl Cyclase and Induction of Brain-Derived Neurotrophic Factor and TrkB mRNA by NKH477, a Novel and Potent Forskolin Derivative. J Neurochem 72: 2198-2205. Link: https://goo.gl/10ryLk
- Tiwari Neha, Mishra Ashutosh, Bhatt Ganesh b, Chaudhary Anil (2014) Anti Stress Activity (in-vivo) of Forskolin Isolated from Coleus forskohlii. Int J Pharm Phytopharmacol Res 4: 201-204. Link: https://goo.gl/GMfYWp
- Russo R, Adornetto A, Cavaliere F, et al. (2015) Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Molecular Vision 21: 718-729. Link: https://goo.gl/pm4yzQ
- Forte Leonard R (1983) Activation of Renal Adenylate Cyclase by Forskolin: Assessment of Enzymatic Activity in Animal Models of the Secondary Hyperparathyroid State. Archives of Biochem and Biophysics 225: 898-905. Link: https://goo.gl/lMpMzK
- Edlund A, Esguerra JL, Wendt A, Flodstrom-Tullberg M, Eliasson L (2014) CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. Edlund et al. BMC Medicine 12: 1-12. Link: https://goo.gl/KOypfE
- Krall JF (1984) A Kinetic Analysis of Activation of Smooth Muscle Adenylate Cyclase by Forskolin. Archives of Biochem and Biophysi 229: 492-497. Link: https://goo.gl/jYxrbb
- Geng D, Li C, Yi LT, Weng LJ, Han YY (2014) Hepatoprotective effects of Coleus forskohlii (wild.) Briq. Against carbon tetrachloride-induced hepatotoxicity in mice. J Chem Pharm Res 6: 130-135. Link: https://goo.gl/fz3Uh2
- Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF (2007) Octreotide Inhibits Hepatic Cystogenesis in a Rodent Model of Polycystic Liver Disease by Reducing Cholangiocyte Adenosine 3’,5’-Cyclic Monophosphate. Gastroenterology 132: 1104–1116. Link: https://goo.gl/7W8thu
- Catalli A, MacDonald C, Pundir P, Kulka M (2012) Inhibitory effects of resveratrol on human mast cell degranulation, cytokine, chemokine and leukotriene release. Open Journal of Immunology 2: 187-194. Link: https://goo.gl/d4Kbzw
- Chang Joseph, Hand James M (1984) Schwalm Susan, Dervinis Alphonse and Lewis Alan J. Bronchodilating activity of forskolin in vitro and in vivo. Eur J Pharmacol 101: 271-274. Link: https://goo.gl/FxrAuu
- Gchlert IR, lason TM, Yan'Lamura HI, Wamslcy JK (1984a) Locallization of qtl forskolin binding sites in lhe ral brain using quantitative auloradiography. Eur J Pharmacol 106: 223- 225.
- Wachtel H, Loschmann PA, Schneider HH, Rettig KJ (1987) Effects of forskolin on spontaneous behavior, rectal temperature and brain cAMP levels of rats: interaction with rolipram. Neuroscience letters 76: 191-196. Link: https://goo.gl/uiaUlA
