Open Journal of Orthopedics and Rheumatology
A review on finite element analysis of the anterior cruciate ligament reconstruction
Simões1, O. J., Ramos2, A., Oliveira3, J. P., Noronha3, J. C., Simões2,4, J. A
2University of Aveiro, Aveiro, Portugal
3Order of Saint Francisco Hospital, Porto, Portugal
4ESAD-College of Art and Design, ESAD.IDEA, Matosinhos, Portugal
Cite this as
Simões, O.J, Ramos, A, Oliveira, J.P, Noronha, J.C, Simões, J.A (2021) A review on finite element analysis of the anterior cruciate ligament reconstruction. Open J Orthop Rheumatol 6(1): 001-011. DOI: 10.17352/ojor.000031A significant number of papers relatively to the investigation made on Anterior Cruciate Ligament (ACL) Reconstruction (ACLR) has been published in orthopaedic related journals. Finite Element (FE) Analysis (FEA) has been used to predict the performance of biomechanical-biomedical systems as well as the effect of clinical factors on the ACLR success. This research tool presents some advantages relatively to experimental studies in assessing stresses and strains in soft tissues of the knee joint. By interpreting correctly FE results, it is possible to extrapolate them to clinical situations. This article reviews papers published from 2016 until nowadays on FEA for ACLR studies searched in Google Scholar, Medline and PubMed databases. Only studies that addressed surgery techniques, type and size of grafts, tunnel geometry and orientation, and fixation devices are reviewed and presented.
Introduction
Anterior Cruciate Ligament (ACL) injuries are common in accidents and sports activities and its reconstruction is necessary to restore the static and dynamic stability of the knee. It is also performed in patients with functional instability following ACL injury, resulting in surgical repair or reconstruction [1-3]. The yearly incidence rate is of over two million injuries worldwide [4].
The knee joint is composed of structures with multiple body articulations that produce biomechanical complex responses to loads resulting from physical activities. The ACL is one of the main ligaments that connects the femur to the tibia and is often torn during certain pivot movements resulting in knee instability. The ACL after rupture leads to abnormal loading of the knee joint and does not have the biological ability to repair itself (self-healing) due to the intricate complexity of its structure and lack of vascular supply. Knee osteoarthritis can develop or progress under abnormal gait after ACL Reconstruction (ACLR). ACLR can be performed using different surgical techniques that need to be known to understand the mechanisms that lead to its failure.
Multiple underlying causes can be associated to the graft reconstruction failure like pain, stiffness or instability [5]. In this sense, many published studies have improved our understanding of the etiology, surgical reconstruction techniques and prevention of ACL injuries. Excessive knee valgus, poor trunk control, excessive quadriceps forces and leg asymmetries have been identified as high risk biomechanical factors for ACL tear [6]. Some studies have emphasized the importance of an anatomical ACLR to restore normal knee anatomy and kinesiology [7].
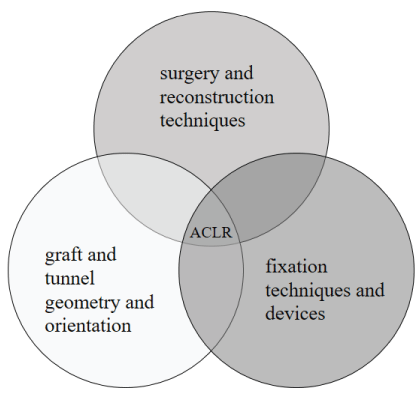
Due to the complexity of the ACL anatomy and function, its reconstruction is not a single solution. Scientific advances have been made and we have more knowledge on the anatomical, biological and biomechanical issues regarding the incorporation of grafts, new fixation devices, materials and techniques, as well as more effective and faster rehabilitation devices and protocols. Literature puts in evidence three main topics of research on ACLR: surgery, biomechanics and clinical outputs [6-64]; types of grafts (autograft versus allograft versus synthetic grafts, Hamstring Tendon (HT) versus Patellar Tendon (PT) versus Quadriceps Tendon (QT) and single bundle versus double bundle) and tunnel geometry and orientation [65-102]; and fixation techniques and devices [103-109]. Other issues have also been addressed such as rehabilitation programs [6,21,44,54], tunnel drilling and thermal necrosis [55,57,110], bone remodeling/healing [36,42], virtual simulation [7,22,27,68] and specific Finite Element Analysis (FEA) [31,77].
The advantages and disadvantages of surgical techniques have been reported [69]. ACLR depends on many factors that can contribute for surgery failure/non-failure. Factors like anatomical positioning in the footprint, fluid leakage, knee hyper flexion and hyperextension, graft size, surgical techniques, tunnel length and geometry, IKDC scores, cortical damage, tunnel enlargement, stress-strain states, Lachman test, Lysholm scales, screw-bone interference, impingement, long-term osteoarthritis, costs, etc., have been discussed in a significant number of scientific papers. Some are related to reaming of the tibial and femoral tunnels (positioning, alignment and geometry), quality of bone tissue, anatomy of the knee; others are related to the performance of graft fixation [45].
The tibial and femoral tunnel placements are of primordial importance in achieving adequate knee functioning. Loads transferred at the bone-ligament interface are also a relevant problem. Bone remodeling depends on the presence of friction and magnitude of stress, strain and/or elastic strain energy, particularly in cancellous bone. Stress/strain shielding causes non physiological biomechanical-biological environments that can lead to bone erosion at the tunnel periphery with excessive osteolysis (enlargement) of the tunnel and ultimately provoke premature failure. A comprehensive understanding of the ACL anatomy has led to the development of new surgical techniques supplemented by more robust biological and mechanical concepts.
Different modeling techniques have been used to model and replicate the functional and structural characteristics of the knee joint ligaments. Some of the factors that influence the success or failure of the ACLR are the integrity of secondary restraints, preoperative laxity of the knee, status of the articular and meniscal cartilages, graft material, surgical technique, graft tension, tibial slope, knee alignment, combined ligament injury and postoperative rehabilitation [92]. FEA is a numerical tool that can be used to evaluate the performance of different types of research problems in orthopaedics and has been used for many years by researchers to determine how devices or structures may behave under different circumstances [49,50,111]. It is a powerful tool that can be used to predict biomechanical-biological performance, optimize design, screening, prediction, and treatment in orthopaedics [49]. FEA can also be used to anticipate complications or failures to prevent similar occurrences. This has been greatly enhanced by more powerful and advanced computer systems and has benefitted the field of orthopaedics. Surgical devices that have been developed using this technology are safer and more effective [112].
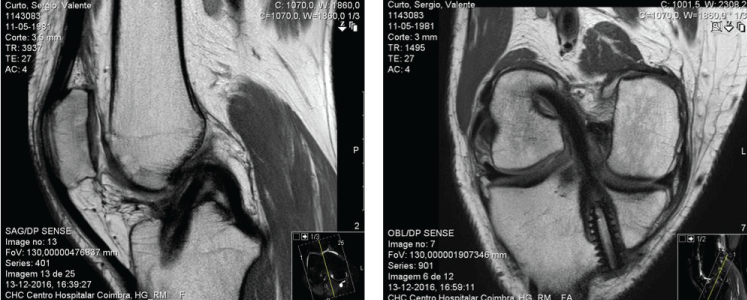
A significant number of papers have addressed the modelling of the ACL, not so much the ACLR [64,82,83]. It is still unclear how ACLR techniques and materials affect knee joint motion and mechanics. As the in vivo measurement of knee joint loading is not possible, FEA are used to assess the influence of these in the outcomes of ACLR [72]. FEA has become an increasingly popular technique for the study of human joint biomechanics, as it allows detailed analysis of the joint/tissue behavior under complex clinically relevant loading conditions. The potential of FE models to define optimal surgical parameters like graft positioning (insertion sites and fixation tension) in combination with graft type to restore the kinematic and kinetic behavior of the knee has been demonstrated [47,76]. Three-dimensional FE models based on Magnetic Resonance Images (MRI) (Figure 1) are a reliable way to build FE models of the knee joint (Figure 2) [56].
