Open Journal of Pediatrics and Child Health
Diaphragmatic Eventration Misdiagnosed as Diaphragmatic Hernia in a Preterm Infant with Respiratory Distress: A Case Report and Review of Diagnosis and Management
John Bishara1*, Sathyaprasad Burjonrappa2, Melodi Pirzada1 and Claudia Halaby1
2Department of Surgery, Winthrop University Hospital, USA
Cite this as
Bishara J, Burjonrappa S, Pirzada M, Halaby C (2015) Diaphragmatic Eventration Misdiagnosed as Diaphragmatic Hernia in a Preterm Infant with Respiratory Distress: A Case Report and Review of Diagnosis and Management. Open J Pediatr Child Health 1(1): 001-004. DOI: 10.17352/2640-7612.000001Introduction: Eventration of diaphragm is a congenital anomaly that results from a failure of muscular development of part or all of the hemidiaphragm. Clinically, eventration of diaphragm refers to an abnormal elevation of an intact diaphragm. In some cases, it may be difficult to distinguish it from congenital diaphragmatic hernia (CDH).
Case Presentation: A three-week-old male, born prematurely at 30 weeks GA, who was weaned off respiratory support on the first DOL, developed respiratory distress. A chest X-ray obtained at that time when compared with the one from DOL#1 showed a new right lower lobe (RLL) opacity, suggestive of lobar atelectasis. Chest MRI revealed the “atelectatic” RLL to be the liver, raising the suspicion for CDH. Thoracoscopic evaluation revealed instead a diaphragmatic eventration, for which a plication procedure was performed.
Discussion: Respiratory distress is the most common clinical manifestation of CDH and diaphragmatic eventration. As in the case of CDH, diaphragmatic eventration can be associated with various degrees of pulmonary hypoplasia due to the compression of the developing lung by the abdominal viscera. The degree of pulmonary hypoplasia and respiratory distress vary depending upon the size of the defect. Patients may be asymptomatic with small localized defects, whereas large defects in neonates can cause respiratory distress.
Conclusion: Symptoms of diaphragmatic eventration can be misleading, becoming a diagnostic dilemma despite a proper evaluation. Eventration must be considered in the differential diagnosis of a newborn with respiratory symptoms and a new CXR image suggestive of lower lobe infiltrate.
Abbreviations
CDH: Congenital Dhernia; NICU: Neonatal Intensive Care Unit; DOL: Day Of Life; RLL: Right Lower Lobe; CPAP: Continuous Positive Airway Pressure; ABG: Arterial Blood Gas; CBC: Complete Blood Count
Introduction
Eventration of diaphragm is a disorder in which part of the diaphragm muscle has an abnormal function and structure, resulting in displacement into the thorax. In the case of diaphragmatic hernia, the diaphragm continuity is disrupted allowing the abdominal content to protrude into the thorax.
Diaphragmatic eventration is a congenital abnormality that results from failure of muscular development of part or all of the hemidiaphragm due to abnormal myoblast migration to the septum transversum and the pleuroperitoneal membrane [1]. The muscular portion of the diaphragm is characterized microscopically by absence of muscular fibers and diffuse fibro elastic changes.
Congenital eventration is rare, occurring in 1 out of 10,000 live births with male sex preponderance and may be associated with other anomalies and syndromes [2]. It can occur in association with chromosomal abnormalities (especially trisomies) and other congenital syndromes like Kabuki makeup syndrome, Beckwith-Wiedemann syndrome, Poland Syndrome, and Jarcho Levin Syndrome. It can be secondary to infections like fetal rubella and cytomegalovirus infection. Eventration can be associated with other congenital anomalies like congenital heart disease, tracheomalacia, cerebral agenesis, renal ectopia, malrotation , Meckel’s diverticulum, and Werdnig Hoffman disease [3].
Management of the diaphragmatic eventration depends upon the severity of respiratory distress. Various surgical techniques are used for correcting the eventration. We present a case of a congenital diaphragmatic eventration with a delayed presentation, which was diagnosed in the operating room despite multiple imaging modalities.
