Open Journal of Orthopedics and Rheumatology
Ozone therapy with local cellular immune modulation and disc progenitor cell implant is safe, effective and efficient
Grangeat AM1, Crocce EA1, Erario MA1, Moviglia Brandolino MT2, Piccone SL2, Lopez MA2 and Moviglia GA2*
2Consultant and Foreign Representative, Research Center for Tissue Engineering and Cell Therapy (CIITT), Civil Association for Research and Development of Advanced Therapies (ACIDTA), Argentina
Cite this as
Grangeat AM, Crocce EA, Erario MA, Moviglia Brandolino MT, Moviglia GA, et al. (2020) Ozone therapy with local cellular immune modulation and disc progenitor cell implant is safe, effective and efficient. Open J Orthop Rheumatol 5(1): 024-033. DOI: 10.17352/ojor.000023Introduction
Low Back Pain (LBP) is a major cause of morbidity with significant economic impact through loss of work (15% in the United Kingdom). Additionally, there is the cost of health care and social support for the affected individual and their family. It is estimated that more than half the population (49%-70%) will experience significant LBP during their lives. Point prevalence from 12% to 30% is reported in western countries [1,2].
LBP is usually defined as pain, muscle tension, or stiffness localized below the costal margin and above the inferior gluteal folds, with or without leg pain (sciatic pain). These symptoms cause significant disability. LBP is defined as acute when it persists for less than six weeks, subacute between six weeks and three months, and chronic when it lasts longer than three months [2,3].
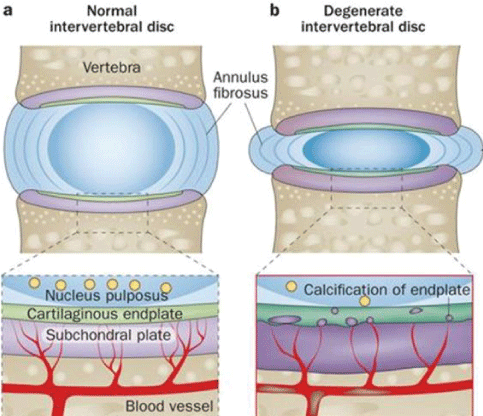
The anatomical basis of this pathology is associated with degeneration of the intervertebral disc (IVD), disc endplates and subchondral bones [4-8]. Etiology has been attributed to, weight overload of the affected joins (through excessive physical labor and/or extreme sport activity), aging, obesity and genetic predisposition [3].
The pathobiology of this condition assumes that an initial acute trauma or degeneration induces a strong inflammatory response. This inflammatory response causes the attraction and activation of macrophages in the IVD structures and adjacent bones [9-13]. The concept of this initial inflammation is to promote healing of damaged tissues [14,15]. While inflammation removes the damaged tissues [16-20], additionally through cytokines secretion and the developing of Th1 specific response the differentiation of local progenitor cells replace the damaged tissues and rebuild the Extracellular Matrix (ECM) of each affected tissue [16-25]. When the pathogenic factors persist, the inflammatory pro regenerative reaction become profibrotic and the activated macrophages lead to a chronic inflammation developing a Th2 [25-28] immune reaction, increasing their phagocytic and lysosomal activity [29]. These events are responsible for the damage of the Annulus Fibrosus (AF) of the IVD. Then, the AF matrix loses the normal organization of the collagen fibrils [26-28]. The Nucleus Pulposus (NP) dehydrates and decreases in overall height with a large loss of cell numbers, whereas the collagen type 2 fibers became type 1 with increasing concentration and thickness. End-plate changes are associated with subchondral sclerosis. These late findings are clear signs of the loss of normal IVD function. (Figure 1). In late stages of degeneration, the IVD has less elasticity and becomes thin; the NP and AF regions become indistinguishable from each other [10,11,26-28].
Initially the treatment for LBP is limited to the use of short-term pain relief with Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) which are helpful. In addition to the NSAID’s a second treatment approach is physiotherapy with the aim to improve movements of the spine and correct posture. When conservative treatments fail, more invasive therapies are considered including epidural injection of corticosteroids and anesthetics or trigger point injections. When less invasive approaches fail, surgical interventions are considered to decompress the spine and or do a spinal fusion. Unfortunately, surgical treatments do not reverse the Degenerative Disease (DDD) or restore the IVD tissues [2,3].
Oxygen-ozone therapy, used to treat this problem, mainly as local but also as systemic administration, has been shown to induce an up regulation of antioxidant enzymes resulting in improved blood circulation delivering more oxygen to ischemic tissues. The improved oxygen delivery up regulates the activity of the immune system by macrophage activation [30-38].
These multifactorial effects of the Oxygen-Ozone therapy are the rational explanation for the high clinical efficacy on chronic DDD. It is highly effective controlling, and even abolishing, the LBP [39-49]. The limitation of this therapy is that it does not affect fibrosis of the affected area, which is mainly composed of collagen type 1 which inhibits the re growing of new cartilage.
Wick G and co-workers [25], has established that the fibrosis process is induced under a Th2 immune environment and the Th1 atmosphere may induce fibroblast and other mesenchymal cells to produce a more pro-regenerative Extra Cellular Matrix (ECM). Th1 and Th2 immune polarization are reciprocally controlled by Macrophage Type 1 (Mo1) and Mo2. These 2 types of cells are different phenotype expressions of the same cell according the microenvironment characteristics [18].
The single use of IVD injections of chondrocytes or different kind of stem cells has been studied in the past decade in various animal models of induced DDD. The preclinical success of these treatments has failed to produce a functional and prolonged effects in the clinical setting. Detailed basic research on IVD cells and their niche indicates that transplanted cells are unable to survive and adapt in the avascular, collagen 1 fibrotic and highly pro-apoptotic in the niche of the DDD. As confirmation of these assumptions, animal and human data on the regenerative potential of injected chondrocytes or disc cells are promising for regeneration of early IVD degeneration [50-58]. It is felt that the microenvironment of the IVD in acute patients remains healthy and allows the engraftment, growth and differentiation of the implanted cells. [56-60]
Based on these concepts, combining local cellular immune modulation with specific cellular progenitor cells [60-68], for chronic fibrotic pathologies, have induced the simultaneous recovery of parenchymal cells and ECM as well as their mutual integration in different clinical settings [69-73].
