Open Journal of Orthopedics and Rheumatology
Complex Regional Pain Syndrome Type 1 Produced by Hematoma Formation after Blood Donation: A Case Report
Shin-Tsu Chang1,2* and Cheng-Chiang Chang1
2Department of Physical Medicine and Rehabilitation, Taichung Veterans General Hospital, Taichung, Taiwan
Cite this as
Chang ST, Chang CC (2021) Complex Regional Pain Syndrome Type 1 Produced by Hematoma Formation after Blood Donation: A Case Report. Open J Orthop Rheumatol 6(1): 012-015. DOI: 10.17352/ojor.000032The occurrence of hematoma and bruise formation, accounting for the majority of donation-related complications in the arm, rarely results in Complex Regional Pain Syndrome (CRPS). We report a 24-year-old man who presented with CRPS on his right upper limb two months later due to hematoma and bruising formation just after a blood donation following with immediate performance of strenuous exercise in the upper limbs. Triple phase bone scan, one of the bone scintigraphic studies, revealed positive findings and was compatible with the symptoms of CRPS, e.g. hyperalgesia, swelling and discoloration. The potentially disabling condition, however, ended up with a thankfully benign outcome because of our early finding and proper treatment that included three-day oral prednisolone and two-week physiotherapy and occupational rehabilitation. To our knowledge, CRPS produced by donation-related complications with subsequent hematoma and bruise due to vigorous exercise is rare. CRPS should be taken into consideration in a blood donor who demonstrated allodynia because of performing heavy exercise immediately after blood donation.
Introduction
Approximately one third of whole-blood donors have an adverse physical event during or after whole-blood donation. Most of the common adverse effects associated with arm findings after blood donation are bruise (22.7%), soreness (10.0%), and hematoma (1.7%) [1]. The reasons of the upper limbs adverse events due to blood donation are multifactorial, including genetic factors, inflammatory process, peripheral/central dyssensitization, sympathetic malregulation, somatosensory cortex reorganization, and psychophysiologic interactions, and all symptoms are likely a result of different combinations following with time elapse [2]. Complex Regional Pain Syndrome (CRPS) induced by hematoma might be another severe complication of blood donation.
Anatomically speaking, sensory branches of the musculocutaneous nerve locate below the antecubital veins, as is classically taught, although they are also above the antecubital veins or intertwine with them. Local nerve injuries are unavoidable after phlebotomy because nerve branches are situated so close to the vessels and are impalpable. The frequency of nerve irritation is relatively high. Newman reported an occurrence of 40% of the nerve injuries after a straightforward phlebotomy [3]. Another study based on a donor interview reported that sensory changes in the forearm and hand occur in approximately 1% of whole-blood donors [1].
The nerve distribution in the donor’s arm might play an important role on the possibility of occurrence of CRPS, which has been shown to be developed due to abnormalities in the central and peripheral nervous systems after a nociceptive painful event. The characteristic of CRPS develops unproportionately with the painful event and is not limited to a single nerve course. Pathophysiological aspects including neurogenic inflammation, impairment of sympathetic function, and coupling between sympathetic efferents and nociceptive afferents should all be taken into consideration [4,5]. The diagnosis is ordinarily made on a clinical basis. There is no pathognomonic laboratory finding for CRPS. An alternative way in bone scintigraphy, the Triple Phase Bone Scan (TPBS), can show increased uptake in the involved limb earlier in the process. Multidisciplinary treatment combining Transcutaneous Electrical Nerve Stimulation (TENS), physical therapy, psychotherapy using behavior modification techniques, and oral medications are sometimes helpful [6].
CRPS induced by formation of hematoma followed blood donation has never been described in the literature before. We present a 24-year-old patient, which developed CRPS two months after blood donation. It was considered that hematoma with bruise on the antecubital fossa or antecubital cutaneous nerve injury could probably have led to the development of CRPS.
