Open Journal of Environmental Biology
Increased biomass of free-living marine nematodes may be indicative of disturbances in the ecosystem of the San Antonio Bay
Gabriela Villares1,2* and Catalina Pastor de Ward2
2Meiobenth Laboratory LAMEIMA-IDEAus (CENPAT-CONICET), Argentina
Cite this as
Villares G, Pastor de Ward C (2020) Increased biomass of free-living marine nematodes may be indicative of disturbances in the ecosystem of the San Antonio Bay. Open J Environ Biol 5(1): 001-006. DOI: 10.17352/ojeb.000014An ecological study of free-living marine nematodes in salt marshes of San Antonio bay was carried out to determine whether they are affected by anthropic disturbances. Samples were collected during the summer of 2009. Three sites were selected, one with urban disturbance and two with possible control. In each site the samples were taken in the 3 areas of the mesolittoral. Nematodes were separated, counted, measured and identified at species level. San Antonio bay presented high salinity values in all sites. “Ciudad” showed the highest values of heavy metals and nematode volumetric biomass. “Baliza Camino” presented the highest salinity values and the lowest volumetric biomass values. “Banco Perdices” presented low organic matter and values intermediate of volumetric biomass.
The result indicated that volumetric biomass acted as good indicator of the state of the bay, since the presence of high values of this nematodes would be contributing information about the presence of disturbances.
Introduction
Free-living nematodes are essential for the functioning of estuarine and marine ecosystem, and could be used more widely as biological indicators of marine environmental pollution [1-7].
Nematodes are the most diverse and numerically dominant metazoans in aquatic ecosystem, and, because of their rare ability to survive in extremely polluted conditions, they ase ususally the only persistent taxon in heavily polluted/disturbed habitats [8].
Benthic organisms make a significant contribution to the regulation of carbon, nitrogen and sulfide cycles, water column processes, the distribution and destination of pollutants, secondary production and transport and stability of sediments [9]. In the coastal zones the meiofauna biomass is smaller than the macrofauna biomass. However, due to its higher renewal rates (B/W), meiofauna plays a proportionally larger role in energy transfer. Therefore, the metabolic requirements of meiofauna (secondary production and respiration) could reach values even higher than those recorded for the macrofauna, particularly in environments where the ratio of macrofauna: meiofauna biomass approaches or is less than 5:1 [10,11]. From an ecological perspective and according to their adaptive and behavioral characteristics as r-k strategists, families of nematodes can be ordered on a scale as colonizers (corresponding to type r strategists), persistent (corresponding to type k strategists) and those with behaviors intermediates [12]. The maturity index is used as an effective measure for the evaluation and quantification of the impact of soil contamination in the nematode community.
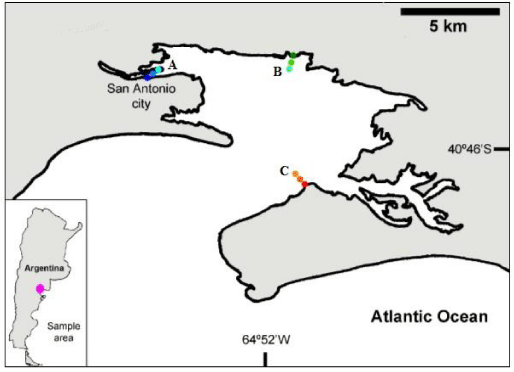
San Antonio bay (located at the northwest end of San Matías Gulf), is a semi-enclosed basin with maximum depths close to 200 m in the center of the bay. Fishing is carried out in that city and in the port of San Antonio Este, one of its main activities is the export of products from the Río Negro valley.
For this work we chose an urbanized beach (“Ciudad") where fishing activities are carried out and the products exported from the Black River Valley and two pristine beaches ("Baliza Camino" and "Banco Perdices").
The objective of this work was to carry out an ecological study of the free-living marine nematode communities of the salt marshes of San Antonio bay, in order to be able to evaluate the volumetric biomass could be indicative of the disturbances produced by the man.
Thus, two main questions have been addressed: is the volumetric biomass a useful tool to detect the effects of a human event? and the volumetric biomass differs between the three sites studied in San Antonio bay (Río Negro)?