Moreover, pre-clinical [24] data suggested that the triple approach of oxygen-ozone therapy, followed by intradiscal injection of Effector Immune cells and Disc Progenitor Cells has a synergistic effect, recovering the damaged vertebral body and IVD structure.
Objectives
In patients with symptomatic DDD Ozone therapy, and Disc Progenitor Cells will be used to control LBP syndrome and recover the damaged vertebral and IVD structures. We hypothesized that ozone therapy, associated to local immune modulation, and local implant of Disc Progenitor Cells, has a synergic effect that will improve the results of the treatment of severe chronic degenerative disc disease.
Materials and methods
Patients population
Patients, men or women, older than 18-year-old, that consulted for LBP. Body weight greater than 45 Kg. Blood parameters: Hematocrit ≥ 35 %; MCV ≥ 70%; MCH ≥ 31%; Leukocytes ≥ 4000/mm3; Platelets: 150000 to 400000/mm3. Compensated Cardiac, liver, respiratory and renal functions.
Inclusion Criteria: Positive DDD diagnosis, supported by clinical symptoms and X ray IVD abnormalities [2,3] and confirmed by MRI of lumbar spine (disc loss or diminishing of its height, dehydration of the nucleus pulposus, alteration of vertebral endplate, vertebral body inflammation or clear signs of osteoporosis and architectural deformities) [74,75]. The pain associated to the MRI characteristics should mark greater than or equal to 5 in the Visual Analogue Scale (VAS) [77] and/or deconditioning due to pain greater than 80% in the ODI scale (Oswestry Disability Index). Patients must sign the Informed Consent Form [78].
Exclusion Criteria: Active neoplasm. Viral, bacterial or fungal (internal) active systemic infection at the time of admission. Patients HIV, hepatitis B or hepatitis C positive. Patients with jaundice or liver failure. Pregnant patients. Dependence on alcohol or any other type of addiction.
Ethical considerations
This is a report of therapeutic results of clinical cases treated with an innovative approach. Therefore, this is not a clinical trial. The treated patients suffered from severe vertebral disc disease secondary to osteoarthritis of the spine that have not responded to standard therapy. The treatment was done under Compassionate Use Conditions. The therapy used is an adaptation of a clinical trial protocol already accepted for evaluation and which is registered with ANMAT (National Administration of Medicines, Food and Technology of Argentina) with the file number 1-0047-0002-000209- 17-6. This protocol was presented to treat patients with severe muscular atrophy. The same cellular methodology was used, whose safety and efficacy have been demonstrated and approved by ANMAT (see Animal Safety results and reference 24). The clinical protocol in particular and the text of the Informed Consent Form were approved by the respective IRB of the two intervening institutions, CIITT and IAOT. 48 hours prior to the start of treatment, patients signed the aforementioned informed consent form.
Ozone therapy
Selected Patients received 10 sessions of ozone therapy. It is an outpatient bases using a portable generator of ozone from medical liquid oxygen (Ozomed®, Kastber Praxisbedarf GMBH-Medizintechnik), 10 ml of Oxygen-Ozone gas at 10 μg/ml, twice a week. It was administered through an Intramuscular injection into the muscles adjacent to the involved IVD [39-41].
The day of the cell implant, (under image guidance) the patient received a dose oxygen-ozone into the most affected discs. This procedure was done to mark the site of cellular implants and preconditioning the local microenvironment [42-49].
After the cell implant, patients continue receiving additional paravertebral IM oxygen-ozone injections (twice a week) for 2 weeks [39-41].
Preparation of immune effector cells (EC)
Autologous Mononuclear Cells (MNC) were obtained from the patient by removing 250 ml of blood. The MNCs were purified by a Ficoll Hypaque gradient. Cells were washed and cultivated in DMEM enriched with 1% enzymatic hydrolysate (Laboratorio Villar, Argentina) of complete calf spine cervical tissues (cartilage, muscles and bones), ranitidine (5 ng/ml), and indomethacin (5 ng/ml). After 4 days of culture, as described previously [23,34,69-73], only lymphocytes pre-sensitized in vivo against the antigen present in the medium were activated and amplified by the clonal selection principle of Burnet [78]. The rest entered apoptosis. The addition of ranitidine and indomethacin made it difficult to enrich the culture in Mo2 and regulatory lymphocytes (Tregs) [79]. Consequently, the suspension was characterized by Fluorescence-Activated Cell Sorting (FACS) as CD3+ CD20+ CD68+ CD183+ CD163- CD25- [79-81]. As evidence of the immune-specificity of the ECs, different EC aliquots were cultured separately in the presence of muscle, brain, or kidney hydrolysate. After 24 hours, the in vitro reaction of tetrazolium blue [81] had a selective increase for calf spine cervical tissues, with values greater than 25% of those for the other antigens.
MSC preparation
Adipose Mesenchymal Stem Cells (aMSC) were obtained from abdominal subcutaneous fat tissue by minimal lipectomy. The tissue was mechanically and enzymatically dissociated (Collagenase IV, Thermo Fisher). The obtained cell suspension was cultivated in DMEM enriched with recombinant human insulin (INULIN® and 2% of human platelet lysate supernatant (homemade). After 3 days of culture, the cells were reseeded. They were cultured until a total amount of 5x107 per disc to be treated was obtained [71-73]. According to International Society for Cellular Therapy (ISCT) criteria, their identity and purity were monitored by flow cytometry, which showed CD73+ CD90+ CD105+ CD34- CD45- [82]. As a test of multipotency, their ability to differentiate into cartilage, bone, and nerve tissue was demonstrated [69-73].
Obtaining disc progenitor cells
According to the results in other biological scenarios, we hypothesized that the co-incubation of anti-muscle EC with MSC was sufficient for the latter to differentiate into DPC [23,24,69-73]. To test this hypothesis, EC and aMSC were incubated at a 1:1 ratio. No other substance was added to the culture medium. For controls, immunohistochemical staining was performed to detect the phenotypic expression of CD 271 (Nerve Growth Factor Receptor) [83], Collagen Type 2, and Aggrecan [84,85]. As controls of this co-culture, MSC were cultured in IVD tissues-enriched medium with inactivated MNC and medium enriched with the supernatant of the EC.