Case report
A 24-year-old man experienced progressive painful swelling of the right upper extremity two months after blood donation. He had an unremarkable past medical history, and is enthusiastic about donating blood. Voluntarily, he had already donated blood two times during his service in the army. Two months prior to this admission, he donated blood for the third time via the right antecubital vein as usual in the morning. An amount of 250 ml whole blood was collected from the vein with a 16-gauge needle using aseptic technique. Eight hours later in the early evening, he was ordered to join routine military training that consisted of chin-ups, push-ups and running and he practiced them vigorously. Unknowingly, he found bruising and hematoma appearing on his forearm, which spread from the site of venipuncture with slight pain during the resting period. From that day on, he began to suffer intermittent painful swelling and discoloration in the right upper extremity after every training course, which became more severe when he performed grenade-throwing. Two days before the admission, he developed a burning pain on the right hand with obvious cyanosis, swelling, weakness and limited active Range of Motion (ROM) after the strenuous exercise. According to his statement these problems were not related to any trauma.
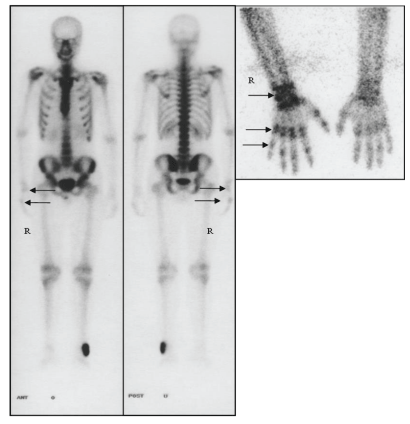
On admission, the patient had a pain Visual Analogue Scales (VAS) of 8/10, and the pain in the forearm progressively worsened and was unrelieved by rest, with a hematoma on the antecubital fossa combined with a large area of bruise by 15x7 cm2. Together with were extensive swelling, mild hyperpigmentation, allodynia, coldness, cyanosis and limited ROM of the right upper limb, especially on the right wrist. The hyperalgesia on the affected muscle-guarding limb was so intense that to undergo even a slight touch could induce a burning and tingling pain out of proportion to the injury and extend beyond the confines of dermatomal distribution. All of the following studies were within normal limits, including C-reactive protein, hemoglobin, white blood cell count, blood chemistry, rheumatoid factor and nerve conduction studies. The TPBS showed the increased uptakes in the right forearm, wrist and hand, and is consistent with the finding of CRPS type 1. He was undergone physiotherapy with whirl-pool hydrotherapy and silver spike point stimulation, as well as occupational therapy such as daily living training, hand function training, and motor-sensory training. A significant improvement was noted followed by three-day prednisolone administration with 15 mg four times a day on the first day of admission, and was weaned by 20 mg a day over three-day period.
All symptoms gradually disappeared in two weeks. The patient had a VAS of 1/10 before being discharged from our ward. Three months since outpatient follow-up, he has made fairly good recovery with regards to his overall general condition.
Discussion
CRPS type 1 or 2 are both clinical syndromes with symptoms including burning pain, hyperalgesia, allodynia, edema, sudomotor or vasomotor changes in the distal extremity. CRPS type 1 most often occurs following soft-tissue trauma to a limb, while CRPS type 2 occurs after injury to a peripheral nerve trunk. The mechanisms underlying these two disorders are poorly understood, but may be related to changes in the sympathetic nervous system and/or neurogenic inflammation [6]. Sasano, et al. reported a 61-year-old woman who presented with CRPS type 2 in the hand after transradial coronary intervention for coronary catheterization [7]. Papadimos and Hofmann reported a rare case of CRPS type 1 following transradial coronary intervention, which etiology was that the radial artery was occluded for a distance of 12 cm proximal to the puncture site, possibly due to the longer period of hemostatic compression [8]. A five-year Japanese study revealed 133 cases of resultant persistent pain and 19 cases of neuropathic pain after performing venipunctures [4].
It has also been shown that venipunctures may cause CRPS type 2 [9]. Horowitz reported 11 patients with injury to upper extremity cutaneous nerves after routine venipuncture developed causalgia (CRPS type 2), and proposed that nerve injury appeared secondary to direct trauma via “inappropriate” needle or bolused material entry into the plane of the nerves beneath the veins, or nerves overlying the veins [10]. Unek et al reported a patient with end-stage renal disease presenting with reflex sympathetic dystrophy syndrome (CRPS type 1) on the patient’s left hand 1 month after Arteriovenous Fistula (AVF) surgery. Magnetic resonance angiography confirmed a steal syndrome at the AVF level and the bone scintigraphy confirmed RSDS in early stage [11]. Genc, et al. also reported an 11-year-old young girl with CRPS type 1 precipitated by rubella vaccination [12]. Therefore, intramuscular injections, percutaneous venous catheter insertion, or percutaneous arterial catheter insertion might induce CRPS on the affected limbs, which may or may not be related to the nerve injury adjacent to these punctured vessels. However, blood donation with subsequent vigorous exercise induced extensive hematoma and bruise triggering CRPS has never been reported in the literature. The symptom of allodynia in our patient was compatible with the image of TPBS (Figure 1) and was worse on the right hand and wrist instead of the forearm, the exact site of punctured injury by the insertion of a 16-gauge needle to the antecubital vein.
