Open Journal of Chemistry
Antioxidant activity assay and determination of phenolic and flavonoid content of Libho (Ficus Septica Burm. F) fruits
Yamin1*, Rina Andriani2, Sabarudin1, Nur Haijah1 and Henny Kasmawati1
2Department of Pharmacy, Mandala Waluya University, Indonesia
Cite this as
Yamin, Andriani R, Sabarudin, Haijah N, Kasmawati H (2022) Antioxidant activity assay and determination of phenolic and flavonoid content of Libho (Ficus Septica Burm. F) fruits. Open Journal of Chemistry 8(1): 008-013. DOI: 10.17352/ojc.000029Copyright
© 2022 Yamin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: One of the plants used in Indonesian traditional medicine Libho (Ficus Septica Burm. F) is traditionally used to treat some diseases, including malaria, diarrhea, diabetes, analgesic, antifungal, dysentery, anthelmintic, antioxidant, hemostatic and anti-inflammatory.
Purpose: The purpose of the study is to investigate the potential in vitro antioxidant activity assay and phytochemical content of Libho fruits.
Methods: Libho leaves powder was extracted with the maceration method Antioxidant activity was evaluated using ABTS cation and CUPRAC radicals. Total phenolic content was determined using the Folin-Ciocalteau method. Meanwhile, the total flavonoid content was determined using the aluminum chloride complex colorimetric method;
Results: Extract and fraction of Ficus Septica Burm. F fruits have the strongest antioxidant potential. The ethyl acetate fraction showed the strongest antioxidant activity on both ABTS and CUPRAC radicals with IC50 values of 6.33 ± 0.01 µg/mL and 11.64 ± 0.28 µg/mL, respectively. Ethyl acetate fraction also showed high phenolic and flavonoid content with values of 28 ± 0.05 mg GAE/100 mg sample and 43.08 ± 0.48 mg QE/100 mg sample, respectively.
Conclusion: Ethyl acetate fraction has the potential to be used as a source of natural antioxidants and has the potential to be used as a nutraceutical.
Background
Free radicals are molecules or compounds with one unpaired electron in their outer shell, making them relatively unstable and highly reactive. Because of their reactive nature, free radicals are always trying to attract electrons from other molecules or other cells in the body that are around them. Its ability to oxidize other molecules can result in oxidative damage in the body. One example of free radicals is reactive oxygen species (ROS). ROS can react and disrupt macromolecules, such as proteins, lipids and nucleic acids in the body. If it cannot stop the damage caused by ROS, it causes oxidative stress [1,2]. Oxidative stress is an essential factor in various types of pathogens in chronic disease. As a result, oxidative stress is the causative agent of various diseases such as arthritis, cancer, asthma, atherosclerosis, diabetes and Parkinson’s and Alzheimer’s diseases [3,4].
Antioxidants are molecules or compounds that play a role in preventing and stabilizing damage caused by free radicals by donating electrons or protons from antioxidant molecules to damaged cells. Antioxidants can also convert free radicals into waste products, which are excreted from the body [5-7]. Antioxidant compounds such as phenolics, flavonoids, and polyphenols can scavenge free radicals such as hydroperoxides, peroxides, or lipid peroxyl so that they can inhibit oxidative mechanisms that cause degenerative diseases [7-9]. Natural sources have been considered good antioxidants since ancient times. Plants in traditional medicine are used to treat chronic and infectious diseases [10,11]. The abundance of plant materials that exist in nature causes the interest of researchers to seek new medicinal sources from plants to increase. The plant contains chemical components that can provide certain physiological effects on the human body, such as alkaloids, flavonoids, tannins and other phenolic groups [12,13]. Traditional medicines from plants and their extracts, usually used as a treatment for various infectious diseases, are being modified and refined into modern formulations that can be used in various diseases such as ischemic heart disease, diabetes, atherosclerosis, liver disease and initiation carcinogenesis [14] Libho (Ficus Septica Burm. F) is one of the plants used in traditional medicine, among others, as an effective therapy in treating hypertension, liver, and diabetes. Degenerative diseases such as cancer and aging are caused too often exposed to free radicals [15],. This plant reported secondary metabolites, including alkaloids, tannins, saponins, terpenoids, and flavonoids [16]. The flavonoid group is found in E. odoratum L plants, is flavonols, flavanone and chalcones. In addition, it also contains phenolic acids, such as ferulic acid and protopathic acid [17]. The presence of phenolic compounds in the Libho plant allows it to have the potential as an antioxidant. Because the antioxidant activity in plants is generally related to the total phenolic and flavonoid content [18]. The ability of phenolic and flavonoid compounds as antioxidants is due to the ability of flavonoid and phenolic compounds to donate hydrogen atoms both in the form of glycosides and in the free state or aglycones [19]. This study aimed to evaluate the ability of phenolics and total flavonoids as antioxidants.