A review on FEA of the ACLR was made and is presented in this paper. The information searched (in Google Scholar, Medline and PubMed databases) was grouped in three main topics (Figure 3): surgery and reconstruction techniques; graft and tunnel geometry and orientation; and fixation techniques and devices. Under several options to organize the literature review, we decided to organize the information in those inter-related topics which seem to be the most important ones concerning performance and outcomes of ACLR. The retrieved papers were screened in order to determine which suited adequately for FEA of ACLR. Table 1 presents the list of papers identified for each subtopic of the main topics considered.
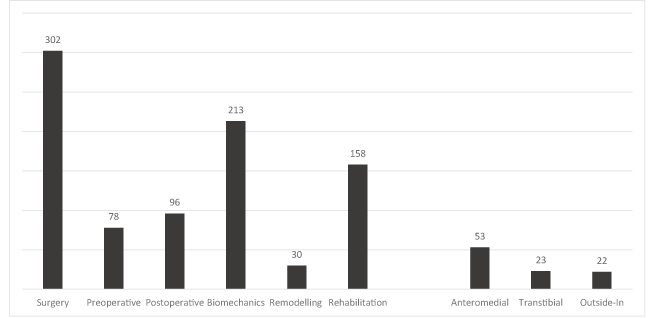
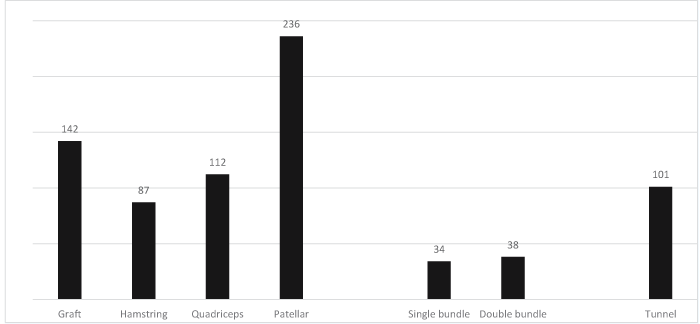
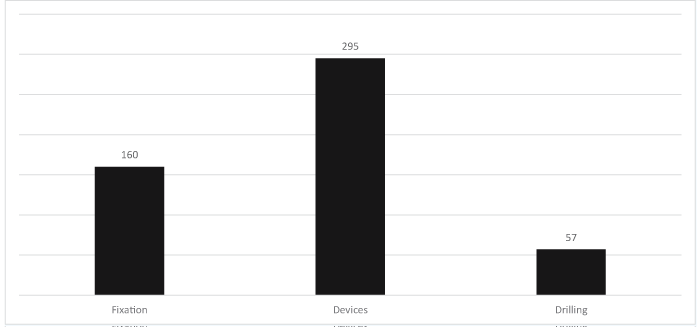
A total of 1180 hits was obtained with intersection of keywords “FEA” and “ACL” and “reconstruction”, but only 190 papers were analyzed since these were the most suitable for the purpose of the study. Figures 4-6 identify the number of hits and keywords considered. These hits were obtained intersecting the respective keywords identified in the graphs.
ACLR techniques include arthroscopic or open surgery, intra or extra-articular reconstruction, femoral and tibial tunnel placement, graft types, single or double bundle and fixation methods and devices.
The two major techniques for ACLR fixation are open surgery (arthrotomy) and arthroscopically assisted. Arthroscopically assisted ACRL is widely accepted as the standard of care for active individuals with functional instability of the knee joint related to ACL injury [113] and is superior to open surgery [114] and provides faster rehabilitation [115].
A femoral tunnel can be created using the Transtibial (TT), Anteromedial (AM) portal, or Outside-in (OI) Technique [116]. TT technique has a tendency to produce a femoral tunnel in non-anatomic location and the graft may be placed too anteriorly and vertically and therefore might not be able to center the graft near the anatomic center of the ACL [117]. Postoperative complications including graft failure and rotational instability have been reported with this technique [116]. ACL reconstruction techniques have been transformed into anatomical and tibial tunnel-independent techniques and more anatomic reconstruction of the ACL can restore normal joint function and kinematics, since femoral tunnel placement has shown to play a vital role in the biomechanics, stability and clinical outcomes after ACLR [118]. Relatively to the TT approach, the independent AM portal technique is thought to better position the femoral tunnel within the native ACL footprint and leave the graft more posteroinferior on the wall of the lateral femoral condyle [118]. However, this technique has complications such as short femoral socket, posterior wall blowout of the femoral socket [116]. The OI technique allows more freedom with positioning of the femoral tunnel and can be performed in retrograde fashion [116].
Extra-articular ACLR has been used over the last century to address ACL deficiency but has not gained favor due to residual instability and the subsequent development of degenerative changes in the lateral compartment of the knee. The intra-articular reconstruction has become the technique of choice but does not restore normal knee kinematics. Some authors have recommended the extra-articular reconstruction in conjunction with the intra-articular technique [119].
Allografts and autografts are the two main groups of grafts used in ACLR [120]. The theoretical advantages of an allograft are elimination of donor site morbidity, decreased pain, shorter operating and rehabilitation times, and better cosmesis [121]. Three autograft options are commonly used. The Bone–Patellar Tendon–Bone (BPTB) allows for bone-to-bone healing within the tibial and femoral tunnels and has theoretical advantages of faster healing. Semitendinosus and gracilis tendons (quadrupled Hamstring Tendon [HT]) minimize donor site morbidity compared with the BPTB autograft and thus theoretically cause less anterior knee pain. A third option is the Quadriceps Tendon (QT), which can include a bone Block from the patella (BQT) [121].
The most common treatment strategy for the injured ACL is either Single-Bundle (SB) or Double-Bundle (DB) ACLR [122]. Both surgical management approaches are relatively effective in restoring the native anatomy and kinematics of the joint [123]. The choice for SB or DB remains controversial. Some published studies compared the two procedures on human cadavers and have demonstrated better results for DB ACLR [124,125]. Several clinical studies have reported that anatomic DB ACL reconstruction might improve pivot-shift resistance, increase rotational knee control, decrease the rate of meniscal tears and postpone progression toward arthritis [126-128]. Other studies found no significant differences between clinical outcomes [129].
Although ACLR can fail for a variety of reasons, the most common technical error is incorrect tunnel placement, with the femoral tunnel more commonly misplaced than the tibial tunnel. In fact, even small changes in tunnel placement have been shown to significantly affect knee kinematics after ACLR [130]. The localization of the femoral tunnel is particularly important in terms of isometric placement of the graft. More anatomic placement of the tunnels can lead to greater knee stability and a more accurate reproduction of native knee kinematics. The all-inside AM portal technique requires only minimal surgical incisions and allows precise femoral tunnel placement. The OI technique may be more beneficial in obese patients, skeletally immature patients or revision cases [131].
There are several methods to assess tunnel placement that include post-operative computed tomography scan, post-operative radiographs, post-operative MRI and intra-operative fluoroscopy. Radiographs of the knee are useful and cost effective in determining the anatomic placement of a graft and have been shown to accurately predict graft placement when validated with three dimensional CT scans [132].
Fixation methods mainly involve fixing soft tissue and bone and can be classified mainly into four types: tissue fixation in the femoral site, tissue fixation in the tibial site, bone fixation in the femoral site, and bone fixation in the tibial site [133]. Devices identified from literature search are described in the work of Wang, et al. [134]. According to these authors, devices for femoral fixation can be divided according to their underlying mechanisms: compression (producing compressive loads to the longitudinal axis of the graft), expansion (producing a bulging of the graft) or suspension (suspending the graft into the femoral tunnel). Examples of compression devices are interference screws (bioabsorbable or metallic) and bone plug. The cross pin system is a popular technique among expansion mechanisms. There are also some other devices which adopt a suspension mechanism and are fixed more or less far away from the knee joint, including three subdivisions according to the type of bone [134].