- Schmidt Kurt, Baer Hans P (1983) Forskolin binding sites in rat liver and brain membranes. EJ P 94: 337-340. Link: https://goo.gl/7p2g9H
- Seamon KB, JW Daly (1981a) Forskolin, a unique diterpene activator of cyclic AMP-generating systems. J Cycl Nucl Res 7: 201. Link: https://goo.gl/I9xVS1
- X Du L (1997) Iacovitti. Multiple signaling pathways direct the initiation of tyrosine hydroxylase gene expression in cultured brain neurons. Mol Brain Res 50: 1–8. Link: https://goo.gl/bwGMu2
- X Du L (1995) Iacovitti, Synergy between growth factors and neurotransmitters required for catecholamine differentiation in brain neurons. J Neurosci 15: 5420–5427. Link: https://goo.gl/Me4dHa
- Wanga Xuan, Lib Xiaoxia, Wang Kun a, Zhou Huifang a, Xue Bing a, et al. (2004) Forskolin cooperating with growth factor on generation of dopaminergic neurons from human fetal mesencephalic neural progenitor cells. Neuroscience Letters 362: 117–121. Link: https://goo.gl/xBtvaB
- Sibley DR, Monsma FJ, Shen Y (1993) Molecular neurobiology of dopaminergic receptors. Int Rev Neurobiol 35: 391–415. Link: https://goo.gl/jHiZOT
- Stoof JC, Kebabian JW (1981) Opposing-roles for D1 and D2 dopamine receptors in efflux of cyclic AMP from rat striatum. Nature 294: 366–368. Link: https://goo.gl/s52Sh5
- Zhuang X, Belluscio L, Hen R (2000) Golf mediates dopamine D1 receptor signaling. J Neurosci 20: 1–5. Link: https://goo.gl/h0dz6x
- Seamon KB, Daly JW (1996) Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Ret 20: 1-150. Link: https://goo.gl/dmh3h7
- Zhao AZ (2005a) Control of food intake through regulation of cAMP. Curr Top Dev Biol 67: 207–224. Link: https://goo.gl/i6bSHl
- Johannessen M, Delghandi MP, Moens U (2004) what turns CREB on? Cell Signal 16: 1211-1227. Link: https://goo.gl/GZ6rMw
- Dumaz N, Marais R (2005) Integrating signals between cAMP and the RAS/ RAF/MEK/ERK signalling pathways. FEBS J 272: 3491–3504. Link: https://goo.gl/TV903s
- Albuquerque EX, Deshpande SS, Aracava Y, Alkondon M, Daly JW (1986) A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. Federation of European Biochemical Societies 199: 113-120. Link: https://goo.gl/Wpc4JP
- Hoffman PW, Ravindran A, Huganir RL (1984) Role of phosphorylation in desensitization of acetylcholine receptors expressed in Xenopus oocytes. J Neurosci 14: 4185-4195. Link: https://goo.gl/d0I1rA
- Caratsch CG, Grassi F, Eusebi F (1992) Functional regulation of nicotinic acetylcholine receptor-channels in muscle. In Narahashi T (Ed) Ion channels Plenum Press New York 3: 177-206. Link: https://goo.gl/QBLrgB