Because subjective cutaneous hypoesthesia and hyperalgesia in the vicinity of the antecubital fossa mainly on the wrist and hand instead of the punctured site or the path of the nerve trunk, we thought that the regional reaction did not result from punctured trauma but from hematoma stasis which could be an initiating noxious event and lead to CRPS type 1. Whereas, a negative result of nerve conduction study could not exclude the contribution of a nerve injury, because the nerve conduction study only detects dysfunction in larger peripheral nerves, and also cannot address the issue of whether there are possible differences of signs or symptoms between patients with and without dysfunction in smaller nerve fibers.
The scintigraphic imaging is valuable in the evaluation and early detection of CRPS. Adult patients with CRPS characteristically have a TPBS pattern consisting of diffusely increased tracer uptake with juxtaarticular accentuation of tracer uptake on images. However, Hod and Horne, presented an uncommon adult case of CRPS, whose bone scintigraphy demonstrated decreased activity on early and late phase images of in the affected limb [13]. The imaging of TPBS in our case demonstrated increased uptake in the right forearm, wrist and hand that was consistent with the finding of CRPS type 1. The early finding of CRPS can also be seen in brain image as increased uptake in the contralateral thalamus [14,15], but our case did not have the perfusion scan at the time.
Diagnosing a CRPS at an early stage is important because treatment in the early stage may lead to a good outcome. The best treatment for CRPS is not known yet. For the relief of symptoms, many kinds of treatment, such as TENS, corticosteroids, adrenergic blocking agents, calcium channel blockers, anti-depressants, hyperbaric oxygen therapy and so on, have been used with varying effects. Two small randomized controlled trials have reported that a 4- to 12-week course of high-dose glucocorticoids administered orally 3 or 4 times daily, can alleviate pain, edema and hyperalgesia in CRPS patients [16,17]. A latest review article described the various medical treatment and disease management [18]. Our case was given analgesics and corticosteroids orally (prednisolone) 60 mg on the first day after admission, 40 mg on the second day, and 20mg on the third day. He presented a good response to the prednisolone administration in combination with rehabilitation therapy, such as physiotherapy with applying hydrotherapy and TENS and occupational therapy with hand function training, fine motor and sensory motor therapy on the affected right upper limb.
In the present patient, insufficient rest after blood donation resulted in the hematoma on the antecubital fossa combined with a large area of bruising. Furthermore, the ensuing fierce military training including push-ups, chin-ups and a long-distance running made the ecchymosis even more serious. Two painful episodes on the forearm following vigorous training within two months between the time of blood donation and the first attack of swelling with allodynia might already denote CRPS. Moreover, two days prior to his admission, he kept running at least 2000 meters long together with his right upper limb immobilized in a guarded position, which may exacerbate the vasomotor changes in the affected forearm, wrist and hand because of pain induced by the previous hematoma. This reminds us that longer and more adequate resting period after blood donation before facing intense exercise, the possibility of occurrence of CRPS on account of the formation of hematoma is reduced.
In summary, we present a case of CRPS type 1 due to blood donation complications which was exacerbated with hematoma and bruising. Early intervention plays an important role in reducing the long-term sequelae. Whenever performing a blood collection, donors must be informed to be aware of the possibility of the formation of hematoma if inadequate resting period, which may also be noxious for the emergence of CRPS. In order to shorten the clinical course, to reduce the possibility of disability, and to prevent long-term morbidity medical personnel should make an early finding and provide proper treatment as soon as possible.
Concise Paragraph
1. CRPS type 1 or 2 are both clinical syndromes with symptoms including burning pain, hyperalgesia, allodynia, edema, sudomotor or vasomotor changes in the distal extremity. CRPS type 1 caused by hematoma per se has rare been reported before, while CRPS type 2 can occur after venipuncture due to nerve injury.