Surgery and reconstruction techniques
Different surgery techniques are commonly applied for ACLR and have been investigated using FE models to predict clinical performance [23,24,30,35,48,51,72,90,95,96]. Traditional TT and AM techniques have been extensively studied with FE models to analyze anatomical placement of the femoral and tibial tunnel within the native ACL footprint and to determine forces of the graft during functional motion [23,24,41,96]. Some mixed findings exist when comparing TT and AM techniques. The systematic review of clinical and biomechanical studies comparing AM and TT techniques published by Chalmers, et al. show that some studies refer superior rotational stability and clinical outcomes with the AM technique and others find no difference [25]. Even though, no studies showed significantly better results with the TT technique. It looks like that the AM portal technique for ACLR may be more likely to produce improved clinical and biomechanical outcomes, but that the TT technique is capable of producing similar ones [25]. Based on the mean value of the von Mises stresses on a HT graft, Bhat, et al. refer that the AM portal technique is a better technique than the TT technique [24]. This conclusion is corroborated by Geng, et al. [80]. According to Tampere, et al. the AM technique places tunnels with less variance, close to the anatomical center of the ACL footprints, with significantly shorter femoral tunnels and smaller inter-tunnel angle [96].
FEA have showed the occurrence of higher, but non-significant, reaction forces in the graft, especially on the femoral side and lower, but statistically not significant, reaction moments with the AM technique. Forces and moments within the graft are technique-dependent. Bae, et al. concluded that the anatomic TT technique places the femoral tunnel to the anatomic position of the native ACL femoral attachment site and decreases the peak contact pressure and the maximum principal stress at the full extension position of the graft compared with the AM portal technique [23]. In this sense, the anatomic TT technique may be regarded as a superior surgical technique when compared with the conventional TT or AM portal techniques. Rezazadeh, et al. suggest that performing a well-done technique is more important than choosing a technique [52].
Single-bundle hamstring ACLR using the AM technique showed superior surgeon-recorded stability according to the IKDC knee score, Lachman test, and pivot-shift test. But no difference in patient-reported functional outcome (Lysholm score) was observed [26]. Guler at al. analyzed 48 patients who underwent arthroscopic ACLR with ipsilateral HT autograft and concluded that precise reconstruction on the sagittal plane cannot be obtained with either the AM or the TT technique [81]. Even though the AM technique is superior to the TT technique in terms of anatomical graft positioning. In a follow-up performed by Franceschi, et al. AM portal provided better rotational stability and anterior translation than drilling the femoral tunnel using the TT technique, but this difference does not seem to be relevant from the clinical and functional viewpoints [33]. Lee, et al. concluded that ACLR using the AM portal and OI femoral drilling techniques resulted in a shorter length and greater coronal obliquity of the femoral tunnel than did the TT technique [43].
ACL is comprised mainly of two bundles: AM and posterolateral (PL) bundles. FE models have been developed to analyze the stress distribution in the internal fibers under load [29]. There is a lack of knee joint FE models which include both AM and PL bundles to predict changes of articular cartilage contact pressures resulting from ACL injuries [28]. The type of reconstruction can include either using single bundle reconstruction, double-femoral-tibial tunnel reconstruction, single-femoral double-tibial tunnel reconstruction or double-femoral single-tibial tunnel reconstruction, and certainly ones will present biomechanical and clinical advantages over others. In this sense, besides rotational stability, stresses of soft tissues can play a major role in the success of ACLR.
The effects of different ACLR techniques on the knee joint mechanics were studied by Halonen, et al. with six FE models during gait: healthy ACL; ACL rupture; single bundle ACLR; double bundle ACLR; weakened (softer) single bundle reconstruction; and single bundle reconstruction with less pre-strain [72]. The results of the study of these authors suggest that rather than the choice of a reconstruction technique, stiffness and pre strain of the ACLR affect the motion and mechanics of the operated knee and an optimal choice of graft properties might help restore normal knee joint function and cartilage responses, thus, minimizing the risk of osteoarthritis [72].
The biomechanical properties of the ACL, tibial, femoral articular cartilage and meniscus in knee joints receiving computer aided or conventional ACLR were determined using 3D knee joint FE models of healthy volunteers (normal group) and patients receiving computer-aided surgery or conventional (traditional surgery) ACLR by He and co-authors [35]. The results evidence that computer-aided ACLR has advantages over conventional surgery approach in restoring the biomechanical properties of knee joints, thus reducing the risk of damage to the cartilage and meniscus after ACLR.
Graft and tunnel geometry and orientation
The importance of size and type of autograft, stiffness and tensioning, optimization placement, tunnel geometry and orientation are research issues in ACLR that have been addressed through FEA [60-62, 72, 73, 75, 92, 100]. The type of grafts and tunnel location are probably the most relevant research questions in ACLR, since it seems that they play a key role in the success of the joint reconstruction. Different types of grafts have been compared using FEA and clinical research concerning knee stability and patient-reported scores. It has been demonstrated that, based on the selected graft type (HT, PT or QT) or surgical technique (single-bundle versus double-bundle), numerical optimizations can be implemented prior to the surgery to find the most optimal graft positioning parameters (insertion sites and fixation tension) to replicate as much as possible the intact knee behavior [91]. The change in HT graft length provokes different strains and stresses in the grafts, but do not greatly influence joint stability [71]. Bogdan, et al. studied the behavior of an artificial ACL made from nitinol using FEA [78].
The performance and success of ACLR depends on osseointegration at the graft-tunnel interface and intra-articular ligamentization [36]. The advantages and disadvantages of allografts have been published in a significant number of papers. The mechanical strength during ligamentization of autografts is highlighted in the work of Marieswaran, et al. [3]. Marrale et al. presented a literature review comparing allograft versus autograft reconstruction for the selection of the most adequate graft source for ACLR [46].
One of the doubts is concerned with the use of single bundle reconstruction, double-femoral-tibial tunnel reconstruction, single-femoral double-tibial tunnel reconstruction, and double-femoral single-tibial tunnel reconstruction with respect to biomechanical characteristics such as rotational stability and stresses in the graft [64]. The use of a QT autograft is supported by current orthopedic literature, since it is a safe, reproducible and versatile graft [15]. Chee, et al. compared clinical results of 4-strand HT and PT reconstructions and indicate that primary ACLR with 4-strand HT achieves clinical results that are comparable with the PT reconstruction and with less postoperative complications [17]. Yoon, et al. used a 3D FE model that include the four major ligaments and found that the posterior stability and ligament stresses following double bundle augmentation were superior to those of single and double bundle reconstructions, especially after secondary deficiency in the reconstructed grafts [101]. The aim of the study of Kim, et al. was to determine the change in length and tension of the reconstructed ACL double bundles at different knee flexion angles using a 3D FE model. Unlike previous descriptions, both bundles functioned throughout the arc of flexion with consistency in tension, although their lengths decreased and the two ACL grafts did not function in a reciprocal manner [88].
Some studies have investigated the irregular geometry and the spirally oriented fiber bundle organization with a realistic ACL geometry obtained using a digitizer and with an ACL geometry reconstructed by directly connecting the femur and tibia insertion sites. When evaluating the effect of fiber bundle orientation, the models with unrealistic and realistic fiber bundle orientation predicted similar ACL resultant forces and stress distributions. The results revealed that ACL geometry has a significant effect on the FE model, while fiber orientation does not [75].
Stiffness and graft tensioning on the knee joint biomechanics has also been studied using FE models. It has been shown that after reconstruction, the closest anterior tibial translation to that of the intact knee is obtained with a bone-PT-bone graft with a pretension of 60 N. But, the initial tension produces an important additional stress in the graft during the knee movement which may cause problems in revascularization and remodeling during the postoperative healing process [92]. As for the femoral graft malposition, it may lead to clinical instability and graft failure. Westermann, et al. used a nonlinear contact FE model to evaluate 25 distinct tunnel loci representing primary ACLR [62]. In their study, knee flexion and a simulated Lachman maneuver was used to assess knee joint laxity, meniscal stress, in situ graft loading, and peak articular cartilage contact pressure for each of the tunnel positions. These authors observed an increased anterior tibial translation during Lachman testing when the femoral graft was moved from anterior, anterior/inferior and posterior/inferior relative to the anatomic footprint. With significant posterior and inferior placement (5–7.5 mm) from the anatomic location, the graft peak stresses increased and may subject grafts to increased pressures. Global joint biomechanics are lease favorable with anterior graft placement [100]. The size of the graft affects significantly the stress magnitudes in the soft tissues and contact pressure at the articular surfaces, since larger grafts are associated with lower meniscal stresses, decreased joint laxity, and less articular cartilage contact stresses. Having said so, we can refer that increased graft size confers a biomechanical advantage in the ACLR [62]. Orsi, et al. investigated how graft geometry and tibial and femoral insertion site location may affect ACL intercondylar notch interactions post ACLR using 3D FE models [73]. These authors simulated three ACL graft sizes and polar arrays of tibial and femoral insertion locations and concluded that minor surgical variations may increase ACL impingement and notchplasty may help to improve the ACLR success rates.