Materials and methods
Study site and sample collection
Sampling was carried out in San Antonio bay in the province of Río Negro (40°46'S, 64°52'W) in February 2009 (Figure 1). Three sites were selected, one with urban settlement and two with no nearby settlements. Sampling sites in San Antonio bay: A) “Ciudad” (40°43'S, 64°57'W): Located on the coast of the estuary on the bank that borders the city. The upper level of the mesolitoral with patches of Sarcocornia sp., the medium level with bare sediment and lower level with presence of polychaetes; B) “Baliza Camino” (40°42'S, 64°50'W): Located in front of the mouth of the bay, is a semi-desert Patagonian site. The upper level with patches of Sarcocornia sp., the medium and the lower level with bare sediment; C) “Banco Perdices” (40°46'S, 64°51'W): Located east of San Antonio East. The upper level with patches of Spartina sp., the medium and lower level with bare sediment.
Sample collection and treatment
The samples were collected with a cylindrical Plexiglas corer, 10cm high and 2.9cm in diameter. They were preserved in 5% formaldehyde in filtered seawater, sieved through both 500µm and 50µm mesh sieves. The nematodes present on the 50 µm were separated by the sediments by means of LUDOX TM [13]. Then, 200 specimens were separated from each sample, which were mounted in glycerin between slide and coverslip and sealed with CANADAX resin. Then, we proceeded to the identification to species level.
The maturity index (IM) can be calculated for each site and level using the C-P values of the species found using the formula of Bongers, et al., 1991 [14]:
IM=∑ vxf
Where v is the C-P value of each taxon and f is the frequency taxon.
The length (L) and width (W) of each of the nematodes were measured. To determine the biomass of the different groups, the Andrassy [15], formula was used:
V=CxLxW2
Where V is the volume in ml, C is a constant according to each taxon, L is the organism length and W is the width. The value of the constant C was obtained from Feller and Warwick [16] , where C=530. The volume was converted to wet weight by multiplying it by the specific gravity of the organism (1.13 g·cm-3).
For each sample the following environmental variables were measured: 1) dissolved oxygen (O2, mg/l), water salinity (S, g/l) and temperature (T,°C) (they were measured in situ with a multiparameter equipment), 2) the depth of the anoxic layer (cm), 3) penetrability (P, cm), 4) organic matter (MO), 5) grain size classification of sediment and 6) heavy metals. The samples of heavy metals (Zn, Pb, and Cu) were analyzed by the chemistry service of the “Centro Nacional Patagónico” (CENPAT).
For the characterization of the sediment each sample was weighed, then all of them were dried in a stove at 60°C, after they were sifted and weighed again. The difference between the original weights after sifted was analyzed to determine the Fine Fraction (FF). The samples of organic matter were dried in a stove at 60°C and then with a mortar were homogenized. From each sample a subsample was taken which was placed in a muffle at 450°C. Subsequently the weight of each subsample was recorded and the value of organic matter was calculated by weight difference [17].
Analysis of nematode distribution patterns
All of the multivariate analyses were performed with the PRIMER v6 software [18].
Cluster analysis derived from Bray-Curtis similarity matrices (fourth-root transformed) was used to view spatial differences in the biomass of the community of nematodes. To evaluate the differences between the samples, the one-way ANOSIM test and the 999 permutations were performed. A Rglobal value is obtained from 0 (when there are no differences between samples) to 1 (when the samples differ from each other) and a probability p, which indicates the significance of the analysis.
Two methodologies were used to analyze the environmental parameters: 1) the main component analysis (PCA) [19]. For this, a matrix was constructed in which the data were transformed using Log(x+1) and then normalized. A graph was obtained that showed the relation between the structure of nematode associations in biomass and the environmental parameters; 2) the BIOENV subroutine, which defines the set of environmental variables that best explain the biotic structure.
Individuals were classified within C-P classes and the maturity index was calculated for each site and mesolitoral level.
Results
Samples taken in San Antonio bay were identified and measured giving a total of 5.140 nematodes. A total of 90 species were identified in 64 genera, 24 families and 8 orders. For the analyzes only the male and female adults were taken into account.