Safety and tumorigenicity tests effect of treatment with human EC and DPC in animals
After the animal experiment protocol was approved by the ethics committee, a group of six nude mice was injected through the tail vein with 1x106 MSC per animal. A second batch was injected with 1×106 EC + 1×106 DPC (both humans). A third batch was injected with 1x104 cells of the 4T1 syngeneic tumor line. The animals were cared for and kept under observation for 120 days in the Argentinean National Commission of Atomic Energy animal facility. Necropsies were performed on animals that developed and died from tumors as well as those that survived the 13-week observation period. The latter were euthanized with carbon monoxide at the end of this period. During the necropsy, the brain, lungs, heart, liver, both kidneys with adrenal glands, and spleen were collected for further study, as were the small intestine and large intestine after visual inspection. These organs were fixed in 10% buffered formaldehyde and processed for pathological study.
Patients safety and clinical follow-up
During the 6 weeks of the schedule therapy the patients were clinical monitored at least once a week to detect the treatment safety, efficacy and effectiveness. Then the controls were done at 60, 90 and 180 days. Safety was evaluated according the National Cancer Institute. USA. Common terminology criteria for adverse events (version 3.0) [86]. VAS and ODI [76,77] were assessed at 3 months and MRI of the spine treated areas at 6 months after the end of the treatment [74,75].
Statistical analysis
All the Statistic Analysis were done with the MedCalc Software from MedCalc Inc. The test used for media, Standard error calculation and graphic plotting was ANOVA for repeat measures. The Significance difference Level established was P< 0.01
Results
Preclinical safety results
After 120 days of observation, all nude mice that were treated with 5×105 4T1/kg of body weight intravenously developed tumors and died from these tumors before day 45. None of the animals injected with 5×107 MSCs or DPCs/kg of body weight, both of human origin, died during the observation period. While the autopsy of the organs injected with 4T1 showed metastases and parenchymal alteration figures, no histological alterations were observed in the post-euthanasia organs at 120 days after the animals were injected with human MSCs and human MPCs.
All the organ anatomopathological results and laboratory study related to the adverse events associated with the treatment of the animals showed no abnormalities. The only minor event detected was local inflammation at the site of injection but not any sign of animal suffering, controlled under the Grimace scale [87,88], was detected. No other variation in the recorded parameters was found in any of the treated animals.
Clinical results
Clinical individual patient conditions, as well as VAS and ODI evaluation results, are summarized in Table 1.
Between March 2016 and May 2019, 27 patients, with an age range between 48 to 85, who met the admission criteria where treated with the proposed combined schema. The patients were admitted at “Instituto Argentino de Ozonoterapia (IAOT)”. Therefore, 27 have the baseline MRI. The clinical evaluations allow the VAS and ODI baseline assess. Only 10/27 have the 6-month post treatment MRI evaluation (Figure 2); 24/27 The 90-day post treatment ODI assets and 27/27 the 90-day post treatment VAS assets.
The only adverse event registered was local pain (13/27) that was controlled with an analgesic program and last a variable lapse of 2 to 21 days after intervention. 4/27 patients experience grade 2 pain in the injected disks and the pertinent dermatome territory. This more severe condition did not require any hospitalization and was controlled with regular pain killers and disappear without any sequela. No other variation in the recorded parameters was found in any of the treated patients.
The MRI images show significant differences between the beginning of the treatment and at 6 months (Figure 2, patient 2). The main changes were seen in most of the signs of diagnostic criteria. The more frequents were referred to the vertebral body bone: recovery of the cartilage endplate as well as subchondral bone, change in the pattern of the bone inflammation, that suggest bone reconstruction, phenomenon well documented in the comparative change of the vertebral body shape (Figure 2 see T12, L1, and L2) with the consequent enlargement of intervertebral neural foramen. Rehydration of the previously dehydrated IVD. Occasionally, recovery of the IVD height (data non-Shown). The improvements persist and increase after a year of patient follow up.
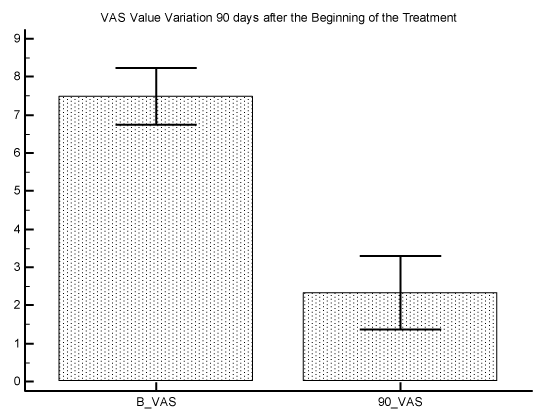
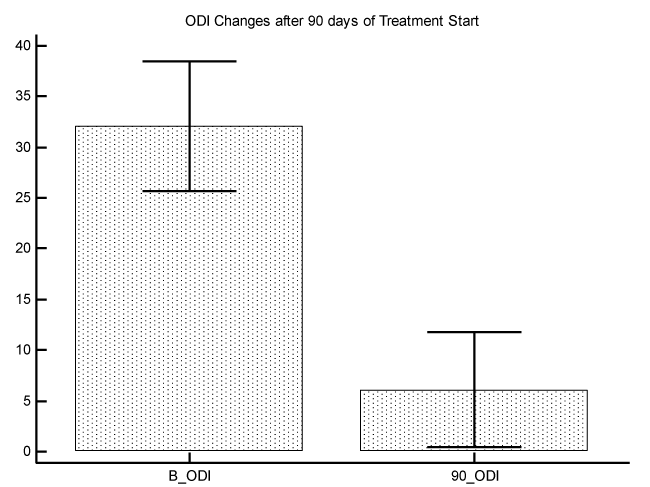
The initial average of VAS value was 7.5 with a Standard Error of 0.4, and the final average of VAS value was 2.3 with a Standard Error of 0.4 P difference between the 2 measures was P<0.0001 (graphic 1). The initial average of ODI value was 32.1 with a Standard Error of 3.1, and the final average of ODI value was 6.1 with a Standard Error of 2.7. P difference between the 2 measures was P<0.0001 (graphic 2).
Discussion
The analysis of the 27 patients who suffered of severe spine osteoarthrosis and were treated with the combination of O3 therapy followed by local cellular immune modulation and IVD implant of DPC were found to be safe, effective and efficient.