2. Blood donation with subsequent vigorous exercise induced extensive hematoma and bruise triggering CRPS type 1 has never been reported in the literature.
3. Whenever performing a blood collection, donors must be informed to be aware of the possibility of the formation of hematoma if inadequate resting period, which might also be noxious for the emergence of CRPS.
- Newman BH, Pichette S, Pichette D, Dzaka E (2003) Adverse effect in blood donors after whole-blood donation: a study of 1,000 blood donors interviewed 3 weeks after whole-blood donation. Transfusion 43: 598–603. Link: https://bit.ly/36ycnwe
- Shah A, Kirchner JS (2011) Complex regional pain syndrome. Foot and Ankle Clinics 16: 351-366. Link: https://bit.ly/2NLLFty
- Newman B (2001) Venipuncture nerve injuries after whole-blood donation. Transfusion 41: 571-572. Link: https://bit.ly/2MFWblN
- Kato J, Araki H, Kimura M, Takahshi K, Ueda K, et al. (2012) Incidence and prognosis of persistent pain induced by venipuncture for blood sampling: an observational study over a 5-year period. Pain Medicine 13: 1627-1630. Link: https://bit.ly/3r8uyAt
- Elahi F, Reddy CG (2014) Venipuncture-Induced Complex Regional Pain Syndrome: A Case Report and Review of the Literature. Case Reports in Medicine. 2014: 613921. Link: https://bit.ly/2Yz95o8
- Baron R, Levine JD, Fields HL (1999) Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the generation of pain? Muscle Nerve 22: 678-695. Link: https://bit.ly/2MFNgAE
- Sasano N, Tsuda T, Sasano H, Ito S, Sobue K, et al. (2004) A case of complex regional pain syndrome type II after transradial coronary intervention. J Anesth 18: 310-312. Link: https://bit.ly/39z4jNI
- Papadimos TJ, Hofmann JP (2002) Radial artery thrombosis, palmar arch systolic blood velocities, and chronic regional pain syndrome 1 following transradial cardiac catheterization. Catheter Cardiovasc Interv 57: 537-540. Link: https://bit.ly/3j9OuQL
- Horowitz SH (2000) Venipuncture-induced causalgia: anatomic relations of upper extremity superficial veins and nerves, and clinical considerations. Transfusion 40: 1036-1040. Link: https://bit.ly/2NVy3w3
- Horowitz SH (1994) Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology 44: 962-964. Link: https://bit.ly/3pEj07J
- Unek IT, Birlik M, Cavdar C, Ersoy R, Onen F, et al. (2005) Reflex sympathetic dystrophy syndrome due to arteriovenous fistula. Hemodial Int 9: 344-348. Link: https://bit.ly/36uS1UW
- Genc H, Karagoz A, Saracoglu M, Sert E, Erdem HR (2005) Complex regional pain syndrome type-I after rubella vaccine. Eur J Pain 9: 517-520. Link: https://bit.ly/2L7A9b7
- Hod N, Horne T (2004) Decreased uptake on 3-phase bone scintigraphy in posttalar fracture reflex sympathetic dystrophy. Clin Nucl Med 29: 560-561. Link: https://bit.ly/3an3GWL
- Fukumoto M, Ushida T, Zinchuk VS, Yamamoto H, Yoshida S (1999) Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet. 354: 1790-1791. Link: http://bit.ly/3q9Izhu
- Lai MH, Wang TY, Chang CC, Li TY, Chang ST (2008) Cerebellar diaschisis and contralateral thalamus hyperperfusion in a stroke patient with complex regional pain syndrome. J Clin Neurosci 15: 1166-1168. Link: http://bit.ly/3rHPJtv
- Christensen K, Jensen EM, Noer I (1982) The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand 148: 653-655. Link: https://bit.ly/3anzRoV
- Braus DF, Krauss JK, Strobel J (1994) The shoulder-hand syndrome after stroke: a prospective clinical trial. Ann Neurol 36: 728-733. Link: https://bit.ly/3oFkUnk
- Cutts S, Gangoo S, Srinivasan SH, Modi N, Pasapula C, et al. (2020) Complex regional pain syndrome: an evolving perspective. Postgrad Med J postgradmedj-2020-13780. Link: https://bit.ly/3ctl5zD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