Wan, et al. used a 3D FE model that included cartilages, menisci and four main ligaments to investigate the effect of the ACLR on the knee joint biomechanics [60]. The material of the menisci was assumed to be transversely isotropic and the ligaments to be hyperelastic. These authors concluded that due to the stability and stresses in other tissues, the quadruple semitendinosus graft reconstruction was better than the others (bone-PT-bone and double semitendinosus) but can only restore the ACL function partially. Higher stresses induced in the medial collateral ligament and menisci may cause damage or degeneration in these tissues [60].
Tibial tunnel is an important factor for accurate anatomic graft tunnel positioning and adequate knee functionality. How graft-tunnel friction affects the FEA of the ACLR is still unclear. Apparently it does not affect joint kinematics and the maximal principal strain of the graft. By contrast, both the relative graft-tunnel motion and equivalent strain for the bone tunnels are altered, which corresponded to different processes of graft-tunnel integration and bone remodeling [60,71]. This implies that the graft-tunnel friction should be defined properly for studying the graft-tunnel integration or bone remodeling after ACLR.
The effect of tibial tunnel orientation on the graft-bending angle and stress distribution in the ACL graft was investigated by Bracht, et al. [97]. These authors refer that the risk of graft rupture was similar for medial tibial tunnels and lateral tibial tunnels, but the location of graft rupture changes from the femoral tunnel aperture towards the tibial tunnel aperture respectively [97].
Graft tissues within bone tunnels maintain dynamic for a long time after ACLR. Simulation of graft-tunnel friction with FE models is a challenge. Different friction coefficients have been simulated and results show that friction does not affect joint kinematics, neither the maximal principal strain of the graft. However, the graft-tunnel motion and equivalent strain for the bone tunnels are changed indicating different mechanisms of graft-tunnel integration. In fact, friction must be defined properly to study the graft-tunnel integration or bone remodeling after ACLR when doing numerical simulations [60].
Fixation techniques and devices
There are a variety of fixation devices to secure grafts within the femur and tibia and have also called attention in FEA because they can provide relevant information. The review of Hawkins. et al. gives an overview of ACL interference screw usage and design as well as an in depth review of studies that have used FEA to assess ACL interference screw performance [106].
FEA have compared the strength of fixation devices with mechanical testing and showed that FE models may be used to define the optimum placement of the tunnel in ACLR by predicting biomechanical parameters such as stress, strain and displacement at regions in the tunnel wall. Stresses in the screw head are an important factor in the stripping mechanism of interference screws and can be a weak point in the assembly during early postoperative period. The strength of the interference screw fixation is dependent on bone quality and stability of the fixation because it can damage the graft through the screw threads. Mau, et al. performed a FEA and compared six fixation designs (hexagonal, quadrangle, torx, trigonal, trilobe, and turbine) [107]. They concluded that it is possible to improve the designs of biodegradable interference screws for greater torque to be applied and greater screw fixation between host bone and the graft for better integration, better patient healing, and improved patient outcomes [107]. The study of Cheng, et al. compares the biomechanical properties of the GraftMax® with the EndoButton® and TightRope® to investigate whether knotting the free end of latter could improve biomechanical properties [105]. The study of Abdullah, et al. shows that the maximum von Mises stress that occurs on interference screws is less than 40 MPa at the femoral and tibial fixation [104]. A stiffer screw is more prone to higher stress variations. According to Krasnoperov, cortical fixators provoke widening of the canals that are larger than in those where interferent screws are used, but the difference does not seem to be significant, only 5% for femoral side and 4% for tibia canals [89].
Conclusions
ACLR surgery is commonly performed using AM and TT techniques. Different results have been published and it seems that the AM surgical technique gives superior stability and clinical outcomes; others found no differences in terms of clinical function and knee joint stability. Based on the von Mises stresses on HT grafts, the AM portal technique is better than the TT technique in terms of the anatomical graft positioning. However, anatomic TT technique may be regarded as a superior surgical technique when compared with conventional TT and AM portal techniques. Stiffness and pre strain of the ACLR affect the motion and mechanics of the operated knee and suggest that an optimal choice of graft properties might help restore the normal knee joint function and cartilage responses, thus, minimizing the risk of osteoarthritis.
Graft performance has been studied using FE models, since these structures play an important role in the kinematics of the ligament reconstruction. Stresses occurring in the soft tissues, as well as contact pressures at the articular surfaces were found to be highly sensitive to graft size. Single-bundle hamstring ACLR using the AM technique has shown superior surgeon-recorded stability according to IKDC knee score, Lachman test, and pivot-shift test. But no difference in patient-reported functional outcome (Lysholm score) has been observed. The change in HT graft length can cause different strain and stress results in the grafts, but does not greatly influence joint stability. More graft tissues inside the femoral and tibial tunnels decrease stresses and strains at the femoral and tibial fixation sites. The posterior stability and ligament stresses following double bundle augmentation is superior to those of single and double bundle reconstructions, especially after secondary deficiency in the reconstructed grafts. Primary ACLR with 4-strand HT gives clinical results that are comparable with those obtained with PT and with less postoperative complications.
Stresses depend on the site placement and peak stresses and pressure in the ACL grafts increase with higher posterior and inferior placement from the anatomic location. The anterior femoral placement is less favorable for the knee joint biomechanics.
Graft-tunnel friction does not affect joint kinematics and the maximal principal strain of the graft. But relative graft-tunnel motion and equivalent strain for bone tunnels change corresponding to different processes of graft-tunnel integration and bone remodeling.
Stresses in the screw head of fixation devices are an important factor in the stripping mechanism of interference screws and can be a weak point in the assembly during early postoperative period. The fixation strength of interference screw fixation is dependent on the bone quality and stability of the fixation because it can damage the graft through the threads of the screw.
This work was supported by the projects UIDB/00481/2020 and UIDP/00481/2020 - Fundação para a Ciência e a Tecnologia; and CENTRO-01-0145-FEDER-022083-Centro Portugal Regional Operational Programme (Centro2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund.