Safety and efficacy of this therapy has been supported by preclinical animal work and corroborate the clinical findings of treated patients.
The safety of the preclinical work was based on the negative tumorgenicity results on the nude mice, as well as the negative anatomopathological results obtained from the organs of the autopsied reactive and non-reactive animals.
The efficacy results were supported by the animal experiments carried out by our group [24]. 30 male Sprague Dawley rats were treated with an IVD injection of high dose of O3, to induce disc reabsorption and spine fusion. A week after, and controlled through X rays for the success of spine fusion, animals were divided into five different groups: the first was kept without any treatment as control, the second was treated with homologous bone marrow MSC (BMMSC) only, the third with O3 only, the fourth with homologous BMMSC co incubated with EC against the Disc Tissues, and the fifth with O3 followed by the BMMSC co incubated with EC against intervertebral disc and followed for local IM )3 Injections. The group with the triple combined treatment was the only that achieved morphologic full recovery of the rat IVD. These results supported, in the animal setting, our proposed therapeutic approach [24].
When this treatment was translated to the clinical setting, beside the highly controlled laboratory procedures in a GMP facility, there was evaluated the clinical safety of treated patients with one of the most validated safety scales: “Common terminology criteria for adverse events, v3.0” [86]. The fact that no one of the treated patients had any adverse event that showed values over degree 2, of the referred scale, is coincident with the safety preclinical studies and are a clear suggestion for innocuity of this new therapeutic approach.
To study the proposed mode of action (efficacy evaluation) MRI studies were done previous and 6 months after the treatment. Several authors have stated the correlation between the T2 MRI images and the degree of chronic DDD: opacity of the IVD, lose of visibility of NP (which are signs of AF and NP fibrosis), lose of disc endplate and sclerosis of the subchondral bone (both as sign and cause of the lose of nourishment of IVD) followed by osteoporotic changes of the vertebral bodies with architectural collapse [74, 75]. The fact that all of the treated patients have some degree of reversions of these MRI changes suggests that the combined treatment helps to regenerate the damaged tissues of the disc and adjacent bones.
Two well validated and accepted scales: VAS and ODI [76,77] were used to determine any impact on the functionality of the spine as well as the quality of life of the treated patients (efficiency evaluation). The effectiveness of this combined therapy is supported by the reduction of values in both scales, previous and after 3 months after treatment. VAS values decreased around 70% and ODI values around 90 %. The ANOVA test demonstrate that these differences were highly significant.
In order to shed light on the mode of action of this combined therapy is necessary to analyze the individual mode of action for each therapy, as well as the reported results when they are applied as individual therapies.
O3 was used for more than a century as a disinfectant for its oxidative power. However, about 30 years ago, it was reported that when it is locally applied in the tissues at low concertation, O3 up regulate the production of antioxidant enzymes becoming a powerful stimulating of oxygen metabolism and activating the immune system [30,33,37]. Moreover, O3 may reach many other tissues for its ability to dissolve in the aqueous component of plasma [37,38]. There O3 reacts with polyunsaturated fatty acids (PUFA) and water, creating hydrogen peroxide (H2O2), which is one of the reactive oxygen species (ROS). Simultaneously, O3 forms a mixture of Lipid Ozonation Products (LOP). The LOPs created after O3 exposure include lipoperoxyl radicals, hydroperoxides, malonyldialdeyde, isoprostanes, ozonide and alkenals, and 4-hydroxynonenal (4-HNE). This moderated oxidative stress caused by O3 increases activation of transcriptional factor mediating nuclear factor-erythroid 2-related factor 2 (Nrf2). Nrf2 domain is responsible for activating the transcription of Antioxidant Response Elements (ARE). Upon induction of ARE transcription, an assortment of antioxidant enzymes gains increased concentration levels in response to the transient oxidative stress of O3. The antioxidants induced include Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), Glutathione S-transferase (GST), Catalase (CAT), Heme oxygenase-1 (HO-1), NADPH quinone-oxidoreductase (NQO-1), Heat Shock Proteins (HSP), and phase II enzymes of drug metabolism. Many of these enzymes act as free radical scavengers clinically relevant to a wide variety of diseases [33,37,38].
Several clinical reports and clinical trials have proved that O3 therapy is effective to control LBP associated to NP Herniated and/or DDD [34,39-49]. The two main route of administration used were the intramuscular injection in the paravertebral muscles of the muscles innervated for the nerves of affected segments [39-41] and the percutaneous Intra disc injection [42-49]. Both administration route showed efficiency to control the LBP in around 60 to 80% of treated patients with a diminishing of 60 to 75% in the VAS index. Both routes seem to control the local pathology stress but only diminishing of the IVD volume (measured on CT scan or MRI images) was observed when the percutaneous intradiscal route was used [42,43]. No other morphological change was detected on the pre and post image analysis. The major cause of medical failure was and advanced degree of lumbar-sacrum osteo arthrosis related to a continuous and severe pain that lasted more than one year. Because these morphologic and clinical limitations were described by the first reported treated patients [39-43], these 2 conditions were exclusions in the subsequent series of clinical trials [44-49].
The question arose; may the observed improvements be related with this O3 treatment or is it the rehabilitation applied to these patients? To shed some light on this question, Appuzo, et al. [45], performed an observational retrospective/horizontal study to compare O2-O3 therapy and/or Global Postural Reeducation (GRP) in complicated chronic LBP. The epidemiology study was done on population who were seen at a single clinic between 1995 and 2014. 546/923 patients that presented with LBP met the restricted admission criteria. 54 were only treated with GRP, 109 with O2-O3 only and 383 patients received both therapies. Analysis of changes in pain in each treatment group revealed an approximately 6-point reduction in VAS score at the end of the treatment in the groups that underwent ozone therapy, as opposed to a reduction of 3.3 VAS points in the GPR group, which, in addition, had significantly lower baseline pain severity. The percentage of recurrence for each group was 27%, 59.6% and 64.2% respectively. Disc Herniation (DH), as observed on MRI, was found to be stable or enlarged in most patients at the end of treatment (64%). Furthermore, no difference in pain reduction was found in the patients with a reduced DH size compared with those showing no change. These results supported the hypothesis that O2-O3 has a positive effect reducing the LBP and, even with this therapy may, in the third of treated patients reduce the DH through its nucleolysis effect, this anatomical change did not influence the positive therapeutic effects.