Case Presentation
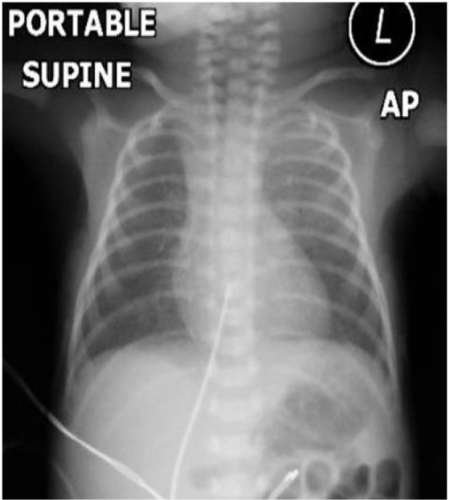
Three-week-old male, born prematurely at 30 weeks GA by vaginal delivery, was weaned off respiratory support on the first DOL. CXR on DOL #1 was normal (Figure 1). Prenatal labs and sonograms were unremarkable. While growing and feeding in the NICU he became tachypneic. Coughing was not present; however there was choking and gagging with oral feeds.
On physical examination, he was afebrile, tachypneic (70-80 breaths per minute) with oxygen saturation of 89-93%. Nasal flaring was not noticed; on lung exam he had no retractions, but had decreased air entry on right side.
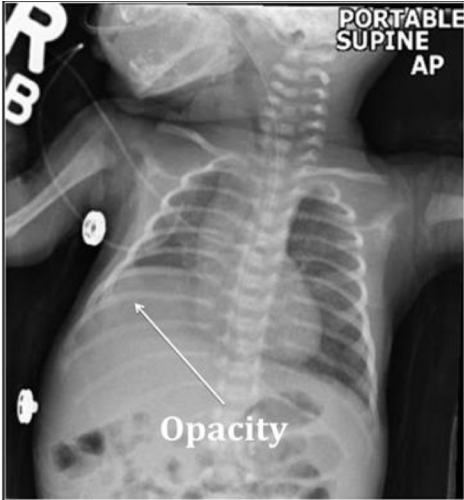
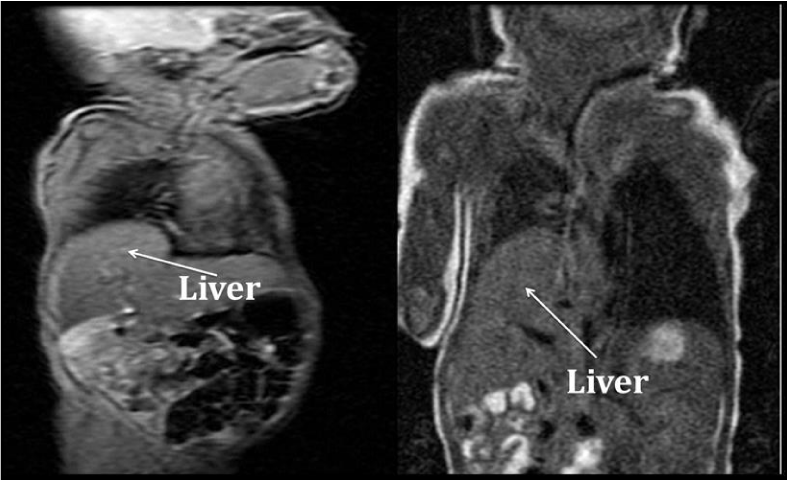
ABG, CBC, and blood culture were normal. To evaluate for tachypnea, a CXR was ordered and showed a new RLL opacity suggestive of atelectasis (Figure 2). For lung recruitment, he was placed on nasal CPAP of 5cmH2O without radiologic or clinical improvement. For concerns of aspiration, he was switched from oral feeds to naso-gastric tube feeds. To evaluate for the RLL opacity, a chest ultrasound was performed, but was limited in evaluation due to rib shadows obscuring the image of the right diaphragm and right lung base. A chest MRI revealed the RLL atelectasis to be the protrusion of the posterior aspect of the liver (Figure 3). Due to the posterior location of the abnormality and to the fact that the diaphragm was not visualized, it raised the suspicion for a Bochdalek CDH. Genetic evaluation revealed a normal karyotype. Echocardiogram, abdominal sonogram and an upper GI series were within normal limits.
However, the baby continued to be tachypneic despite CPAP support and further developed a new right upper lobe atelectasis. With the family consent, the pediatric surgeon elected to repair the suspected right sided CDH. Thorascopy on DOL#56 revealed a right side diaphragmatic eventration instead of CDH. It was also noted that the atelectasis in the right upper lobe was significant. Therefore to prevent potential complications a right diaphragmatic plication was performed. The baby was taken back to the NICU for post-operative care. His respiratory distress improved after diaphragmatic plication and he was tolerating oral feeds by post-operative day #5. He was discharged home on post-operative day #8. Follow-up in the clinic thereafter showed appropriate weight gain and no respiratory distress at 2, 3, and 6 months of age.
