Open Journal of Orthopedics and Rheumatology
Muscle Vanishing in Poliomyelitis Manifested on F-18 FDG PET/CT: An Interesting Imaging Finding
Rong-Hsin Yang1 and Yum-Kung Chu1,2*
2National Yang-Ming University School of Medicine, Taipei, Taiwan
Cite this as
Yang RH, Chu YK (2017) Muscle Vanishing in Poliomyelitis Manifested on F-18 FDG PET/CT: An Interesting Imaging Finding. Open J Orthop Rheumatol 2(1): 009-011. DOI: 10.17352/ojor.000007Muscle atrophy is the loss of muscles bulk; it can be a partial or complete wasting away of muscle. Herein, we describe a 56-year-old man with diffuse large B-cell lymphoma underwent F-18 FDG PET for postchemotherapy evaluation. Fused PET/CT image showed a successful response to treatment and an interesting instance of complete muscle vanishing, attributed to poliomyelitis sequelae. Muscle atrophy seen in polio patients is the result of destruction of motor neurons caused by the poliovirus. CT and MRI are prevalent tools for assessing muscular pathology. As a glucose analog, F-18 FDG PET can provide greater insight into glucose metabolism of the skeletal muscle. F-18 FDG distribution is the crucial information to make it possible for clinicians to fully interpret the functional integrity of the muscles, and to determine if specific procedures are beneficial. Straightforward exhibition of F-18 FDG uptake appears to add validity of donor muscle selection before polio-corrective surgery, and of monitoring after treatment.
Abbreviations
DLBCL: Diffuse large B-cell lymphoma; FDG: Flurodeoxyglucose; PET: Positron emission tomography
Introduction
Although poliomyelitis has been almost eradicated in the developed world, orthopedic surgeons occasionally encounter residual deformities in patients who suffered the disease in childhood. This presentation demonstrates the instance of complete muscle vanishing attributed to poliomyelitis sequelae, on positron emission tomography (PET) image. Muscle or tendon transfer to replace a paralyzed muscle is sometimes of help if the muscle to be transferred is strong enough. As these muscles play an essential role in locomotion, it is critical to assure the functional integrity of muscles prior to polio-corrective surgeries or further rehabilitation regimens. F-18 flurodeoxyglucose (FDG), as a glucose analog, is known to show variable uptake in the skeletal muscles. The technologies of PET may provide a clear status of glucose metabolism of the muscles.
Case Report
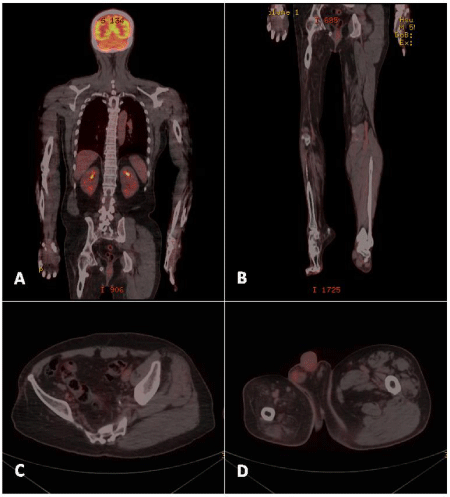
A 56-year-old Chinese male with a history of diffuse large B-cell lymphoma (DLBCL) underwent F-18 FDG PET for postchemotherapy evaluation. The patient had suffered from poliomyelitis at around the age of 5 years. Paralysis remained in his right lower extremity after recovery from the acute stage. Over the last two years, the patient underwent R-CHOP chemotherapy for DLBCL and achieved remission. A control PET/CT obtained after administration of 11 mCi of F-18 FDG revealed a successful response to chemotherapy without FDG-avid lymphadenopathy. Noteworthy the disparity between the bilateral pelvic girdles and the lower limbs was evident, with significant muscle shrinkage on the right side. Vanishing of gluteus maximus, gluteus medius, gluteus minimus, and piriformis muscles was noted. Besides, most of the muscle groups in the right leg were wasting. The preservation of the skeletal structure was noted (Figure 1). These findings were attributed to poliomyelitis sequelae.