Supporting the findings of Apuzzo, et al [45], Rahimzadeh, et al [46], compared percutaneous intradiscal ozone injection with laser disc decompression in patients that suffer of discogenic LBP. VAS reduction in both groups was similar, but with a slight superiority after a year of patient follow up for O3 threated patients [46].
As a result of the analysis of these data, only 60% of patients with LBP qualify to be treated with O3 therapy focusing in the long-lasting resolution of the problem. Between the new approaches intended to solve this medical necessity cellular therapy emerge as one that may have large application [50-58]. The DDD is a problematic scenario for tissue regeneration due to the harsh microenvironment that resident cells are immersed [54, 56]. It is very important to control the interactions between implanted cells and the disc microenvironment (oxygen tension, nutrients, pH, osmolarity, cytokine levels related with the kind of inflammatory cells present, ECM scar and mechanical load). It may be the reason for the poor and transitory result obtained for different cellular treatments [50-58].
Proposed cell-based strategies include the implantation of autologous cells into the degenerate NP [50-56]. Autologous NP cells have demonstrated improvements in terms of pain relief and disc hydration upon injection into degenerate human IVDs [51, 56]. However, harvesting of NP cells yields a limited number of them and requires invasive procedures, which have, themselves, been shown to initiate degenerative changes. Allogeneic juvenile chondrocytes [53-56] have been explored as an alternative cell source. However, caution must be taken as the matrix produced by articular chondrocytes may not be the most appropriate for IVD tissue engineering/repair [56,59,61].
A more immediate autologous progenitor cell source is adult mesenchymal stem cells (MSCs), which can be isolated easily from multiple sources, mainly bone marrow (BM-MSCs) [50-54] or adipose tissue (AD-MSCs) [51, 54-56], divide rapidly, and are able of differentiating into cells of different lineage. They are also capable of differentiating into NP-like cells. Adult MSCs appear to offer a promising cell type for IVD regeneration, due to the relative ease of acquisition and their ability to undergo discogenic differentiation. Autologous MSC-based therapies, in a pilot study, have been shown to reduce the pain induced by IVD [53]. The only opportunity to see a Long-lasting effective result of a single kind of cell therapy was when they were used to treat early stages of disc degeneration [56,58]. There was also reported that tissue specific progenitor cells seem to be more effective than MSC [56-58].
A possible explanation for these discordant effects (efficacy in early stages and poor response in late stages) is to consider, that the DDD is an active process [11], that progressively affect different essential structures of the vertebral to vertebral joint [4-7]. The deterioration of these structures is double: parenchymal cell death and degeneration of the ECM. All the physiologic structure of fibrillar and amorphous components are converted into a fibrotic (scar) structures. This fact may be one of the main causes of the failure of the single cell therapy of DDD. The ECM is necessary to promote the specific differentiation of each of the four different tissues that compose the intervertebral articulation: cartilage of NP, fibrotic cartilage of the AF, hyaline cartilage of cartilage of end plate and non-sclerotic bone structure of the subchondral bone. The fibrotic ECM do not allow an appropriate differentiation of the stem cell [59-61]. Early stages have partially preserved the physiologic structure of ECM allowing cell implant engraftment and differentiation to the appropriated tissues [59-61]. The fibrotic transformation of the ECM is associated with chronic inflammation in DDD pathogenesis [8-11]. Moreover, the destruction of structures that allow the nourish of the tissues [4-7] produce a shift of a healthy microenvironment to a more anoxic and catabolic one with ROS which leads to very low extracellular pH [25-29] which in turn adds more stress to any kind of tissue regeneration.
Many authors suggest that the main important actor of the inflammatory process are macrophages [8-17]. The switch of predominant macrophage phenotype from M1 to M2. However, primary Mo1 tissue infiltration promote tissue specific Th1 immune adaptative response, which promote tissue regeneration [18-19], as well as attract local and systemic progenitor cells. Th1 cells have been shown to promote pro-regenerative processes through at least three different modes of action: promotes fibroblast activity to regenerate a physiologic appropriate ECM of each damaged tissue [25,70], improves stem cell attraction initiated by Mo1 macrophages [21,22] and promote stem cell differentiation into appropriate cells of each tissues [23,24,71-73]. Local (specific progenitor cells) and systemic stem cells (MSC) stablish a cross talk with the present immune cells [62-65]. Mo1 became Mo2 and Th1 switch to Th2 and Treg later. Regenerative process is started by Mo1 and Th1 immune reaction, promoted by Mo2 Th2 immune reaction that slowly switch to profibrotic Mo2 and Treg reaction [15-25].
Following this rational, several authors are proposing to add an immunomodulator element to the MSC or tissue progenitor cells used for cellular therapy [62-68]. Our group have been working in animal and clinical setting since 2003 on different protocols for tissue repair using this combined approach with successful results [69-73]. There is a combined implant of tissue specific immune effector cells with a mixing of MSC and tissue specific progenitor cells, all from autologous or closely immune related cells. Because the cells are autologous the treatment may be repeated several times without eliciting any autoimmune response.
However, the ROS produced by the activated macrophages cause the reparatory function of lymphocytes and stem cells to produce many times a transient grade 2 adverse event [10,28,29].
In order to overcome this adverse effect, we have tested in the animal setting [24] and now in this case report the use of O3 therapy. It is justified because through their secondary antioxidant effect and the immune modulation, specifically focused on the activated macrophages modulate the microenvironment present into the degenerated intervertebral disc and in the implanted cells, allowing a better action of Lymphocytes and stem cells. The suggested mode of action may be related with the fact that Ozone therapy modulates oxidative stress, and the rest of the harmful micro environment of the intervertebral space, allowing the cells to be implanted and survive this micro environment. Local Immune modulation may contribute to improving the local microenvironmental, helping the end terminal plate reconstruction, subchondral bone and vertebra body remodeling, as well as allowing the integration of all these structures. Autologous Disc Progenitor cells may contribute with the necessary cells to recover damaged structures.
The weakness of this study is low numbers 27 patients with only 10 with post op MR studies. In addition, there are no control groups to better understand the interactions of this complex mode of therapy for degenerative disc disease.