Discussion
Eventration of diaphragm is a rare congenital developmental defect of the diaphragm.
As in our case, respiratory distress is the most common clinical manifestation of both, CDH and diaphragmatic eventration. The respiratory distress originates from the direct abdominal organs compression on the lung (resulting in lobar/localized atelectasis and/or development of bronchopneumonia [1]) or from the lung hypoplasia (that results from in utero compression on developing lung).
In the case of diaphragmatic eventration, the thinned, weakened musculature is inadequate to restrain the abdominal viscera, the diaphragm rises, and is upwardly displaced together with the abdominal organs during inhalation [4]. In some cases, it may be difficult to distinguish it from CDH. Posterior eventration of the diaphragm is usually indistinguishable from a Bochdalek hernia, as in our case, where the MRI evaluation of the chest could not distinguish between the two entities [2]. The degree of respiratory distress varies and correlates with the size of the defect. Patients are asymptomatic with small localized defects [4], whereas large defects in neonates can cause respiratory distress [5]. Our case is uncommon, as our patient had right sided eventration, as almost invariably complete diaphragmatic eventration occurs on the left side [1]. The eventration process can have varying degrees of diaphragmatic involvement, from a thin muscle plate to its complete absence, leaving only a thin membrane consisting of pleura, connective tissue and peritoneum [2]. Presentation is dependent on the degree of diaphragmatic muscle involvement and may have a delayed presentation, as in our patient, with a normal chest radiograph on DOL #1 and symptoms starting on DOL#21.
The diagnosis of a CDH is often made on a prenatal ultrasound, around the mean gestational age of 24 weeks, but can be made as early as 11 weeks [6]. Polyhydramnios is the most common finding on ultrasound, reported in up to 80% of prenataly diagnosed CDH cases. Other ultrasound findings suggestive of a CDH include the absence of the stomach in the abdomen and the presence of other solid viscera in the thorax. In contrast with CDH, the diagnosis of congenital diaphragmatic eventration is not suspected prenatally, but after birth, and in many cases the diagnosis may be delayed due to absence of symptoms.
Imaging of the diaphragm can be used to either evaluate the function or its anatomy. Several radiologic modalities, such as chest radiography, ultrasound, CT scan, or magnetic resonance (MR) may be used to evaluate the anatomy of the diaphragm. The function of the diaphragm can be evaluated with the use of fluoroscopy, ultrasound or MR fluoroscopy [7].
With chest radiography, the superior aspect of the diaphragm is seen on frontal and lateral views; however the inferior borders blend with the abdominal viscera beneath. On frontal projection in infants and young children, the right diaphragm is at about the level of the anterior sixth rib. The left diaphragm is usually one intercostal space lower than the right. On lateral views, the anterior part of the left hemidiaphragm is obscured by cardiac shadow, but the right hemidiaphragm is entirely seen. Eventration may be diagnosed by simple radiography revealing abnormally highly displaced hemidiaphragm. However, diaphragmatic elevation can also be caused by other conditions, including normal exhalation. Any process that increases intra-abdominal pressure, (i.e. obesity, ascites, and hepatosplenomegaly) or lung volume loss may pull the diaphragm superiorly (i.e. atelectasis, lung resection, and pulmonary fibrosis) [8] making the diagnosis more challenging.
Traditionally, fluoroscopy has been used for evaluation of diaphragmatic movement. In fluoroscopy, diaphragmatic movement can be evaluated with the patient in various positions and in frontal and lateral views [7]. Evaluation is performed during quiet respiration and deep breathing. Comparative movement of the hemidiaphragms, excursion of an individual hemidiaphragm, and shift of the mediastinum are evaluated. Special maneuvers such as coughing or the sniff test can also be performed in patients able to cooperate. Reduced, absent, or paradoxical movement of a hemidiaphragm (elevation during inspiration and vice versa), is suggestive of diaphragm paralysis or eventration. Fluoroscopic evaluation is limited for the least mobile anterior third of the hemidiaphragm on anteroposterior views and potentially may misinterpret bilateral diaphragmatic paralysis, due to their movement in tandem. Portability, ionizing radiation, visualization of structures above and below the diaphragm, and ability to quantify diaphragmatic motion has limited fluoroscopy in functional evaluation of the diaphragm [9]. Ultrasound replaced fluoroscopy in functional evaluation of the diaphragm, especially in children [7-9].