Discussion
Poliomyelitis is an acute disease of the anterior horn motor neurons of the spinal cord and brain stem caused by poliovirus, with the classic manifestation of asymmetric flaccid paralysis and muscle atrophy. Normal motor function depends on the transmission of signals from the cerebral cortex to the brainstem or spinal cord by upper motor neurons; and from there directly to innervate skeletal muscle by lower motor neurons. According to Agamanolis [1], damage of the lower motor neuron at any point can cause myofiber atrophy. Motor neurons influence tissue growth and maintenance in muscle. The trophic influence is mediated by induced contractions and by chemical substances (trophic factors) released at neuromuscular synapses, which modulate protein synthesis in muscle. Myofibers that lose their innervation become angular and shrink. At an extreme stage of atrophy, virtually all sarcoplasm is lost and the myofiber is reduced to a cluster of nuclei. In the process of denervation, there is loss and disarray of myofilaments but no myonecrosis occurs [1]. The most characteristic feature of polio paralysis is its asymmetric distribution, which affects some muscle groups while sparing others.
Based on the study in 1356 children with 1800 poliomyelitic limbs, Sharma et al. observed that more than two-third (68%) of the muscles was affected. The muscles most frequently affected were tibialis anterior (1516), quadriceps (1465) and tibialis posterior (1435) [2]. Utility of CT or MRI in assessment of muscle affection is prevalent. Fatty replacement can easily be displayed on CT by its low-density appearance [3]. MRI is also useful in detection and characterization of changes in muscle signal intensity caused by variable disorders. In chronic denervation, muscle atrophy and fatty infiltration demonstrate high signal changes on T1-weighted sequences in association with volume loss [4]. Furthermore, recognition of the affected extent and metabolic integrity of the muscles and bones is critical for appropriate approach to the particular part of the body affected by poliomyelitis [5]. Skeletal muscle has a relatively high-glucose metabolism. The technologies of positron emission tomography (PET) can provide greater insight into the status of glucose metabolism of the muscular system.
The skeletal muscle and liver are capable of absorbing glucose and storing it as glycogen in response to insulin. Glycogen serves as a buffer to maintain blood-glucose levels. When plasma insulin is low between meals, glucagon increases glucose availability. Moreover, the glucose from glycogen is readily mobilized and fast glycolysis is therefore a major energy source for bursts of activity [6]. F-18 flurodeoxyglucose (FDG) is an analog of glucose with substitution of the oxygen in C-2 position with fluorine-18. F-18 FDG is transported into the cells mediated by glucose transporters (GLUT-1 through GLUT-4) and gets trapped there after phosphorylation [7]. Glucose uptake is therefore a main indication of functional integrity. In a series of 14 patients referred for evaluation of muscle graft viability, Smith et al. observed three patterns of FDG uptake on PET imaging:
a) Good FDG uptake indicative of a viable transfer graft.
b) Absence of FDG activity in the extent of the graft with nonviable tissue.
c) No FDG activity in much of the area suggestive of a nonviable graft related to peripheral vascular disease [8].
Uptake ratio, defined as FDG activity in the region of interest versus that in the contralateral limb, was also used as a semi-quantitative analysis in Smith’s study. They found that for all patients, the uptake ratio of viable muscle (mean 2.26 ± 1.81; n= 26) showed significantly higher than that of nonviable muscle (mean 0.27 ± 0.12; n= 6; p < 0.02). A clinically realistic threshold of 0.5 yielded sensitivity and specificity of 96% (25/26) and 100% (6/6), respectively [8]. FDG-PET for assessment of skeletal muscle viability in trauma or following muscle transfer is applicable. Normal F-18 FDG uptake of skeletal muscles is frequently symmetric, and mild and homogeneous in intensity on PET [9]. Observation of an asymmetric pattern of F-18 FDG distribution even after proper patient preparation should raise the suspicion for pathologic involvement [7]. As illustrated in our case (Figure 1), absence of muscle uptake of F-18 FDG, attributed to denervated muscle atrophy, is perceptible and profound in polio patient. Knowledge of pathoanatomy by means of F-18 FDG imaging is crucial before embarking on further therapy.