Conclusion
These pilot cases support the hypothesis that ozone therapy, associated to local immune modulation, and local implant of Disc Progenitor Cells, has a synergic effect that improve the functional and structural results of the treatment of severe chronic degenerative disc disease.
- Friedly J, Standaert C, Chan L (2010) Epidemiology of Spine Care: The Back-Pain Dilemma. Phys Med Rehabil Clin N Am 21: 659–677. Link: https://bit.ly/2Ckrv45
- Koes BW, van Tulder MW, Thomas S (2006) Diagnosis and treatment of low back pain. BMJ 332: 1430-1434. Link: https://bit.ly/2YiVO40
- Taher F, Essig D, Lebl DR, Hughes AP, Sama AA, et al. (2012) Lumbar Degenerative Disc Disease: Current and Future Concepts of Diagnosis and Management. Adv Orthop 2012: 970752. Link: https://bit.ly/2AR61vi
- Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, et al. (2012) Diversity of intervertebral disc cells: phenotype and function. J Anat 221: 480-496. Link: https://bit.ly/2zRaw8Q
- Moore RJ (2006) The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J 15: S333–S337. Link: https://bit.ly/3fC2Aaw
- Wen C, Lu WW, Chiu KY (2014) Importance of subchondral bone in the pathogenesis and management of osteoarthritis from bench to bed. Journal of Orthopaedic Translation 2: 16-25. Link: https://bit.ly/2NaUERw
- Huang YC, Jill PG, Urban JPG, Luk KDK (2014) Intervertebral disc regeneration: do nutrients lead the way? Nat Rev Rheumatology 10: 561-566. Link: https://bit.ly/2YTnB9X
- Findlay DM, Kuliwaba JS (2016) Bone–cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res 4: 16028. Link: https://bit.ly/3dkVcip
- Lee SH, Choi Y (2015) Communication between the skeletal and immune systems. Osteoporosis and Sarcopenia 1: 81-91. Link: https://bit.ly/2ARcHcQ
- Weber A, Chan PMB, Wen C (2017) Do immune cells lead the way in subchondral bone disturbance in osteoarthritis? Prog Biophys Mol Biol 1-11. Link: https://bit.ly/2V1nFDA
- Boisson M, Lefèvre-Colau MM, Rannou F, Nguyen C (2018) Active discopathy: a clinical reality. RMD Open 4: e000660. Link: https://bit.ly/3epzhrS
- Kokubo Y, Uchida K, Kobayashi S, Yayama T, Sato R, et al. (2008) Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 in bloc surgical samples. Laboratory investigation. J Neurosurg Spine 9: 285-295. Link: https://bit.ly/37M3KOj
- Molinos M, Almeida CR, Caldeira J, Cunha C, Gonc¸alves RM, et al. (2015) Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface 12: 20141191. Link: https://bit.ly/2YPRcBn
- Gu Q, Yang H, Shi Q (2017) Macrophages and bone inflammation. Journal of Orthopaedic Translation 10: 86-93. Link: https://bit.ly/2Yfj9TU
- Cunha C, Silva AJ, Pereira P, Vaz R, Gonçalves RM, et al. (2018) The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther 20: 251. Link: https://bit.ly/2NkrJKJ
- Nahrendorf M, Pittet MJ, Swirski FK (2010) Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121: 2437-2445. Link: https://bit.ly/37JD8NT
- Koh TJ, DiPietro LA (2011) Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13: e23. Link: https://bit.ly/3dmLGLv
- Novak ML, Koh TJ (2013) Phenotypic Transitions of Macrophages Orchestrate Tissue Repair. Am J Pathol 183: e1352-e1363. Link: https://bit.ly/3fIehwx
- Forbes SJ, Rosenthal N (2014) Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 20: 857-869. Link: https://bit.ly/3hKy8wO
- Minutti CM, Knipper JA, Allen JE, Zaiss DM (2017) Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol 61: 3-11. Link: https://bit.ly/2zOvlSn
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, et al. (1999) Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med 5: 49-55. Link: https://bit.ly/2Ymuz8J
- Barouch R, Schwartz M (2002) Autoreactive T cells induce neurotrophin production by immune and neural cells in injured rat optic nerve: implications for protective autoimmunity. FASEB J 16: 1304-1306. Link: https://bit.ly/3dhYtyU
- Moviglia GA, Varela G, Gaeta CA, Brizuela JA, Bastos F, et al. (2006) Autoreactive T cells induce in vitro BM mesenchymal stem cell transdifferentiation to neural stem cells. Cytotherapy 8: 196-201. Link: https://bit.ly/3enIBwc
- Moviglia GA, Grangeat AM, Croce EA, Erario MA, Blasetti N, et al. (2009) Mesenquimal Stem Cells combined with Effector T Cells and low doses of Ozone therapy produce regeneration of damaged inter vertebral discs in rats. Cytotherapy 11
- Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, et al. (2010) The immunology of fibrosis: innate and adaptive responses. Trends Immunol 31: 110-119. Link: https://bit.ly/2AVMTwd
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, et al. (2002) Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A 99: 15620-15625. Link: https://bit.ly/2CqdpOR
- Hartupee J, Mann DL (2016) Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol 93: 143-148. Link: https://bit.ly/2ALUP3k
- Ramos G, Hofmann U, Frantz S (2016) Myocardial fibrosis seen through the lenses of T-cell biology. J Mol Cell Cardiol 92: 41-45. Link: https://bit.ly/3fCaLDK
- Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, et al. (2013) A role for TNFα in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem Biophys Res Commun 433: 151-156. Link: https://bit.ly/3hIsE5L
- Wolff HH (1979) editor. Das medizinische Ozon: Theoretische Grundlagen, Therapeutische Anwendungen [Medical ozone: theoretical bases, therapeutic applications]. Heidelberg: Verlag für Medizin; German.