Ultrasound visualizes the diaphragm as a thick echogenic line due to reflection of the ultrasound waves [7]. Portions of both hemidiaphragms can be seen together on an oblique transverse subxiphoid view obtained at the midline, where their movement can be assessed and compared in real time or in M-mode [10]. The individual domes can be assessed in different ultrasound planes. In M-mode, diaphragmatic movement is assessed quantitatively by using two parameters: direction of motion and amplitude of excursion. Diaphragmatic movement is considered normal if the diaphragm moves toward the transducer during inspiration, with excursion of greater than 4 mm and difference in excursion between the domes of less than 50% [7,10]. Ultrasound limitations are due to poor acoustic contrast obscuring the image and operator experience. Focal diaphragmatic eventration may be missed due to location of the defect.
Chest CT scan and MR imaging are excellent modalities for evaluating the diaphragm due to multiplanar capability and soft-tissue resolution. MR imaging additionally provides excellent soft-tissue resolution and demonstrates the diaphragm as a thin sheet of muscle separating the thoracic and abdominal cavities [7]. Multiplanar imaging allows easier understanding of the orientation of diaphragmatic anatomy and pathologic conditions. MR can use gradient-echo sequences for quantification of diaphragmatic excursion in conditions such as paralysis [7]. Additionally, MR imaging does not carry the risk for ionizing radiation as a CT scan does for the pediatric patient. However, as in our case, it may not be possible to differentiate radiologically between focal eventration and diaphragmatic hernia (particularly if there is a pleural sac with the hernia) or between complete eventration and diaphragmatic paralysis [7-10]. We hypothesize that the rapid respiratory rate of our patient impaired the resolution of the MR imaging.
In our case, appropriate diagnostic imaging techniques were not able to differentiate the diaphragmatic eventration from a CDH. When the diagnosis is elusive or unclear, surgical evaluation may provide further information, as in our case. Thoracoscopy or thoracotomy approach can evaluate the integrity of the diaphragm and the diaphragmatic motion. Surgical correction can be provided depending on the pathology found.
The management of eventration depends upon the extent of respiratory distress. If respiratory distress is mild, the management is supportive. Support may include supplemental oxygen and/or pressure support. Surgical intervention is indicated only in the presence of severe or persistent respiratory distress, resulting in the need for mechanical ventilation [11,12] or failure to thrive. The established surgical treatment is diaphragmatic plication that can be achieved by various techniques and through various approaches: open transthoracic approaches (various thoracotomies, sternotomy, and hemi clam shell incision), thoracoscopic approaches, and minimally invasive approaches (small thoracotomies and thoracoscopic assisted), and open trans abdominal, or laparoscopic approaches [13]. Various techniques of diaphragmatic plication have also been applied for both diaphragmatic eventration and diaphragmatic paralysis. All techniques aim to reduce the abundant diaphragmatic surface and lower the hemidiaphragm [14]. Various suturing methods have been used, including (buttressed or not) interrupted mattress, multiple parallel U, figure of eight, or continuous running sutures. Endostaplers have also been used for plication. Various non-absorbable but also absorbable sutures have been used [14]. There are no studies directly comparing the results of the various approaches and plication techniques [13]. The choice between thoracoscopy versus thoracotomy approach depends on the size of the patient, presenting symptoms and surgeon expertise.
Thirteen observational studies comparing VATS and thoracotomy showed lower mortality rate with the VATS procedure (0% in the VATS group vs. 4% in the thoracotomy group) [15]. The thoracoscopic approach appeared to have more advantages regarding pulmonary functional tests, dyspnea score, length of hospitalization, and postoperative complications [15]. In other reports, laparoscopic diaphragmatic plication has demonstrated significant short-term and mid-term improvements in respiratory quality of life and pulmonary function test results [16]. Thoracoscopic plication (mainly in patients with unilateral paralysis), had good long term results with complete relief of symptoms, absence of radiologic relapse at a mean follow-up of 64.4 (+/- 46) months, and improvement in functional tests sustained for 5 years [17]. Although the short-term outcomes after minimally invasive plication are promising, more long-term results have yet to be assessed [13]. Subjective and objective improvement achieved after open transthoracic plication in patients with unilateral diaphragmatic paralysis was sustained for 5 or more years [18]. The appropriate timing for surgical correction is uncertain and controversial. If the eventration is focal and causes no symptoms, some authors advocate conservative follow-up [19]. But others discuss the adverse effects of this approach on lung growth and suggest that an operation without delay is necessary. As pulmonary development continues in the first few years of life, it seems reasonable to provide space for future lung growth, although no comparative data on this subject is available [19]. In one report, early surgical intervention resulted in reduced respiratory rate and improved weight gain [20]. Performing early surgery may prevent the progression of pathological changes in the lung. In a report that included both congenital and acquired eventration, lung biopsies at the time of surgery showed that atelectasis and pneumonia increased in extent and severity with age [20].