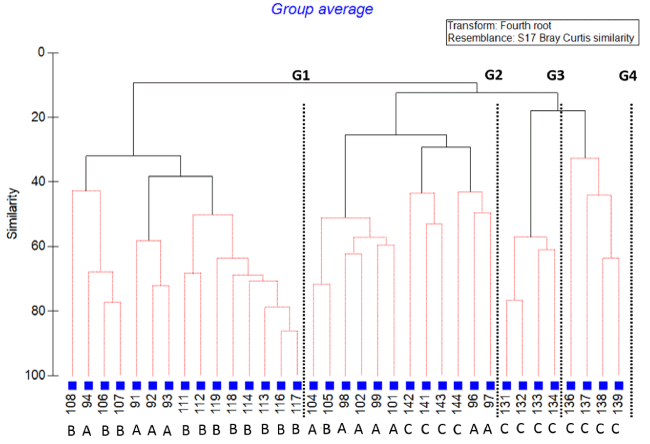
ANOSIM analysis for volumetric biomass data using factor sites showed significant differences between the samples of the sites (p=0.001; Rglobal=0.600). These differences were mostly reflected between the B-C sites and to a lesser extent between the A-B and A-C sites. The upper level of site A was grouped with the 3 levels of site B while the middle and lower levels of the site A were clustered closer to the upper level of the site C. The middle and lower levels of the site C were shown to be more separated from the rest. This was also revealed by the cluster analysis (Figure 2) that highlighted the presence of four mains groups: the first represented by the upper, middle and lower level of the site B and upper of the site A(G1), the second one represented by the middle and lower of the site A and upper of the site C(G2), the third(G3) represented by lower of the site C and the fourth (G4) represented by the middle of the site C.
Taking into account the level factor of the mesolitoral, the ANOSIM test found significant differences (p=0.001; Rglobal=0.736). The differences were reflected between the upper and the middle level, and between the upper and lower levels; while they did not do it between the middle and lower levels.
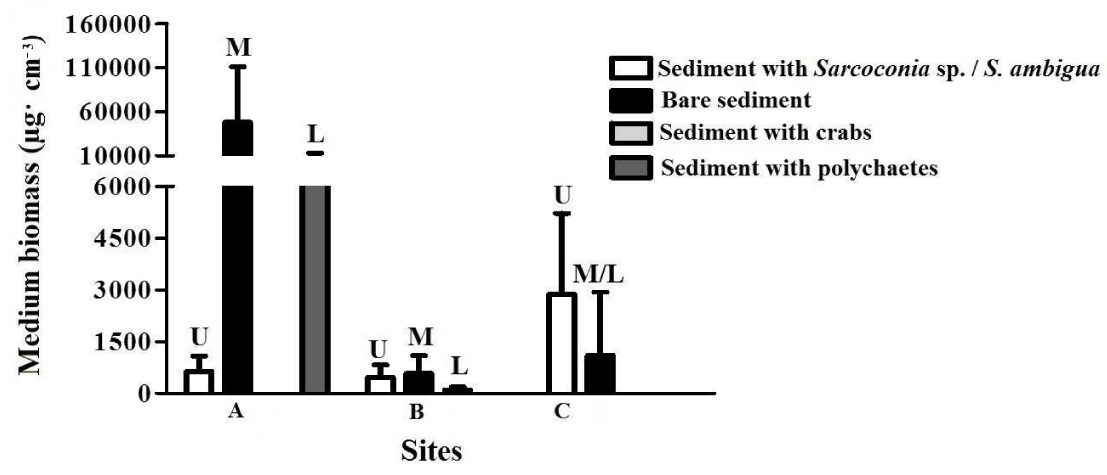
The highest biomass was found at site A(341.333μg·cm-3), followed by site C(49.625μg·cm-3) and B(2.313μg·cm-3), which was the lowest biomass (Figure 3).
To determine which species generate dissimilarity between sites based on volumetric biomass, those that contributed up to 60% were considered.
The site A was differentiated from the site B by the species Neochromadora papillosa, Nudora crepidata, Spirinia septentrionalis, Polysigma sp., Viscosia macramphida, Diplolaimelloides oschei and Onyx sp. It was differentiated from site C, by species Spirinia septentrionalis, Onyx. sp., Polysigma sp., Paraethmolaimus dahli, Viscosia macramphida, Nudora crepidata, Neochromadora papillosa, Microlaimus decoratus, Diplolaimelloides oschei, Diplolaimella gerlachi, Terschellingia longicaudata and Calyptronema maxweberi. The site B differed from the site C by Paracyatholaimus sp., Paraethmolaimus dahli, Thalassomonhystera parva and Diplolaimella gerlachi.