- Larini A, Bocci V (2005) Effects of ozone on isolated peripheral blood mononuclear cells. Toxicol In Vitro 19: 55-61. Link: https://bit.ly/37Jzx24
- Bocci V, Di Paolo N (2009) Oxygen-Ozone Therapy in Medicine: An Update. Blood Purif 28: 373-376. Link: https://bit.ly/37N2U3S
- Bocci V (2012) How a calculated oxidative stress can yield multiple therapeutic effects. Free Radic Res 46: 1068–1075. Link: https://bit.ly/2Yi4Vli
- Bocci V, Emma Borrelli E, Zanardi I, Travagli V (2015) The usefulness of ozone treatment in spinal pain. Drug Des Devel Ther 9: 2677-2685. Link: https://bit.ly/2VirrJ5
- Buyuk SK, Ramoglu SI, Sonmez MF (2016) The effect of different concentrations of topical ozone administration on bone formation in orthopedically expanded suture in rats. Eur J Orthod 38: 281-285. Link: https://bit.ly/2YhJYad
- Francis M, Sun R, Cervelli JA, Choi H, Mandal M, et al. (2017) Editor's Highlight: Role of Spleen-Derived Macrophages in Ozone-Induced Lung Inflammation and Injury. Toxicol Sci 155: 182-195. Link: https://bit.ly/3djdoZV
- Smith NL, Wilson AL, Gandhi J, Vatsia S, Khan SA (2017) Ozone therapy: an overview of pharmacodynamics, current research and clinical utility. Med Gas Res 7: 212-219. Link: https://bit.ly/2YNYrcW
- Mehraban F, Seyedarabi A, Seraj Z, Ahmadian S, Poursasan N, et al. (2018) Molecular insights into the effect of ozone on human hemoglobin in autohemotherapy: Highlighting the importance of the presence of blood antioxidants during ozonation. Int J Biol Macromol 119: 1276-1285. Link:
- Paoloni M, Di Sante L, Cacchio A, Apuzzo D, Marotta S, et al. (2009) Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: a multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine 34: 1337-1344. Link: https://bit.ly/2YhTdqP
- Biazzo A, Corriero AS, Confalonieri N (2018) Intramuscular oxygen-ozone therapy in the treatment of low back pain. Acta Biomed 89: 41-46. Link: https://bit.ly/2V27m9u
- Andreula CF, Simonetti L, De Santis F, Agati R, Ricci R, et al. (2003) Minimally invasive oxygen-ozone therapy for lumbar disk herniation. AJNR Am J Neuroradiol 24: 996-1000. Link: https://bit.ly/2NcngK3
- Muto M, Andreula C, Leonardi M (2004) Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. J Neuroradiol 31: 183-189. Link: https://bit.ly/3djdHE3
- Lehnert T, Naguib NN, Wutzler S, Nour-Eldin NE, Bauer RW, et al. (2012) Analysis of disk volume before and after CT-guided intradiscal and periganglionic ozone-oxygen injection for the treatment of lumbar disk herniation. J Vasc Interv Radiol 23: 1430-1436. Link: https://bit.ly/2YkMpsF
- Magalhaes FN, Dotta L, Sasse A, Teixera MJ, Fonoff ET (2012) Ozone therapy as a treatment for low back pain secondary to herniated disc: a systematic review and meta-analysis of randomized controlled trials. Pain Physician 15: E115-E129. Link: https://bit.ly/2NdmpZL
- Apuzzo D, Giotti C, Pasqualetti P, Ferrazza P, Soldati P, et al. (2014) An observational retrospective/horizontal study to compare oxygen-ozone therapy and/or global postural re-education in complicated chronic low back pain. Funct Neurol 29: 31-39. Link: https://bit.ly/311FvKG
- Rahimzadeh P, Imani F, Ghahremani M, Faiz SHR (2018) Comparison of percutaneous intradiscal ozone injection with laser disc decompression in discogenic low back pain. J Pain Res 11: 1405-1410. Link: https://bit.ly/3evKPtH
- Ozcan S, Muz A, Yildiz Altun A, Onal SA (2018) Intradiscal ozone therapy for lumbar disc herniation. Cell Mol Biol (Noisy-le-grand). 64: 52-55. Link: https://bit.ly/3fIcIP4
- Elawamy A, Kamel EZ, Hassanien M, Wahba OM, Amin SE (2018) Implication of Two Different Doses of Intradiscal Ozone-Oxygen Injection upon the Pain Alleviation in Patients with Low Back Pain: A Randomized, Single-Blind Study. Pain Physician 21: E25-E31. Link: https://bit.ly/2Ngl44w
- Ezeldin M, Leonardi M, Princiotta C, Dall'olio, Tharwat M, Zaki M, et al. (2018) Percutaneous ozone nucleolysis for lumbar disc herniation. Neuroradiology 60: 1231-1241. Link: https://bit.ly/2YS7zNJ
- Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, et al. (2007) Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng 24: 5-21. Link: https://bit.ly/2YR6VAb
- Hohaus C, Ganey TM, Minkus Y, Meisel HJ (2008) Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 17: S492-S503. Link: https://bit.ly/316f72h
- Orozco L, Soler R, Morera C, Alberca M, Sanchez A, et al. (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92: 822-828. Link: https://bit.ly/3emcE7m
- Gilbert HTJ, Hoyland JA, Richardson SM (2013) Stem Cell Regeneration of Degenerated Intervertebral Discs: Current Status (Update). Curr Pain Headache Rep 17: 377. Link: https://bit.ly/2YRpeFf
- Sakai D, Andersson GBJ (2015) Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol 11: 243-256. Link: https://bit.ly/3dj1L54
- Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, et al. (2016) Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 99: 69-80. Link: https://bit.ly/3djent5
- Smith LJ, Silverman L, Sakai D, Le Maitre CL, Mauck RL, et al. (2018) Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine 1: e1036. Link: https://bit.ly/37JFKvb
- Sadat-Ali M, Al-Dakheel DA, AlMousa SA, AlAnii FM, Ebrahim WY, et al. (2019) Stem-cell therapy for ovariectomy-induced osteoporosis in rats: a comparison of three treatment modalities. Stem Cells Cloning 12: 17-25. Link: https://bit.ly/37WmIlr
- Mancuso P, Raman S, Glynn A, Barry F, Murphy JM (2019) Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front Bioeng Biotechnol 7: 9. Link: https://bit.ly/3fFeikU
- Lim SH, Mao HQ (2009) Electrospun scaffolds for Stem Cell engineering. Advanced Drug Delivering Reviews 61: 1084-1096. Link: https://bit.ly/2BqFm8w