Materials and methods
Plant materials
Fresh Libho (Ficus Septica Burm. F) fruits collected from Kabangka District, Muna Regency, Southeast Sulawesi Province, Indonesia in September - October 2020. Fruits material was authenticated at the Laboratory of Biology, FKIP, University of Halu Oleo, Indonesia by Mrs. Murni Sabilu and deposited in the herbarium of the same laboratory with a voucher specimen number BIO 268 (Figure 1), The fresh fruits (10 kg) were washed under running tap water to remove sand and debris and were airdried under shade for 10 days. The final weight after drying was 1.4 kg and powdered using a mechanical grinder. DPPH (Sigma-Aldrich®, USA), ABTS (Sigma-Aldrich®, USA), Galic Acid (Sigma-Aldrich®, USA), Quercetin (Sigma- Aldrich®, USA), methanol (E. Merck, Germany), ethyl acetate (E. Merck, Germany), chloroform (E. Merck, Germany), n-hexane (E. Merck, Germany) and equates.
Extraction
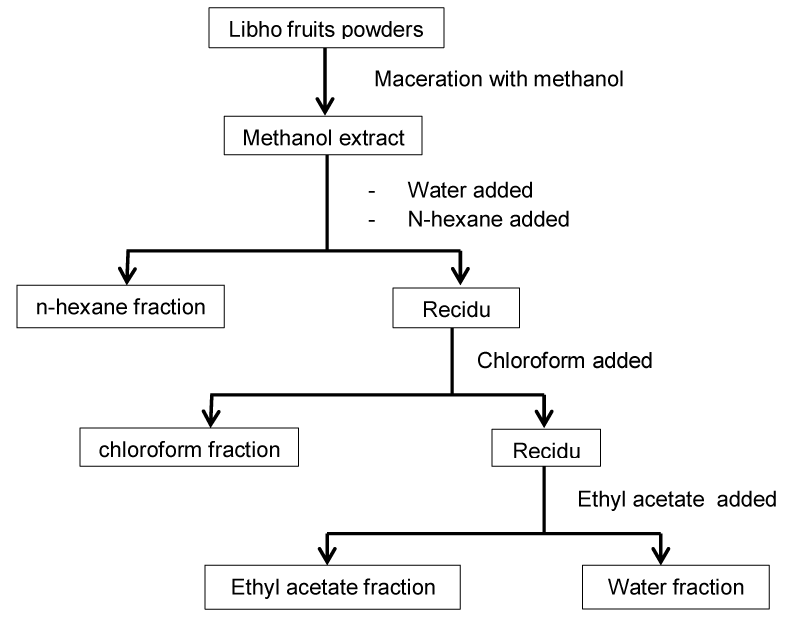
Seven hundred and fifty grams of Libho fruit powder was macerated with 10 L methanol for 72 hours. The extract obtained was concentrated with a rotary evaporator to obtain crude extract. The crude extract (105 g). after the 90-gram crude extract was partitioned with the aid of a separating funnel into hexane-soluble (24 g), chloroform soluble (17.2 g), ethyl acetate soluble (16.6 g) and water fractions (35.9 g). the extraction and fractionation scheme is shown in Figure 2.
Antioxidant activity test by ABTS cation method
Determination of the antioxidant properties of the sample using the ABTS method, the ABTS cation radical was first prepared following the method [20,21], namely, making seven mM ABTS solutions, namely by dissolving 18 mg ABTS in 5 mL solution, then added 5 mL of potassium persulfate solution, incubated in a dark room at 23 0C for 16 hours before use, resulting in ABTS with a dark blue color. Measure the antioxidant capacity of samples with ABTS radicals was carried out by adding 3 mL of methanol to 1 mL of sample and 1 mL of ABTS cation radical solution. Shake until homogeneous and incubated in the dark for 10 minutes at room temperature. The absorbance of the solution was measured at 745 nm.