The BEST analysis that relates the environmental variables to the biomass data showed low correlation values, the highest value being the salinity variable (S), which was 0.404g/l (Table 1).
With respect to the C-P values, in San Antonio bay the strategists type C-P 3(82%) were the most dominant, followed by strategists C-P 4(10%) and C-P 2(8%). No individuals with strategy type C-P 1 and C-P 5 were found. The one-way ANOSIM test showed that there are significant differences between sites in terms of C-P values (p=0,001; Rglobal=0,386). No significant differences were found between the levels (p=0,193; Rglobal=0,032). The maturity index (IM) was calculated for the sites and mesolitoral levels. The values of IM (3.0-3.3) were found in site C. The remaining values were similar to each other, being in the range from IM of 2.1 to 2.8.
Discussion and conclusion
The bay of San Antonio was characterized by high salinity. The bay showed high salinity values in two of the three sites (~50g/l), "Ciudad" (Site A) and "Banco Perdices" (Site C) and very high in "Baliza Camino" (Site B), exceeding 100g/l (Table 2).
The site A ("Ciudad") presented the highest volumetric biomass. Of all the sites studied, it was the one that presented the highest concentration values of heavy metals in sediment. In turn, it was the upper level of this site that presented the highest values. Although these values are below the pollution limits, the values of copper and zinc were close to the threshold level of effect on the organisms and the value of lead exceeded this level. The metals enter the nematodes through the cuticle [20] or trapped in the mucus that the nematodes produce. The species found on this site have not been identified as indicators of heavy metal contamination. Although in some cases if the genres found coincide, Howell (1982) showed that species of the same genus do not accumulate the metals of the environment in the same way. Microlaimus globiceps, Diplolaimelloides oschei and Diplolaimella gerlachi and Paraethmolaimus dahli were the species that contributed most to the volumetric biomass. On this site the epistrato feeders and non-selective deposit feeders were favored, although there were other types of feeding
The values of C-P 2 and C-P 3 were found most frequently in the "Ciudad". It is probable that the nematodes found in this site have developed a certain tolerance to these concentrations of metals, and therefore have the highest volumetric biomass. The middle and lower levels did not present differences in relation to volumetric biomass. Although the metal levels were higher than the rest of the sites, the effect was not notorious. At the lower level the highest amount of organic matter was observed. The process of eutrophication, as a result of port activity [21] and sewage discharges was notorious in this levels.
Site B presented the highest salinity of the 3 sites, together with the lowest volumetric biomass. The number of species was low, standing out as the ones that contributed most to the biomass: Paraethmolaimus dahli, Diplolaimella gerlachi and Paracyatholaimus sp. According to Heip, et al., [22], the species that are in a habitat with high salinity are well adapted, and observed that some species survived a salinity of 123g/l, such as Diplolaimelloides oschei, Monhystera multiseta, Paracyatholaimus intermedius. Species of the genus Diplolaimella Allgen, 1929 and Diplolaimelloides Meyl, 1954 are found in extreme environments around the world, from salt water to brackish water, groundwater and coastal areas [23]. Although there are no reported data on P. dahli, this is a species resistant to extreme environmental conditions, so it is not strange that it is one of the most abundant in this case.
The site C was characterized by sediment fine sand and silt, a high percentage of fine fraction, low organic matter and presented the lowest salinity. The volumetric biomass was intermediate with respect to the other two studied sites, being higher in the upper level.
The species that most contributed to the volumetric biomass were: Nudora besnardi, Microlaimus globiceps, Odontophora peritricha, Neochromadora alejandroi, Paraethmolaimus dahli, Rhips sp., Daptonema laxus y Bathylaimus australis. Most correspond to epistrato feeders, while some are non-selective deposit feeders. This site was characterized by individuals with k strategies, due to the greater stability of the environmental conditions compared to the other sites.