Muscle groups in the limbs work in a synergistic fashion to perform extension or flexion across a joint. If major nerves or muscles in a synergistic group are damaged or destroyed, motion is crippled. When available, local muscles and tendons can be transferred from an adjacent muscle compartment to restore lost function. The mainstay of surgical approaches for polio deformity includes reestablishment of muscle balance to prevent deformities, muscle transfer to replace a paralyzed muscle, stabilization of a relaxed or flail joint by means of various orthopedic techniques [10]. In muscle transplant procedures, unlike in tendon transfer, both the origin and the insertion of a muscle are detached along with its neurovascular pedicle. It is essential to find a normal muscle to transplant and to avoid donor-site morbidity. Delineation of the particular part affected by poliomyelitis is necessary to assist the prognosis and eventual treatment [5,11]. F-18 FDG PET may become the key investigative tool for planning the appropriate management strategy.
Conclusion
The most characteristic feature of polio paralysis is its asymmetric distribution, which affects some muscle groups while sparing others. When available, local muscles and tendons can be transferred from an adjacent muscle compartment to restore lost function. Alone or in conjunction with other modalities, F-18 FDG imaging is helpful in interpreting the muscle viability of the involved limbs, and determining if special procedures are beneficial.
- Agamanolis DP (2007) Myopathology. Neuropathology: An Illustrated Interactive Course for Medical Students and Residents. Link: https://goo.gl/W7ZLb3
- Sharma JC, Gupta SP, Sankhala SS, Mehta N (1991) Residual poliomyelitis of lower limb-pattern and deformities. Indian J Pediatrics 58: 233-238. Link: https://goo.gl/1n6v1G
- Termote JL, Baert A, Crolla D, Palmers Y, Bulcke JA (1980) Computed tomography of the normal and pathologic muscular system. Radiology 137: 439-444. Link: https://goo.gl/9wNdRT
- Kamath S, Venkatanarasimha N, Walsh M, Hughes P (2008) MRI appearance of muscle denervation. Skeletal Radiology 37: 397-404. Link: https://goo.gl/aNfcP2
- Faraj AA (2006) Poliomyelitis: Orthopaedic management. Current Orthopaedics 20: 41-46. Link: https://goo.gl/YVShxb
- Katch VL, McArdle WD, Katch FI (2010) Essentials of Exercise Physiology. 4th revised international edition. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins. Link: https://goo.gl/IDvpcX
- Karunanithi S, Soundararajan R, Sharma P, Naswa N, Bal C, et al. (2015) Spectrum of physiologic and pathologic skeletal muscle F-18 FDG uptake on PET/CT. AJR Am J Roentgenol 205: W141–W149. Link: https://goo.gl/NL5IEy
- Smith GT, Wilson TS, Hunter K, Besozzi MC, Hubner KF, et al. (1995) Assessment of skeletal muscle viability by PET. J Nucl Med 36: 1408-1414. Link: https://goo.gl/g2CjDP
- Liu Y, Ghesani NV, Zuckier LS (2010) Physiology and pathophysiology of incidental findings detected on FDG-PET scintigraphy. Semin Nucl Med; 40:294–315. Link: https://goo.gl/ynDgHo
- Watts HG (2005) Orthopedic techniques in the management of the residua of paralytic poliomyelitis. Techniques in Orthopaedics 20: 179-189. Link: https://goo.gl/nMmYHl
- Khare R, Agarwal AK, Kumar R (2007) Polio rehabilitative surgery camps. IJPMR 18: 21-23. Link: https://goo.gl/nxX3Vs
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