- Kosy JD, Mandalia VI (2018) Anterior cruciate ligament mechanoreceptors and their potential importance in remnant-preserving reconstruction: a review of basic science and clinical findings. J Knee Surg 31: 736-746. Link: https://bit.ly/3pNUp0e
- Sharp JW, Kani KK, Gee A, Mulcahy H, Chew FS, et al. (2018) Anterior cruciate ligament fixation devices: Expected imaging appearance and common complications. Eur J Radiol 99: 17-27. Link: https://bit.ly/3rSGLdI
- Marieswaran M, Jain I, Garg B, Sharma V, Kalyanasundaram D (2018) A review on biomechanics of anterior cruciate ligament and materials for reconstruction. Appl Bionics Biomech 2018: 4657824. Link: https://bit.ly/2MpC4I9
- Gabr AKA (2019) Functional outcomes of Anterior Cruciate Ligament reconstruction surgery (Doctoral dissertation, UCL (University College London)). Link: https://bit.ly/2LkmtsN
- Buller LT, Best MJ, Baraga MG, Kaplan LD (2014) Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med 3: 2325967114563664. Link: https://bit.ly/38VYbhc
- van Melick N, van Cingel RE, Brooijmans F, Neeter C, van Tienen T, et al. (2016) Evidence-based clinical practice update: practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br J Sports Med 50: 1506-1515. Link: https://bit.ly/3ob4UtP
- Mabrey JD, Reinig KD, Cannon WD (2010) Virtual reality in orthopaedics: is it a reality?. Clin Orthop Relat Res 468: 2586-2591. Link: https://bit.ly/3hH3S6k
- Fu FH, van Eck CF, Tashman S, Irrgang JJ, Moreland MS (2015) Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surg Sports Traumatol Arthrosc 23: 640-648. Link: https://bit.ly/2XaoRFa
- Domnick C, Raschke MJ, Herbort M (2016) Biomechanics of the anterior cruciate ligament: Physiology, rupture and reconstruction techniques. World J Orthop 7: 82-93. Link: https://bit.ly/3oc6MCK
- Figueroa D, Calvo R, Figueroa F, Paccot D, Izquierdo G, et al. (2016) Clinical and arthrometric outcomes of an anatomic outside-in single-bundle anterior cruciate ligament reconstruction using a retrodrill. Knee 23: 1098-1105. Link: https://bit.ly/3nc0OAg
- Osaki K, Okazaki K, Matsubara H, Kuwashima U, Murakami K, et al. (2015) Asymmetry in femoral tunnel socket length during anterior cruciate ligament reconstruction with transportal, outside-in, and modified transtibial techniques. Arthroscopy 31: 2365-2370. Link: https://bit.ly/3pQb6rP
- Matsubara H, Okazaki K, Osaki K, Tashiro Y, Mizu-uchi H, et al. (2016) Optimal entry position on the lateral femoral surface for outside-in drilling technique to restore the anatomical footprint of anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 24: 2758-2766. Link: https://bit.ly/38b0vS5
- Noh JH, Kyung HS, Roh YH, Kang TS (2017) Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 25: 1205-1210. Link: https://bit.ly/3b4RKuX
- Shimizu R, Adachi N, Ishifuro M, Nakamae A, Ishikawa M, et al. (2017) Bone tunnel change develops within two weeks of double-bundle anterior cruciate ligament reconstruction using hamstring autograft: a comparison of different postoperative immobilization periods using computed tomography. knee 24: 1055-1066. Link: https://bit.ly/357OxGZ
- Slone HS, Romine SE, Premkumar A, Xerogeanes JW (2015) Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy 31: 541-554. Link: https://bit.ly/3pQb96Z
- Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, et al. (2016) Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med 50: 597-612. Link: https://bit.ly/3odnDVE
- Chee MY, Chen Y, Pearce CJ, Murphy DP, Krishna L, et al. (2017) Outcome of patellar tendon versus 4-strand hamstring tendon autografts for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of prospective randomized trials. Arthroscopy 33: 450-463. Link: https://bit.ly/2X6QPBR
- Bin SI (2017) Have evolving surgical methods improved clinical outcomes after anterior cruciate ligament reconstruction?. Knee Surgery & Related Research 29: 1-2. Link: https://bit.ly/2Mvd02F
- Huang RY, Zheng HG, Xu Q (2012) Biomechanical evaluation of different techniques in double bundle anterior cruciate ligament reconstruction using finite element analysis. Journal of Biomimetics, Biomaterials and Tissue Engineering 13: 55-68. Link: https://bit.ly/391qYRi
- Ramaniraka NA, Saunier P, Siegrist O, Pioletti DP (2007) Biomechanical evaluation of intra-articular and extra-articular procedures in anterior cruciate ligament reconstruction: a finite element analysis. Clin Biomech 22: 336-343. Link: https://bit.ly/3rORqpW
- Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L (2012) Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther 42: 601-614. Link: https://bit.ly/3ofJdcm
- Antonis J, Bahadori S, Gallagher K, Immins T, Wainwright T, et al. (2019) Validation of the Anterior Cruciate Ligament (ACL) module of the VirtaMed Virtual Reality Arthroscopy Trainer. Surg Technol Int 35: 311-319. Link: https://bit.ly/2X739SB
- Bae JY, Kim GH, Seon JK, Jeon I (2016) Finite element study on the anatomic transtibial technique for single-bundle anterior cruciate ligament reconstruction. Med Biol Eng Comput 54: 811-820. Link: https://bit.ly/2Li8ofK
- Bhat BK, Adhikari R, Acharya KKV (2018) A Numerical investigation of anatomic anterior cruciate ligament reconstruction. Open Bioinformatics Journal 11. Link: https://bit.ly/3obwTtF
- Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, et al. (2013) Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy 29: 1235-1242. Link: https://bit.ly/2LlwExe
- Chen Y, Chua KHZ, Singh A, Tan JH, Chen X, et al. (2015) Outcome of single-bundle hamstring anterior cruciate ligament reconstruction using the anteromedial versus the transtibial technique: a systematic review and meta-analysis. Arthroscopy 31: 1784-1794. Link: https://bit.ly/2X3UmRw
- Choi CH, Kim SJ, Chun YM, Kim SH, Lee SK, et al. (2018) Influence of knee flexion angle and transverse drill angle on creation of femoral tunnels in double-bundle anterior cruciate ligament reconstruction using the transportal technique: Three-dimensional computed tomography simulation analysis. knee 25: 99-108. Link: http://bit.ly/3n93mPW
- Czapla NA, Sylvia MK, Lerner ZF, Tuttle DJ, Schueckler OJ, et al. (2015) Human knee joint finite element model using a two bundle anterior cruciate ligament: Validation and gait analysis. In 2015 Summer Biomechanics, Bioengineering, and Biotransport Conference Proceedings. Link: https://bit.ly/2X3Rcgu
- Dai C, Yang L, Guo L, Wang F, Gou J, et al. (2015) Construction of finite element model and stress analysis of anterior cruciate ligament tibial insertion. Pak J Med Sci 31: 632-636. Link: http://bit.ly/2JGCuZL
- Dhaher YY, Salehghaffari S, Adouni M (2016) Anterior laxity, graft-tunnel interaction and surgical design variations during anterior cruciate ligament reconstruction: A probabilistic simulation of the surgery. J Biomech 49: 3009-3016. Link: https://bit.ly/3oby1NV
- Fernandes DJC (2014) Finite element analysis of the ACL-deficient knee. Lisbon: University of Lisbon.