- Huganir R, Greengard P (1990) Regulation of transmitter desensitization by protein phosphorylation. Neuron 5: 555-567. Link: https://goo.gl/qUFb3G
- Aylwin ML, White MM (1992) Forskolin acts as a non-competitive inhibitor of nicotinic acetylcholine receptors. Mol Pharmacol 41: 908-913. Link: https://goo.gl/g80Iry
- Boarder MR, Plevin R, Marriott DB (1988) Angiotensin II potentiates prostaglandin stimulation of cyclic AMP levels in intact bovine adrenal medulla cells but not adenylate cyclase in permeabilised cells. J Biol Chem 263: 11273 -11284. Link: https://goo.gl/xUP0nz
- Sage SO, Rink TJ (1985) Inhibition by forskolin of cytosolic calcium rise, shape change and aggregation in quin2-loaded human platelets. FEBS Lett 188: 135–140. Link: https://goo.gl/L1wlDB
- Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE (1993) 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. Toxicol Environ Health 40: 391-404. Link: https://goo.gl/KzmAE9
- Won JS, Lee JK, Suh HW (2001) Forskolin inhibits expression of inducible nitric oxide synthase mRNA via inhibiting the mitogen activated protein kinase in C6 cells. Molecular Brain Research 89: 1–10. Link: https://goo.gl/cH5RHF
- Koh G, Suh KS, Chon S, Oh S, Woo JT, et al. (2005) Elevated cAMP level attenuates 2-deoxy-d-ribose-induced oxidative damage in pancreatic beta-cells. Arch Biochem Biophys 438: 70-79. Link: https://goo.gl/fXLZf8
- Marsh Laura (2000) Neuropsychiatric Aspects of Parkinson’s disease. Psychosomatics 41: 15–23. Link: https://goo.gl/kqSkDU
- Landsberg G (2005) Therapeutic agents for the treatment of cognitive dysfunction syndrome in senior dogs. Progress in Neuro-Psychopharmacology & Biological Psychiatry 29: 471– 479. Link: https://goo.gl/KqgVtV
- Rutten A, Van Albada M, Silveira DC, et al. (2002) Memory Impairment Following Status Epilepticus in Immature Rats: Time-Course and Environmental Effects. Eur J Neurosci 16: 501–513. Link: https://goo.gl/ChrSfm
- Yena TP, Khoia NN, Ana DP (2014) Down regulation of CREB expressions in trimethyltin-induced hippocampus caused memory dysfunction in mice. Bull. Env. Pharmacol Life Sci 3: 145-153. Link: https://goo.gl/xwKOUJ
- Einstein EB, Patterson CA, Hon BJ (2010) Somatostatin Signaling in Neuronal Cilia Is Critical for Object Recognition Memory. The Journal of Neuroscience 30: 4306–4314. Link: https://goo.gl/8okOPD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

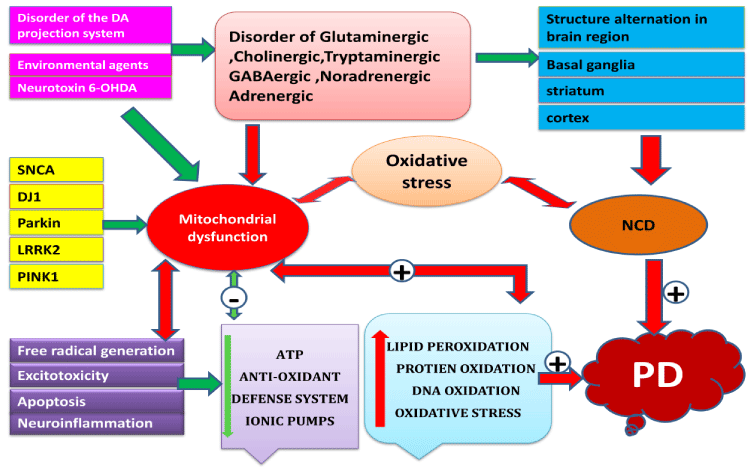
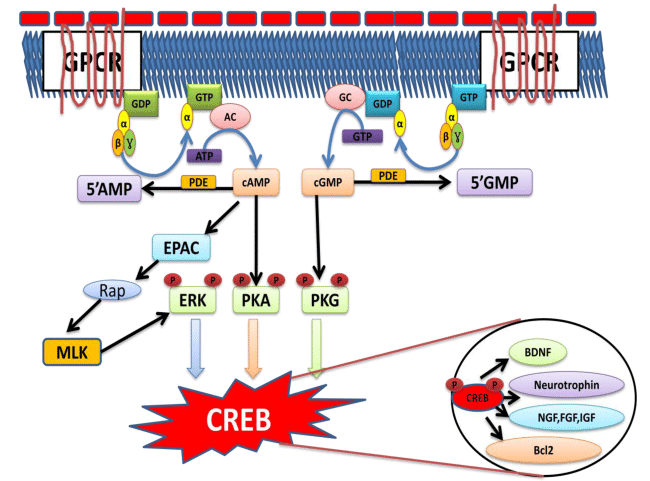
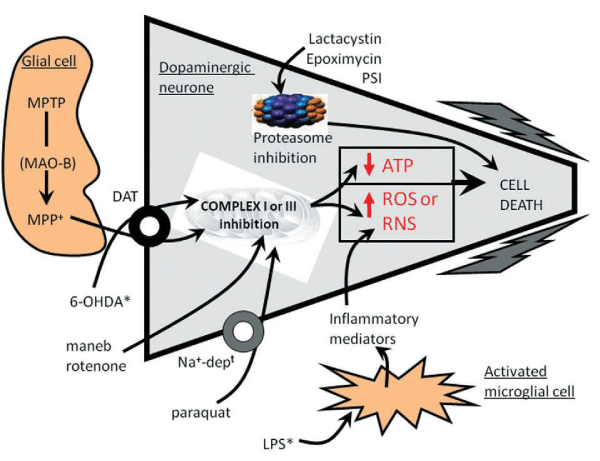

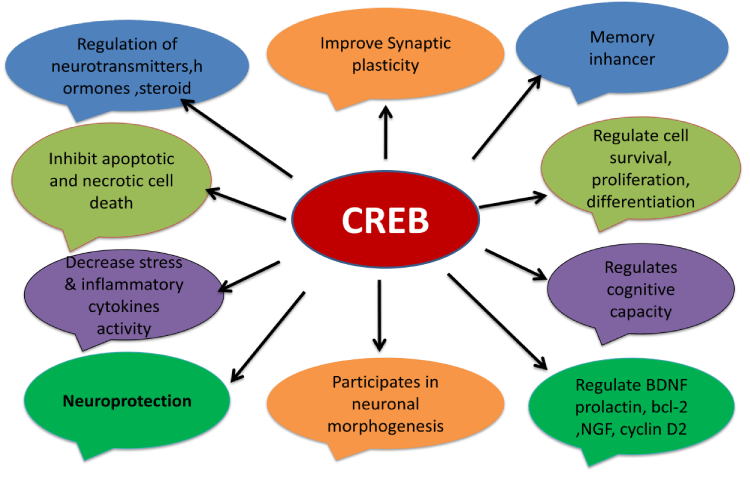


 Save to Mendeley
Save to Mendeley
