Open Journal of Pediatrics and Child Health
Severe immune thrombocytopenic purple in children critical of SARS-CoV-2: Case report
Jesus Dominguez Rojas1*, Mariela Tello Pezo2, Álvaro Coronado Muñoz3, Giancarlo Alvarado-Gamarra4 and Kenny Chonlon Murillo4
2Pediatric Department of the National Institute of Child Health - San Borja, Peru
3Department of Pediatrics, McGovern medical school, University of Texas at Houston, Peru
4Pediatric Department of the Edgardo Rebagliati Martins Hospital, Lima, Peru
Cite this as
Dominguez Rojas JA, Tello Pezo MV, Coronado Muñoz A, Alvarado G, Murillo KC. (2021) Severe immune thrombocytopenic purple in children critical of SARS-CoV-2: Case report. Open J Pediatr Child Health 6(1): 001-004. DOI: 10.17352/ojpch.000029Idiopathic Thrombocytopenic Purpura (ITP) is caused by production of platelet autoantibody. Then ITP can be caused by various viral infections, including Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), cytomegalovirus, herpes simplex virus, Zika virus and others [1]. Episodes of ITP can range from mild to life-threatening.
We reported a pediatric patient with positive molecular test to SARS-CoV-2 associated with severe thrombocytopenia, with active bleeding, not meeting criteria for PIMS, refractory to conventional treatment with immunoglobulin and corticoids for Idiopathic Thrombocytopenic Purpura. The purpose of reporting the case is to provide the hematological manifestations of the new SARS-CoV-2 virus. Idiopathic thrombocytopenic purpura, in our patient the possible cause is the new coronavirus.
Case report
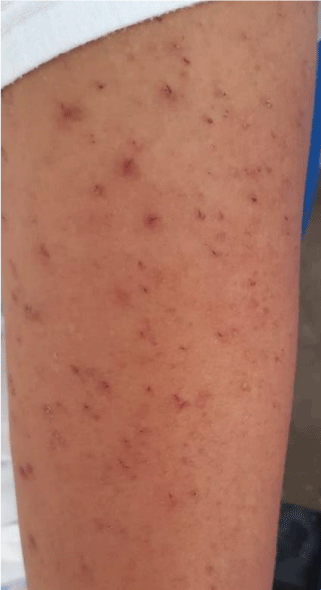
A 13-year-old school patient, female sex, positive epidemiological contact for SARS-CoV-2 (Mother and Maternal Grandmother), enters the emergency department of Lima Reference Hospital in January 2021. Four days of illness, prior to admission you have metrorragias, dysmenorrheas, associated moderate anemia and severe thrombocytopenia 6 x 103/uL (Table 1). Catamenial rhythm: 8/30. On admission RT-PCR SARS-CoV-2 positive. To the preferential clinical examination: oxymetry: 97% environmental, FC: 68 lpm, T: 36.2°C FR: 20 rpm. Weight: 64 Kg, glasgow 15, marked paleness, no breathing difficulty, multiple petechial lesions in upper and lower extremities and ecchymosis (Figure 1), no signs of hypoperfusion, lower hemiabdomen pain, no visceromegalys, hospitalized in isolation area for COVID-19.
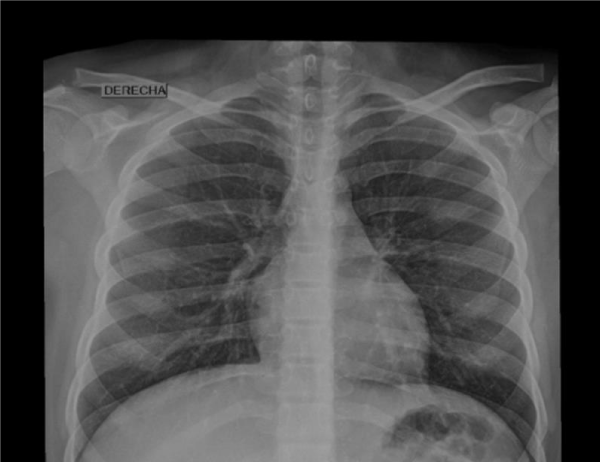
During your hospital stay, platelet count less than 10 x 103/uL and iron profile compatible with iron deficiency anemia are evident. Abdominal and pelvic ultrasound within normal ranges for your age. Increased bronchovascular frame increment chest x-ray, without consolidation (Figure 2).
It is indicated on the fourth day of illness, Human immunoglobulin 2gr/k/total dose, with partial response to therapy with platelet count 105 x 103/ uL (Table 1), with subsequent decreasing controls. He presents headache, nausea, fatigue, and hypermenorrhea, with a decrease in hemoglobin of less than 7g/dl receiving transfusion of blood products. Hormonal profile normal for his age, rheumatological profile pattern ANA 1/160 positive. Factor VIII dosage, normal von Willebrand factor antigen for your age, and elevated ristocetin cofactor. Viral serological tests Epstein Barr, Cytomegalovirus, HIV, negative viral hepatitis. Flow cytometry within normal ranges.