- Fink C, Lawton R, Förschner F, Gföller P, Herbort M, et al. (2018) Minimally invasive quadriceps tendon single-bundle, arthroscopic, anatomic anterior cruciate ligament reconstruction with rectangular bone tunnels. Arthroscopy Techniques 7: e1045-e1056. Link: https://bit.ly/3rPtGCa
- Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, et al. (2013) Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy 29: 1330-1337. Link: https://bit.ly/3hDiyU4
- Grasso S, Linklater J, Li Q, Parker DA (2018) Validation of an MRI protocol for routine quantitative assessment of tunnel position in anterior cruciate ligament reconstruction. Am J Sports Med 46: 1624-1631. Link: https://bit.ly/3ob4SlX
- He C, He W, Li Y, Wang F, Tong L, et al. (2018) Biomechanics of knee joints after anterior cruciate ligament reconstruction. J Knee Surg 31: 352-358. Link: https://bit.ly/3nbt8TA
- Hexter AT, Thangarajah T, Blunn G, Haddad FS (2018) Biological augmentation of graft healing in anterior cruciate ligament reconstruction: a systematic review. Bone Joint J 100: 271-284. Link: https://bit.ly/3ofMGYf
- Hussein M, van Eck CF, Cretnik A, Dinevski D, Fu FH (2012) Individualized anterior cruciate ligament surgery: a prospective study comparing anatomic single-and double-bundle reconstruction. Am J Sports Med 40: 1781-1788. Link: https://bit.ly/3hITdYX
- Inderhaug E, Stephen JM, Williams A, Amis AA (2017) Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med 45: 347-354. Link: https://bit.ly/3rRFeVh
- Johnston PT, McClelland JA, Webster KE (2018) Lower limb biomechanics during single-leg landings following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Sports Med 48: 2103-2126. Link: https://bit.ly/2JMmaXC
- Kamien PM, Hydrick JM, Replogle WH, Go LT, Barrett GR (2013) Age, graft size, and Tegner activity level as predictors of failure in anterior cruciate ligament reconstruction with hamstring autograft. Am J Sports Med 41: 1808-1812. Link: https://bit.ly/3hFJFxS
- Kang KT, Koh YG, Son J, Kim SJ, Choi S, et al. (2017) Finite element analysis of the biomechanical effects of 3 posterolateral corner reconstruction techniques for the knee joint. Arthroscopy 33: 1537-1550. Link: https://bit.ly/3b4i8oB
- Koch M, Mayr F, Achenbach L, Krutsch W, Lang S, et al. (2018) Partial anterior cruciate ligament ruptures: advantages by intraligament autologous conditioned plasma injection and healing response technique—midterm outcome evaluation. BioMed research international. Link: https://bit.ly/2JF6WDx
- Lee DH, Kim HJ, Ahn HS, Bin SI (2016) Comparison of femoral tunnel length and obliquity between transtibial, anteromedial portal, and outside-in surgical techniques in single-bundle anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy 32: 142-150. Link: https://bit.ly/357yclB
- Malempati C, Jurjans J, Noehren B, Ireland ML, Johnson DL (2015) Current rehabilitation concepts for anterior cruciate ligament surgery in athletes. Orthopedics 38: 689-696. Link: https://bit.ly/38aBV3N
- Marques AR (2016) Avaliação clínica e funcional da reconstrução cirúrgica do LCA: técnica" all-inside" vs." outside-in": uma revisão bibliográfica (Master's thesis), University of Coimbra, Coimbra. Link: https://bit.ly/2X49Zby
- Marrale J, Morrissey MC, Haddad FS (2007) A literature review of autograft and allograft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 15: 690-704. Link: https://bit.ly/3560Ljj
- Naghibi Beidokhti H (2018) Personalized Finite element models of the knee joint: a platform for optimal orthopedic surgery pre-planning (Doctoral dissertation), Radboud University Nijmegen, Nijmegen. Link: https://bit.ly/2LfjRMX
- Noronha JC, Oliveira JP (2018) Inside-out Tibial Tunnel Drilling Technique for All-inside Anterior Cruciate Ligament Reconstruction. Arthrosc Tech 7: e373-e377. Link: https://bit.ly/2X6RinB
- Pfeiffer FM (2016) The use of finite element analysis to enhance research and clinical practice in orthopedics. J Knee Surg 29: 149-158. Link: https://bit.ly/2X9upjj
- Popescu R, Haritinian EG, Cristea S (2019) Relevance of Finite Element in Total Knee Arthroplasty. Chirurgia 114: 437-442. Link: https://bit.ly/391Ga13
- Qi Y, Sun H, Fan Y, Li F, Wang Y, et al. (2018) Three dimensional finite element analysis of the influence of posterior tibial slope on the anterior cruciate ligament and knee joint forward stability. J Back Musculoskelet Rehabil 31: 629-636. Link: http://bit.ly/3bhMLar
- Rezazadeh S, Ettehadi H, Vosoughi AR (2016) Outcome of arthroscopic single-bundle anterior cruciate ligament reconstruction: anteromedial portal technique versus transtibial drilling technique. Musculoskelet Surg 100: 37-41. Link: http://bit.ly/358W8oG
- Safari M, Shojaei S, Tehrani P, Karimi A (2020) A patient-specific finite element analysis of the anterior cruciate ligament under different flexion angles. J Back Musculoskelet Rehabil 33: 811-815. Link: http://bit.ly/356D5vv
- Saka T (2014) Principles of postoperative anterior cruciate ligament rehabilitation. World J Orthop 5: 450. Link: http://bit.ly/3pQdRtb
- Shea KG, Cannamela PC, Fabricant PD, Anderson AF, Polousky JD, et al. (2018) Anatomic all-epiphysial tibial tunnels for anterior cruciate ligament reconstruction in skeletally immature knees may be placed without damaging the anterior meniscus root. Journal of ISAKOS: Joint Disorders & Orthopaedic Sports Medicine 3: 3-7.
- Shen GS, Xu YJ, Zhou HB, Guo X, Niu WX, et al. (2008) Construction of three-dimensional finite element model of knee joint based on MRI images and simulation scheme of anterior cruciate ligament reconstruction. Journal of Medical Biomechanics 5. Link:
- Tei MM, Placella G, Sbaraglia M, Tiribuzi R, Georgoulis A, et al. (2020) Does Manual Drilling Improve the Healing of Bone–Hamstring Tendon Grafts in Anterior Cruciate Ligament Reconstruction? A Histological and Biomechanical Study in a Rabbit Model. Orthop J Sports Med 8: 2325967120911600. Link: http://bit.ly/3nbv9iC
- Thaunat M, Fayard JM, Sonnery-Cottet B (2019) Hamstring tendons or bone-patellar tendon-bone graft for anterior cruciate ligament reconstruction?. Orthop Traumatol Surg Res 105: S89-S94. Link: http://bit.ly/2X78MQO
- Vairis A, Stefanoudakis G, Petousis M, Vidakis N, Tsainis AM, et al. (2016) Evaluation of an intact, an ACL-deficient, and a reconstructed human knee joint finite element model. Comput Methods Biomech Biomed Engin 19: 263-270. Link: http://bit.ly/3n94W4k
- Wan C, Hao Z (2018) Does the graft-tunnel friction influence knee joint kinematics and biomechanics after anterior cruciate ligament reconstruction? A finite element study. Comput Methods Biomech Biomed Engin 21: 278-286. Link: http://bit.ly/2LlTkNV
- Wang HD, Gao SJ, Zhang YZ (2018) Comparison of Clinical Outcomes After Anterior Cruciate Ligament Reconstruction Using a Hybrid Graft Versus a Hamstring Autograft. Arthroscopy 34: 1508-1516. Link: http://bit.ly/3hDkUCo
- Westermann RW, Wolf BR, Elkins JM (2013) Effect of ACL reconstruction graft size on simulated Lachman testing: a finite element analysis. Iowa Orthop J 33: 70. Link: http://bit.ly/2X3ZiWB
- Wren TA, Mueske NM, Brophy CH, Pace JL, Katzel MJ, et al. (2018) Hop distance symmetry does not indicate normal landing biomechanics in adolescent athletes with recent anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 48: 622-629. Link: http://bit.ly/3hIcjP8
- Xie F, Lv C, Huang T, Huang C (2017) Effect of anterior cruciate ligament rupture of knee joint on meniscus and cartilage: a finite element analysis of knee joint. International Journal of Clinical and Experimental Medicine 10: 16468-16475.