- Elahimehr R, Scheinok AT, McKay DB (2016) Hematopoietic stem cells and solid organ transplantation. Transplant Rev (Orlando) 30: 227-234. Link: https://bit.ly/2YPV9Gd
- Rufaihah AJ, Cheyyatraivendran S, Mazlan MDM, Lim K, Chong MSK, et al. (2018) The Effect of Scaffold Modulus on the Morphology and Remodeling of Fetal Mesenchymal Stem Cells. Front Physiol 9: 1555. Link: https://bit.ly/2NdHHGD
- Aurora AB, Olson EN (2014) Immune modulation of stem cells and regeneration. Cell Stem Cell 15: 14-25. Link: https://bit.ly/2Ykvh6m
- Godwin JW, Pinto AR, Rosenthal NA (2017) Chasing the recipe for a pro-regenerative immune system. Semin Cell Dev Biol 61: 71-79. Link: https://bit.ly/3dkilkU
- Julier Z, Park AJ, Briquez PS, Martino MM (2017) Promoting tissue regeneration by modulating the immune system. Acta Biomaterialia 53: 13-28. Link: https://bit.ly/3dkZ1UN
- Weiss ARR, Dahlke MH (2019) Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol 10: 1191. Link: https://bit.ly/2YPSmNi
- Schwartz M, London A, Shechter R (2009) Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience 158: 1133-1142. Link: https://bit.ly/2YVkLkK
- Schwartz M, Kipnis J, Rivest S (2013) Prat A. How do immune cells support and shape the brain in health, disease and aging? J Neurosci 33: 17587-17596. Link: https://bit.ly/2YmxBd7
- Tashireva LA, Perelmuter VM, Manskikh VN, Denisov EV, Savelieva OE, et al. (2017) Types of Immune-Inflammatory Responses as a Reflection of Cell-Cell Interactions under Conditions of Tissue Regeneration and Tumor Growth. Biochemistry 82: 542-555. Link: https://bit.ly/2zLs8mm
- Moviglia GA, Fernandez Viña R, Brizuela JA, Saslavsky J, Vrsalovic F, et al. (2006) Combined Protocol of Cell Therapy for Chronic Spinal Cord Injury. Report on the Electrical and Functional Recovery of Two Patients. Cytotherapy 8: 202-209. Link: https://bit.ly/2CkCZod
- Moviglia GA, Picon MC, Huerta J (2015) Local Adoptive Immunotherapy with Th1 Specific Autologous Lymphocytes May Correct the Cutaneous Hypertrophic Scar. Cytotherapy. Link: https://bit.ly/2NdXWU3
- Zarate JO, Folgar M, Pelayes D, Alvarado M, Piccone S, et al. (2016) Study of Choroidal Tissue Neovascularization After AMSC Treatment Through OCT And Digital Biopsy Analysis. Cytotherapy 18.
- Richardson J, Santa Coloma E, Piccone S, Moviglia GA (2016) Combined cell therapy associated with Hyaluronic Acid infusion for ad integrum repair of knee cartilage. Cartilage. Cytotherapy 18. Link: https://bit.ly/2V02l1c
- Moviglia GA, Moviglia-Brandolino MT, Couto D, Piccone S (2018) Local immunomodulation and muscle progenitor cells induce recovery in atrophied muscles in spinal cord injury patients. Journal of Neurorestoratology 136-145. Link: https://bit.ly/2Yj9Fai
- Hwang D, Kim S, Abeydeera NA, Statum S, Masuda K, et al. (2016) Quantitative magnetic resonance imaging of the lumbar intervertebral discs. Quant Imaging Med Surg 6: 744-755. Link: https://bit.ly/2UWwtuv
- Paul CPL, Smit TH, de Graaf M, Holewijn RM, Bisschop A, et al. (2018) Quantitative MRI in early intervertebral disc degeneration: T1rho correlates better than T2 and ADC with biomechanics, histology and matrix content. PLoS One 13: e0191442. Link: https://bit.ly/30ZmZCt
- Smeets R, Ke AK, Lin CW, Ferreira M, Demoulin C (2011) Measures of Function in Low Back Pain/Disorders. Arthritis Care Res 63: S158–S173. Link: https://bit.ly/3ehuW9S
- Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, et al. (2019) Measurement Properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in Patients with Low Back Pain: A Systematic Review. J Pain 20: 245-263. Link: https://bit.ly/2Nh0TDv
- Burnet FM (1976) A modification of Jerne's theory of antibody production using the concept of clonalselection. CA Cancer J Clin 26: 119-121. Link: https://bit.ly/3elTlLx
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A (1998) Flexible programs of chemokine receptor expression on human polarized T Helper 1 and 2 lymphocytes. J Exp Med 187: 875-883. Link: https://bit.ly/2BrFSDb
- Groom JR, Luster AD (2011) CXCR3 in T cell function. Exp Cell Res 317: 620-631. Link: https://bit.ly/2YME0x2
- Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, et al. (2004) Cell viability assays. In: Sittampalam GS, Coussens NP, Brimacombe K, et al., editors. Assay Guidance Manual [Internet]. Bethesda, MD: Eli Lilly & Company and the National Center for Advancing Translational Sciences.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317. Link: https://bit.ly/3eiAK39
- Mifune Y, Matsumoto T, Murasawa S, Kawamoto A, Kuroda R, et al. (2013) Therapeutic Superiority for Cartilage Repair by CD271-Positive Marrow Stromal Cell Transplantation. Cell Transplant 22: 1201-1211. Link: https://bit.ly/3ek3MiO
- Rutkovskiy A, Stensløkken KO, Vaage IJ (2016) Osteoblast differentiation at a glance. Med Sci Monit Basic Res 22: 95-106. Link: https://bit.ly/2V12TUI
- Séguin CA, Chan D, Dahia CL, Gazit Z (2018) Latest advances in intervertebral disc development and progenitor cells JOR Spine 1: e1030. Link: https://bit.ly/3eiuZCh
- NCI-USA (2006) Common terminology criteria for adverse events. v3.0 – CTCAE. Link: https://bit.ly/2BltogH
- Klune CB, Larkin AE, Leung VSY, Pang D (2019) Comparing the Rat Grimace Scale and a composite behaviour score in rats. PLoS One 14: e0209467. Link: https://bit.ly/310bvif
- Cho C, Michalidis V, Lecker I, Collymore C, Hanwell D, et al. (2019) Evaluating analgesic efficacy and administration route following craniotomy in mice using the grimace scale. Sci Rep 9: 359. Link: https://go.nature.com/2YR3E3Q
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