It presented the lowest organic matter content within the three sites, which would explain the low biomass due to the horizontal migration of the nematodes towards patches with greater amount of food. In turn, there could have been a vertical migration of organisms to greater depths to protect themselves from predators [24-32].
In San Antonio bay the different values of volumetric biomass of the free-living nematodes allowed to identify different factors that affect their coasts. The "Ciudad" site showed to be an environment in which the anthropic effect was clearly manifested. The volumetric biomass acted as a good indicator of the state of the bays, since the presence of high values would be contributing information about the presence of anthropic effects, thus demonstrating its capacity as a monitoring tool in disturbed environments. Diplolaimella gerlachi and Paraethmolaimus dahli were the two species that showed great tolerance to heavy metals and high salinity, which is why they can be considered as indicator species.
The authors thank to the Dr. Virginia lo Russo for the help in the collection of the samples and in the classification of the nematodes.
- Lambshead PJD (1986) Sub-catastrophic sewage and industrial waste contamination as revealed by marine faunal analysis. Mar Ecol Prog Ser 29: 247-260. Link: http://bit.ly/38v2k9X
- Austen MC, McEvoy AJ (1997) The use of offshore meiobenthic communities in laboratory microcosm experiments: response to heavy metal contamination. J Exp Mar Bio Ecol 211: 247-261. Link: http://bit.ly/2GetOEy
- Schratzberger M, Gee JM, Rees HL, Boyd SE, Wall CM (2000) The structure and taxonomic composition of sublittoral meiofauna assemblages as an indicator of the status of the marine environment. J Mar Biol Assoc U K 80: 969-980. Link: http://bit.ly/38rqW3l
- Austen MC (2004) Natural nematode communities are useful tools to address ecological and applied question. Nematol Monogr Perspect 2: 1-17. Link: http://bit.ly/2sKTuWf
- Danovaro P, Gambi C, Höes S, Mirto S, Traunspurger W, et al. (2009) Case studies using nematodes assemblage analysis in aquiatic habitats. In M.J. Wilson and T.C. Kakouli-Duarte, (Eds) Nematodes as Environmental Indicators. CAB Internationals. Wallingford, UK 146-171. Link: http://bit.ly/38xTvfl
- Moreno M, Semprucci F, Vezzulli L, Balsamo M, Fabiano M, e al. (2011) The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol Indic 11: 328-336. Link: http://bit.ly/2RHvo78
- Vanaverbeke J, Steyaert M, Soetaert K, Rosseau V, Van Gansbeke D, et al. (2004b) Changes in structural and functional diversity of nematode communities during a spring phytoplankton bloom in the southern North Sea. J Sea Res. 52: 281-292. Link: http://bit.ly/37vp5dP
- Coull BC, Chandler GT (1992) Pollution and meiofauna field, laboratory, and mesocosms studies. Ann Rev Mar Sci 30: 191-271. Link: http://bit.ly/2Re7hhE
- Snelgrove VR, Blackburn TH, Hutchings PA, Alongi DM, Grassle JF, et al. (1997) The importance of marine sediment biodiversity in ecosystem processes. Ambio 26: 578-583. Link: http://bit.ly/36g7rt5
- Gerlach SA (1971) On the importance of marine meiofauna for bentos communities. Oecología 6: 176-190. Link: http://bit.ly/2THjm0t
- Higgins RP, Thiel H (1988) Introduction to the study of Meiofauna. Washington, District of Columbia, USA, Smithsonian Institution Press 488. Link: http://bit.ly/37hym9g
- Bongers T, Ferris H (1999) Nematode communities structure as a bioindicators in environmental monitoring. Tree 14: 224-228. Link: http://bit.ly/2NKvdHa
- De Jonge VN, Bouwman LA (1977) A simple density separation technique for quantitative isolation of meiobenthos using the Colloidal LUDOX-TM. Mar Biol 42: 143-148. Link: http://bit.ly/2tItz1Q
- Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index. Mar Ecol Prog Ser 76:135-142. Link: http://bit.ly/3azeFfa
- Andrassy I (1956) Die Rauminshals-und Gewichtbestmmung der Fadenwürmer (Nematoden). Acta Zoologica Hungaria 2: 1-15. Link: http://bit.ly/2RdRLSN
- Feller RJ, Warwick RM (1988) Energetics. In: Higgins, R.P, Thiel, H. (Eds), Introduction to the study of meiofauna. Washington, DC, Smithsonian Institution Press 181-196. Link: http://bit.ly/30GRWJm
- Byers SC, Mills EL, Steward PJ (1978) Determining Organic Carbon in Marine Sediments, with suggestion for a standard Method. Hidrobiologica58: 43-47. Link: http://bit.ly/37fJza9
- Clarke KR, Warwick RM (2001) Change in marine communities: an approacha to stadistical analysis and interpretation, Plymoth, PRIMER-E Ltd 168.