- Bansal A, Lamplot JD, VandenBerg J, Brophy RH (2018) Meta-analysis of the risk of infections after anterior cruciate ligament reconstruction by graft type. Am J Sports Med 46: 1500-1508. Link: http://bit.ly/3hMyLGQ
- Lopes OV, de Freitas Spinelli L, Leite LHC, Buzzeto BQ, Saggin PRF, et al. (2017) Femoral tunnel enlargement after anterior cruciate ligament reconstruction using RigidFix compared with extracortical fixation. Knee Surg Sports Traumatol Arthrosc 25: 1591-1597. Link: http://bit.ly/3pKr5HJ
- Rizer M, Foremny GB, Rush A, Singer AD, Baraga M, et al. (2017) Anterior cruciate ligament reconstruction tunnel size: causes of tunnel enlargement and implications for single versus two-stage revision reconstruction. Skeletal Radiol 46: 161-169. Link: http://bit.ly/3rJGwBM
- Meuffels DE, Potters JW, Koning AH, Brown CH, Verhaar JA, et al. (2011) Visualization of postoperative anterior cruciate ligament reconstruction bone tunnels: reliability of standard radiographs, CT scans, and 3D virtual reality images. Acta orthop 82: 699-703. Link: http://bit.ly/358w7WN
- Oliveira C (2016) Análise biomecânica da reconstrução do ligamento cruzado anterior (Master's thesis), University of Aveiro, Aveiro. Link: http://bit.ly/3ob7nol
- Rayan F, Nanjayan SK, Quah C, Ramoutar D, Konan S, et al. (2015) Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J Orthop 6: 252. Link: http://bit.ly/3pMkv3q
- Wan C, Hao Z, Li Z, Lin J (2017) Finite element simulations of different hamstring tendon graft lengths and related fixations in anterior cruciate ligament reconstruction. Med Biol Eng Comput 55: 2097-2106. Link: http://bit.ly/3rYcuKX
- Halonen KS, Mononen ME, Töyräs J, Kröger H, Joukainen A, et al. (2016) Optimal graft stiffness and pre-strain restore normal joint motion and cartilage responses in ACL reconstructed knee. J Biomech 49: 2566-2576. Link: http://bit.ly/3hFcXwv
- Orsi AD, Canavan PK, Vaziri A, Goebel R, Kapasi OA, et al. (2017) The effects of graft size and insertion site location during anterior cruciate ligament reconstruction on intercondylar notch impingement. Knee 24: 525-535. Link: http://bit.ly/3900xLJ
- Koga H, Muneta T, Yagishita K, Watanabe T, Mochizuki T, et al. (2015) Effect of initial graft tension on knee stability and graft tension pattern in double-bundle anterior cruciate ligament reconstruction. Arthroscopy 31: 1756-1763. Link: http://bit.ly/2MyRqdL
- Zhang X, Wu C, Jiang G, Woo SL (2008) The effects of geometry and fiber bundle orientation on the finite element modeling of the anterior cruciate ligament. Annu Int Conf IEEE Eng Med Biol Soc 2008: 899-902. Link: http://bit.ly/3bbdw09
- Beidokhti HN, Janssen D, van de Groes S, Hazrati J, Van den Boogaard T, et al. (2017) The influence of ligament modelling strategies on the predictive capability of finite element models of the human knee joint. J Biomech 65: 1-11. Link: http://bit.ly/3naO2Cc
- Bijalwan A, Patel BP, Marieswaran, M., & Kalyanasundaram, D. (2018) Volumetric locking free 3D finite element for modelling of anisotropic visco-hyperelastic behaviour of anterior cruciate ligament. Journal of biomechanics, 73, 1-8. Link: http://bit.ly/3hEztWi
- Bogdan L, Faur N, Natal RJ, Parente M, Pătraşcu JM (2013) Nitinol artificial anterior cruciate ligament: A finite element study. In 2013 E-Health and Bioengineering Conference (EHB) IEEE 1-4. Link: http://bit.ly/3hFNuTZ
- Eysturoy NH, Nissen KA, Nielsen T, Lind M (2018) The influence of graft fixation methods on revision rates after primary anterior cruciate ligament reconstruction. Am J Sports Med 46: 524-530. Link: http://bit.ly/38UDYbz
- Geng Y, Gai P (2018) Comparison of 2 femoral tunnel drilling techniques in anterior cruciate ligament reconstruction. A prospective randomized comparative study. BMC Musculoskeletal Disorders 19: 454. Link: http://bit.ly/3bbdNAd
- Guler O, Mahırogulları M, Mutlu S, Cercı MH, Seker A, et al. (2016) Graft position in arthroscopic anterior cruciate ligament reconstruction: anteromedial versus transtibial technique. Arch Orthop Trauma Surg 136: 1571-1580. Link: http://bit.ly/3hMzKXy
- Hirokawa S, Tsuruno R (1997) Hyper-elastic model analysis of anterior cruciate ligament. Med Eng Phys 19: 637-651. Link: http://bit.ly/388JGHp
- Hirokawa S, Tsuruno R (2000) Three-dimensional deformation and stress distribution in an analytical/computational model of the anterior cruciate ligament. J Biomech 33: 1069-1077. Link: http://bit.ly/3hEzXf4
- Hofbauer M, Soldati F, Szomolanyi P, Trattnig S, Bartolucci F, et al. (2019) Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 27: 130-136. Link: http://bit.ly/2KVJzGo
- Homyk A, Orsi A, Wibby S, Yang N, Nayeb-Hashemi H, et al. (2012) Failure locus of the anterior cruciate ligament: 3D finite element analysis. Comput Methods Biomech Biomed Engin 15: 865-874. Link: http://bit.ly/3n5Jop6
- Hu B, Shen W, Zhou C, Meng J, Wu H, et al. (2018) Cross pin versus interference screw for femoral graft fixation in hamstring anterior cruciate ligament reconstruction: a systematic review and meta-analysis of clinical outcomes. Arthroscopy 34: 615-623. Link: http://bit.ly/3901hR1
- Kiapour AM, Kaul V, Kiapour A, Quatman CE, Wordeman SC, et al. (2014) The effect of ligament modeling technique on knee joint kinematics: a finite element study. Appl Math 4: 91-97. Link: http://bit.ly/2JGFG7H
- Kim HY, Seo YJ, Kim HJ, Nguyenn T, Shetty NS, et al. (2011) Tension changes within the bundles of anatomic double-bundle anterior cruciate ligament reconstruction at different knee flexion angles: a study using a 3-dimensional finite element model. Arthroscopy 27: 1400-1408. Link: https://bit.ly/35nMyib
- Krasnoperov S, Golovakha M (2018) Effect of bone canal widening after anterior cruciate ligament reconstruction. Orthopaedics, traumatology and prosthetics 95-101. Link: http://bit.ly/38cIjI2
- Magnussen RA, Lawrence JTR, West RL, Toth AP, Taylor DC, et al. (2012) Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy 28: 526-531. Link: http://bit.ly/3b1oSnj
- Naghibi H, Janssen D, Van Tienen T, Van de Groes S, Van de Boogaard T, et al. (2020) A novel approach for optimal graft positioning and tensioning in anterior cruciate ligament reconstructive surgery based on the finite element modeling technique. The knee 27: 384-396. Link: http://bit.ly/3pQfuah
- Pena E, Martinez MA, Calvo B, Palanca D, Doblaré M (2005) A finite element simulation of the effect of graft stiffness and graft tensioning in ACL reconstruction. Clin Biomech 20: 636-644. Link: http://bit.ly/2JHhZMH
- Salehghaffari S, Dhaher YY (2015) A phenomenological contact model: understanding the graft–tunnel interaction in anterior cruciate ligament reconstructive surgery. J Biomech 48: 1844-1851. Link: http://bit.ly/38WdKoZ
- Sim JA, Kim JM, Lee S, Song EK, Seon JK (2018) No difference in graft healing or clinical outcome between trans-portal and outside-in techniques after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 26: 2338-2344. Link: http://bit.ly/3nhZ5tC
- Steiner M (2017) Editorial commentary: size does matter—anterior cruciate ligament graft diameter affects biomechanical and clinical outcomes. Arthroscopy 33: 1014-1015. Link: http://bit.ly/38Qe20s
- Tampere T, Devriendt W, Cromheecke M, Luyckx T, Verstraete M, et al. (2019) Tunnel placement in ACL reconstruction surgery: smaller inter-tunnel angles and higher peak forces at the femoral tunnel using anteromedial portal femoral drilling—a 3D and finite element analysis. nee Surg Sports Traumatol Arthrosc 27: 2568-2576. Link: http://bit.ly/3pQfGq1
- Van Der Bracht H, Tampere T, Beekman P, Schepens A, Devriendt W, et al. (2018) Peak stresses shift from femoral tunnel aperture to tibial tunnel aperture in lateral tibial tunnel ACL reconstructions: a 3D graft-bending angle measurement and finite-element analysis. Knee Surg Sports Traumatol Arthrosc 26: 508-517. Link: http://bit.ly/357BB3R
- Vignos MF, Kaiser JM, Baer GS, Kijowski R, Thelen DG (2018) American Society of Biomechanics Clinical Biomechanics Award 2017: Non-anatomic graft geometry is linked with asymmetric tibiofemoral kinematics and cartilage contact following anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 56: 75-83. Link: http://bit.ly/2KZ28cL
- Wan C, Hao Z, Wen S (2011) The finite element analysis of three grafts in the anterior cruciate ligament reconstruction. In 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI) 3: 1338-1342). IEEE. Link: http://bit.ly/3rRJ4h9
- Westermann RW, Wolf BR, Elkins J (2017) Optimizing graft placement in anterior cruciate ligament reconstruction: a finite element analysis. J Knee Surg 30: 97-106. Link: http://bit.ly/391ghy6
- Yoon KH, Kim YH, Ha JH, Kim K, Park WM (2010) Biomechanical evaluation of double bundle augmentation of posterior cruciate ligament using finite element analysis. Clin Biomech 25: 1042-1046. Link: http://bit.ly/3b5xARp
- Zh M, Li S, Su Z, Zhou X, Peng P, et al. (2018) Tibial tunnel placement in anatomic anterior cruciate ligament reconstruction: a comparison study of outcomes between patient-specific drill template versus conventional arthroscopic techniques. Arch Orthop Trauma Surg 138: 515-525. Link: http://bit.ly/2Lfe1eH
- Chivot M, Harrosch S, Kelberine F, Pithioux M, Argenson JN, et al. (2018) Pull-out strength of four tibial fixation devices used in anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res 104: 203-207. Link: http://bit.ly/38YQ8Ac
- Abdullah AH, Rashid H, Mahmud J, Othman MF, Ibrahim MWAJ (2012) Effects of screw materials in anterior cruciate ligament reconstruction using finite element analysis. Procedia Engineering 41: 1614-1619. Link: http://bit.ly/3b6yzku
- Cheng J, Paluvadi SV, Lee S, Yoo S, Song EK, et al. (2018) Biomechanical comparisons of current suspensory fixation devices for anterior cruciate ligament reconstruction. Int Orthop 42: 1291-1296. Link: http://bit.ly/3b4mE6p
- Hawkins KM, Flowers JR, McCullough MB (2013) Finite Element Analyses of ACL Interference Screws: A Review. International Journal of Engineering Research and Technology 2. Link: http://bit.ly/3hCTtZH
- Mau JR, Hawkins KM, Woo SLY, Kim KE, McCullough MB (2020) Design of a new magnesium-based anterior cruciate ligament interference screw using finite element analysis. Journal of Orthopaedic Translation 20: 25-30. Link: https://bit.ly/3534KNx
- Schumacher TC, Tushtev, K., Wagner, U., Becker, C., große Holthaus, M., Hein, S. B., ... & Rezwan, K. (2017). A novel, hydroxyapatite-based screw-like device for anterior cruciate ligament (ACL) reconstructions. The knee, 24(5), 933-939.