It is indicated at 3 weeks, 2nd dose of human immunoglobulin at the dose indicated above, associated with prednisone corticosteroids 1mg/k/d, obtaining platelet control at 35 x 103/uL, currently listed as refractory Idiopathic Thrombocytopenic Purpura for first-line treatment. Latest platelet control than 55 x 103/uL (Table 1), prior to patient home discharge. Viral panel serology was performed being negative, negative rheumatological tests, non-compatible peripheral lamina for neoplasms, considered as a probable etiological agent SARSCOV2 causing Idiopathic Thrombocytopenic Purpura.
Discussion
Different hematological abnormalities have been observed in patients with COVID-19. Most of them have resulted in a state of hypercoagulability that causes thrombotic complications [2]. However, mild thrombocytopenia has been observed in up to one third of patients with COVID-19 [3]. Although thrombocytopenia observed in COVID-19 was associated with longer hospital stay and increased mortality, they were mild and transient (lasted less than 7 days) and not associated with bleeding [4].
Extremely low platelet counts of <20 x 109 /l, or a sudden fall in the platelet count >50% over 24–48 h, may indicate an immune a etiology [5].
During the COVID-19 pandemic, it has become widely recognized that the SARS-CoV-2 virus has the capability of generating an extraordinary immune response with devastating multi-systemic consequences. A case describing this ‘cytokine storm’ was published by Sinha, et al. [6]. This phenomenon is explained by immunogenic virus peptides that correspond to human proteins that play an important role in the adaptive immune system. It is becoming clear that the immune response plays a strong role in severe disease presentations of COVID-19 such as COVID-19-associated ITP, as seen in the study written by Martincic, et al. [7].
The pathogenesis of ITP is not fully understood. However, the thrombocytopenia is hypothesized to come from three mechanisms: autoantibody mediated decreased platelet production, autoantibodies directed against platelet membrane antigens which cause increased splenic clearance of platelets and shortened platelet half-life, and deficient platelet formation in the bone marrow [8].
In our case, the patient presented severe thrombocytopenia due to the mechanisms already mentioned, with signs of active bleeding related to her catamenial regimen, which required support with platelet blood products. Although it is not uncommon for ITP to improve after 7 to 10 days of treatment, late response is not common [9].
There are few data to indicate whether steroids pose a higher risk of the development of COVID-19 infection or worsening symptoms once infected. However, current guidance from the WHO is to avoid steroids if there are alternative treatment options [10].
In our patient, steroids were used as first-line treatment, with the dose and duration to the minimum necessary. An initial dose of 1 mg / kg was considered without response.
Intravenous immunoglobulin (1g / kg) was added, obtaining an immediate partial elevation of the platelet count.
The patient was hospitalized for approximately 4 weeks in the COVID-19 area, two cycles of corticosteroid-associated human immunoglobulin, and no improvement in platelet count above 105 x 103/uL was observed.
In this case, we illustrate the presentation, clinical course, follow-up and public health implications of the first documented case of ITP in a pediatric patient positive for SARS-CoV-2, in our country.
Note
Unlike the first international pediatric reports with idiopathic thrombocytopenic purpura due to the new SARSCoV-2 virus, which indicate weeks after exposure to the virus. The identification and clinical presentation at the beginning of the disease associated with gynecological disorders were the novelty of the case, observing the behavior of the new virus and its hematological repercussion.
Conclusion
Our case of pediatric idiopathic thrombocytopenic purpura was presented during acute COVID-19 involvement, was associated only with idiopathic thrombocytopenic Purpura skin manifestation, had severe bleeding and was relatively resistant to frontline agents. It is a paediatric case resistant to IgIV treatment and its life-threatening bleeding complications that warrant hospital admission and long-term follow-up for infection with the new coronavirus.
- Liebman HA (2008) Viral-associated immune thrombocytopenic purpura. Hematol Am Soc Hematol Educ Program 1: 212-218. Link: http://bit.ly/3lCBmoA
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, et al. (2020) High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicentre prospective cohort study. Intensive Care Med 46: 1089-1098. Link: http://bit.ly/3rbXYgK
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708-1720. Link: https://bit.ly/2PcCVgQ
- Chen W, Li Z, Yang B, Wang P, Zhou Q, et al. (2020) Delayedphase thrombocytopenia in patients of coronavirus disease 2019 (COVID-19). Br J Haematol Link: https://bit.ly/3tCZoCw
- Pavord S, Thachil J, Hunt BJ (2020) Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. British Society for Haematology and John Wiley & Sons Ltd. Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7267627/
- Sinha P, Matthay MA, Calfee CS (2020) Is a ‘cytokine storm’ relevant to COVID-19?. JAMA Intern Med 180: 1152-1154. Link: http://bit.ly/3lEnaeP
- Martincic Z, Skopec B, Rener K, Mavric M, Vovko T, et a. (2020) Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int J Infect Dis 90: 269-271. Link: https://bit.ly/2OU2nbb
- Tsao HS, Chason HM, Fearon DM (2020) Immune thrombocytopenia (ITP) in a SARS-CoV2–positive pediatric patient. Pediatrics Link: https://bit.ly/31ijIxf
- Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, et al. (2019) American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 3: 3829-3866. Link: http://bit.ly/3r6IOtm
- World Health Organisation (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance 19. Link: http://bit.ly/3vL3zy5
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