- Chen AC, Soo CL, Balsamo M, Semprucci F (2017) An approach based on nematode descriptors for the ecological quality (EcoQ) classification of the Malaysian coasts. Mar Biodivers 48: 117-126. Link: http://bit.ly/38xUPPl
- Howell R (1983) Heavy metals in marine nematodes: Uptake, tissue distribution and loss of copper and zinc. Mar Pollut Bull 14: 263-268. Link: http://bit.ly/2THkjG5
- Commendatore MG, Esteves JL, Colombo JC (2000) Hydrocarbons in Coastal Sediments of Patagonia, Argentina: Levels and Probable Sources. Mar Pollut Bull 40: 989-998. Link: http://bit.ly/2uhYn9L
- Heip C, Vincx M, Vranken G (1985) The Ecology of Marine Nematodes. Oceanogr Mar Biol Annu Rev 23: 399-489. Link: http://bit.ly/2NPMEpX
- Pastor de Ward CT, Lo Russo V (2009) Distribution of Diplolaimella and Diplolaimelloides species from Patagonian lagoons and coastal waters (Nematoda: Monhysteridae), Chubut and Santa Cruz provinces. J Mar Biol Assoc U K 89: 711-718. Link: http://bit.ly/2NMATR9
- Steyaert M, Herman PMJ, Moens T, Widdows J, Vincx M (2001) Tidal migration of nematodes on an estuarine tidalflat (the Molenplaat, Schelde estuary, SW Netherlands). Mar Ecol Prog Ser 224: 299-304. Link: http://bit.ly/2NKuk1i
- Gallucci F, Steyaert M, Moens T (2005) Can field distributions of marine predacious nematodes be explained by sediment constraints on their foraging success? Mar Ecol Prog Ser 304: 167-178. Link: http://bit.ly/2tw4jft
- Coull BC, Bell SS (1979) Perspectives of meiofaunal ecology. In. Livingston R.J. (Ed), Ecological Processes in Coastal and Marine Systems. New York: Plenum Press 189-216. Link: http://bit.ly/37hHMl4
- King CK, Dowse MC, Simpson SL, Jolley DF (2004) An assessment of five Australian polichaetes and bivalves for use in whole-sediments toxicity tests: toxicity and accumulation of copper and zinc from water and sediments. Arch Environ Contam Toxicol 47: 314-323. Link: http://bit.ly/2uof57i
- Maloney J (1996) Influence of organic enrichment on the partitioning and bioavailability of cadmium in a microcosm study. Mar Ecol Prog Ser 144: 147-161. Link: http://bit.ly/2NMajr2
- Semprucci F, Balsamo M (2012) Free-living marine nematodes as bioindicator: past, present and future perspectives. Environ Res J 6: 18-35. Link: https://go.aws/37gtZLJ
- Semprucci F, Frontalini F, Sbrocca C, du Châtelet EA, Bout-Roumazeilles V, et al. (2015) Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environ Monit Assess 187: 251. Link: http://bit.ly/38nKxBo
- Semprucci F, Colantoni P, Balsamo M (2016) Is maturity index an efficient tool to assess the effects of the physical disturbance on the marine nematode assemblages? A critical interpretation of disturbance-induced maturity successions in some study cases in Maldives. Acta Oceanologica Sinica 35: 89-98. Link: http://bit.ly/2RfGIbF
- Vanaverbeke J, Steyaert M, Soetaert K, Rosseau V, Van Gansbeke D, et al. (2004) Changes in structural and functional diversity of nematode communities during a spring phytoplankton bloom in the southern North Sea. J Sea Res 52: 281-292. Link: http://bit.ly/2NPJmTD

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