- Wang H, Kang H, Yao J, Cheng CK, Woo SLY (2019) Evaluation of a magnesium ring device for mechanical augmentation of a ruptured ACL: Finite element analysis. Clin Biomech 68: 122-127. Link: http://bit.ly/2JHjYk7
- Gholampour S, Shakouri E, Deh HHH (2018) Effect of drilling direction and depth on thermal necrosis during tibia drilling: an in vitro study. Technol Health Care 26: 687-697. Link: http://bit.ly/3rTkNaz
- Almeida C, Simões JA, Ramos (2020) A Finite element study of short and standard press-fit hip stems. Journal of mechanical engineering and biomechanics 4: 83-89.
- Zhang M, Gong H (2020) Translation of engineering to medicine: A focus on finite element analysis. J Orthop Translat 20: 1. Link: http://bit.ly/3pHTWfV
- Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, et al. (2009) Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am 91: 2321-2328. Link: http://bit.ly/356fsmq
- Laffargue P, Delalande JL, Maillet M, Vanhecke C, Decoulx J (1999) Reconstruction of the anterior cruciate ligament: arthrotomy versus arthroscopy. Rev Chir Orthop Reparatrice Appar Mot 85: 367-373. Link: http://bit.ly/3b6iUBl
- Barzegar H, Mohseni M, Sedighi A, Shahsavari A, Mohammadpour H (2011) Arthroscopically-assisted vs. open surgery in repairing anterior cruciate ligament avulsion. Pak J Biol Sci 14: 496-501. Link: http://bit.ly/2LkuiPb
- Kim NK, Kim JM (2015) The three techniques for femoral tunnel placement in anterior cruciate ligament reconstruction: transtibial, anteromedial portal, and outside-in techniques. Arthroscopy and Orthopedic Sports Medicine 2: 77-85.
- Cain EL, Clancy WG (2002) Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am 33: 717-725. Link: http://bit.ly/3bbg9ix
- Shamah S, Kaplan D, Strauss EJ, Singh B (2017) Anteromedial portal anterior cruciate ligament reconstruction with tibialis anterior allograft. Arthrosc Tech 6: e93-e106. Link: http://bit.ly/3b6HaDA
- Dodds AL, Gupte CM, Neyret P, Williams AM, Amis AA (2011) Extra-articular techniques in anterior cruciate ligament reconstruction: a literature review. J Bone Joint Surg Br 93: 1440-1448. Link: http://bit.ly/38WMQgO
- Maca Macaulay AA, Perfetti DC, Levine WN (2012) Anterior cruciate ligament graft choices. Sports Health 4: 63-68. Link: http://bit.ly/3b8n8IO
- Clark JC, Rueff DE, Indelicato PA, Moser M (2009) Primary ACL reconstruction using allograft tissue. Clin Sports Med 28: 223-244. Link: http://bit.ly/2LmrjWQ
- Chen G, Wang S (2015) Comparison of single-bundle versus double-bundle anterior cruciate ligament reconstruction after a minimum of 3-year follow-up: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 8: 14604-14614. Link: http://bit.ly/3rRPVra
- Desai N, Björnsson H, Musahl V, Bhandari M, Petzold M, et al. (2014) Anatomic single-versus double-bundle ACL reconstruction: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 22: 1009-1023. Link: http://bit.ly/2JMszC8
- Gabriel MT, Wong EK, Woo SLY, Yagi M, Debski RE (2004) Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res 22: 85-89. Link: http://bit.ly/3occxAo
- Woo SL, Kanamori A, Zeminski J, Yagi M, Papageorgiou C, et al. (2002) The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon: a cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg Am 84: 907-914. Link: http://bit.ly/3b601hW
- Lee S, Kim H, Jang J, Seong SC, Lee MC (2012) Comparison of anterior and rotatory laxity using navigation between single-and double-bundle ACL reconstruction: prospective randomized trial. Knee Surg Sports Traumatol Arthrosc 20: 752-761. Link: http://bit.ly/2LlDP8E
- van Eck CF, Kopf S, Irrgang JJ, Blankevoort L, Bhandari M, et al. (2012) Single-bundle versus double-bundle reconstruction for anterior cruciate ligament rupture: a meta-analysis—does anatomy matter?. Arthroscopy 28: 405-424. Link: http://bit.ly/3hH36Xm
- Xu Y, Ao YF, Wang JQ, Cui GQ (2014) Prospective randomized comparison of anatomic single-and double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22: 308-316. Link: http://bit.ly/388F0RS
- Park SJ, Jung YB, Jung HJ, Jung HJ, Shin HK, et al. (2010) Outcome of arthroscopic single-bundle versus double-bundle reconstruction of the anterior cruciate ligament: a preliminary 2-year prospective study. Arthroscopy 26: 630-636. Link: http://bit.ly/3rPwhvR
- Samitier G, Marcano AI, Alentorn-Geli E, Cugat R, Farmer KW, et al. (2015) Failure of anterior cruciate ligament reconstruction. Archives of Bone Joint Surgery 3: 220.
- Burnham JM, Malempati CS, Carpiaux A, Ireland ML, Johnson DL (2017) Anatomic femoral and tibial tunnel placement during anterior cruciate ligament reconstruction: Anteromedial portal all-inside and outside-in techniques. Arthrosc Tech 6: e275-e282. Link: http://bit.ly/3odgPrh
- Illingworth KD, Hensler D, Working ZM, Macalena JA, Tashman S, et al. (2011) A simple evaluation of anterior cruciate ligament femoral tunnel position: the inclination angle and femoral tunnel angle. Am J Sports Med 39: 2611-2618. Link: http://bit.ly/3hBu6r6
- Hapa O, Barber FA (2009) ACL fixation devices. Sports Med Arthrosc Rev 17: 217-223. Link: http://bit.ly/3rPzk7k
- Wang Y, Lei G, Zeng C, Wei J, He H, et al. (2020) Comparative risk-benefit profiles of individual devices for graft fixation in anterior cruciate ligament reconstruction: a systematic review and network meta-analysis. Arthroscopy 36: 1953-1972. Link: http://bit.ly/3hCVTrf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