Conclusion
Symptoms of congenital diaphragmatic eventration can be misleading which represents a diagnostic dilemma despite a proper evaluation. Congenital diaphragmatic eventration must be considered in the differential diagnosis of a newborn with respiratory symptoms and a new CXR image suggestive of lower lobe opacity. Our patient initially had respiratory distress related to prematurity which resolved. Re-onset of respiratory distress was due to a delayed presentation of a large right sided congenital diaphragmatic eventration from the lung compression by the liver and not due to lung hypoplasia. In these cases with delayed presentation, the prognosis tends to be better due to absent/minimal lung hypoplasia.
- Goldstein JD, Reid LM (1980) Pulmonary hypoplasia resulting from phrenic nerve agenesis and diaphragmatic amyoplasia. J Pediatr 97: 282-287.
- Soni A, Singh P, Singh RJ, Sood V (2005) Eventration of diaphragm - Embryologic basis. J Anat Society India 54: 1-9.
- Kulkarni ML, Sneharoopa B, Vani HN, Nawaz S, Kannan B, et al. (2007) Eventration of the diaphragm and associations. Indian J Pediatr 74: 202-205.
- Mohammed Kabir Saleh, Mohammad Abba Suwaid, Sule Kazaure Idris, Abdulkadir Musa Tabari, Kabiru Isyaku (2012) Diaphragmatic eventration mimicking congenital diaphragmatic hernia: The value of chest radiograph and barium meal in diagnosis. Niger J Basic Clin Sci 9: 36-39.
- Deslauriers J (1998) Eventration of the diaphragm. Chest Surg Clin N Am 8: 315-330.
- Langham MR Jr, Kays DW, Beierle EA, Chen MK, Mullet TC, et al. (2003) Twenty years of progress in congenital diaphragmatic hernia at the University of Florida. Am Surg 69: 45-52.
- Chavhan GB, Babyn PS, Cohen RA, Langer JC (2010) Multimodality imaging of the pediatric diaphragm: anatomy and pathologic conditions. RadioGraphics 30: 1797-1817.
- Laura K Nason, Christopher M Walker, Michael F McNeeley, Wanaporn Burivong, Corinne L Fligner, et al. (2012) Imaging of the Diaphragm: Anatomy and Function. Radio Graphics 32: E51-E70.
- Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, et al. (2001) Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med 20: 597–604.
- Epelman M, Navarro OM, Daneman A, Miller SF (2005) M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol 35: 661–667.
- Thomas TV (1970) Congenital eventration of the diaphragm. Ann Thorac Surg 10: 180-192.
- Groth SS, Andrade RS (2009) Diaphragmatic eventration. Thorac Surg Clin 19: 511-519.
- Groth SS, Andrade RS (2010) Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg 89: S2146-2150.
- Leo F, Girotti P, Tavecchio L, Conti B, Delledonne V, et al. (2010) Anterior diaphragmatic plication in mediastinal surgery: the “reefing the mainsail” technique. Ann Thorac Surg 90: 2065-2067.
- Visouli AN, Mpakas A, Zarogoulidis P, Machairiotis N, Stylianaki A, et al. (2012) Video Assisted Thoracoscopic Plication of the Left Hemidiaphragm in Symptomatic Eventration in Adulthood. J Thorac Dis 4: 6–16.
- Groth SS, Rueth NM, Kast T, D'Cunha J, Kelly RF, et al. (2010) Laparoscopic diaphragmatic plication for diaphragmatic paralysis and eventration: an objective evaluation of short-term and midterm results. J Thorac Cardiovasc Surg 139:1452-1456.
- Graham DR, Kaplan D, Evans CC, Hind CR, Donnelly RJ (1990) Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience. Ann Thorac Surg 49: 248-251.
- Mouroux J, Venissac N, Leo F, Alifano M, Guillot F (2005) Surgical treatment of diaphragmatic eventration using video-assisted thoracic surgery: a prospective study. Ann Thorac Surg 79: 308-312.
- Yazici M, Karaca I, Arikan A, Erikçi V, Etensel B, et al. (2003) Congenital eventration of the diaphragm in children: 25 years' experience in three pediatric surgery centers. Eur J Pediatr Surg 13: 298-301.
- Obara H, Hoshina H, Iwai S, Ito H, Hisano K (1987) Eventration of the diaphragm in infants and children. Acta Paediatr Scand 76: 654-658.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