The formula calculates radical scavenging activity
The IC50 value is obtained by replacing y with 50 in the linear regression equation y = bx + a so that the value of x is obtained. the value of x is the IC50 value of the sample.
Antioxidant activity assay by CUPRAC method
Cupric Reducing Antioxidant Capacity (CUPRAC) was evaluated by following the method [22,23] with some modifications, using copper(II)- neocuproine reagent as the chromogenic oxidizing agent and ascorbic acid as the standard. This assay carefully mixed 1 ml of the sample with 1 ml of neocuproine (7.5 mM) and CuCl2 (10 mM) reagents, and the absorbance was measured at 450 nm after 30 min.
Measurement of total phenolic content
Extracts and fractions were analyzed for TPC by the Folin-Ciocalteau assay as described by [24-26] using gallic acid as standard. Briefly, put 1 ml of the sample in a test tube, and then 0.4 mL of Folin- Ciocalteau reagent was added, then allowed to stand for 5-8 minutes. Then 4 mL of 7% Na2CO3 solution was added and then shaken until homogeneous. Then it was allowed to stand for 30 minutes at room temperature, and then the absorbance was measured using a UV-Vis spectrophotometer at 750 nm. The total phenolic content was calculated as milligrams of gallic acid equivalent (mg GAE/gram sample).
Determination of total flavonoids
Measurement of flavonoid content using the aluminum chloride method, as described by [27-29], with minor modifications. Briefly, added as much as 1 ml of the sample and 3 mL of methanol p.a, then 0.2 mL of 10% AlCl3 and 0.2 mL of 1 M potassium acetate. Then made up 10 ml with distilled water. Shake until homogeneous and incubated at room temperature for 30 minutes. Then the absorbance was measured using a spectrophotometer at 417 nm. The total flavonoid content was calculated as milligrams of quercetin equivalent (mg QE/gram sample).
Data analysis
All data were represented as mean and standard deviation of three parallel measurements and analyzed with SPSS version 16.0 (SPSS, Chicago, IL, USA) and Microsoft Excel (American corps). The difference between the means was calculated by one-way analysis of variance and was considered significant if p < 0.05. The antioxidant activity test was carried out in at least three replications. Pearson correlation coefficient (r) was determined to determine the relationship between phenolic and flavonoid content and antioxidant activity.
Results and discussion
Antioxidant activity assay by ABTS method
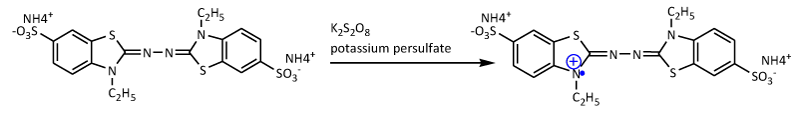
Determination of antioxidant strength using the ABTS method is obtained from the oxidation of potassium persulfate with ABTS salt [30], as shown in Figure 3. This method measures the relative ability of antioxidants to scavenge ABTS. The ABTS method has greater sensitivity in detecting antioxidant capacity because its response to antioxidants is higher than the DPPH method and has faster reaction kinetics [31]. The principle of the ABTS method is based on the ability of antioxidant compounds to stabilize radicals by donating protons to radical compounds. The process of capturing protons by radical compounds is characterized by a change in the color of the radical solution from blue to colorless. The speed of decreasing color intensity indicates the strength of antioxidant compounds in reducing free radicals [2,32,33]. ABTS assay can be carried out on lipophilic and hydrophilic compounds observed at 750 nm. Based on the results of the ABTS method, showed that the extract and fraction of Libho fruits had very strong radical scavenging abilities, as shown in Table 1.
Table 1 shows that the ethyl acetate fraction has the strongest antioxidant activity compared to the methanol extract, chloroform, n-hexane, and water fractions, with IC50 values of 6.33 ± 0.01 µg/mL (ethyl acetate fraction), 6.76 ± 0.04 µg/mL (methanol extract), 6.83 ± 0.02 µg/mL (chloroform fraction), 7.17 ± 0.02 µg/mL (n-hexane fraction), and 10.24 ± 0.05µg/mL (water fraction), respectively. Ascorbic acid was used as standard. This is in line with several studies showing that the ethyl acetate fraction has very strong antioxidant potential, including the ethyl acetate fraction of Clerodendrum cyrtophyllum Turcz leaves [34], and the ethyl acetate fraction of Rambutan (Nephelium lappaceum L) peel [20].
Antioxidant activity assay with the CUPRAC method
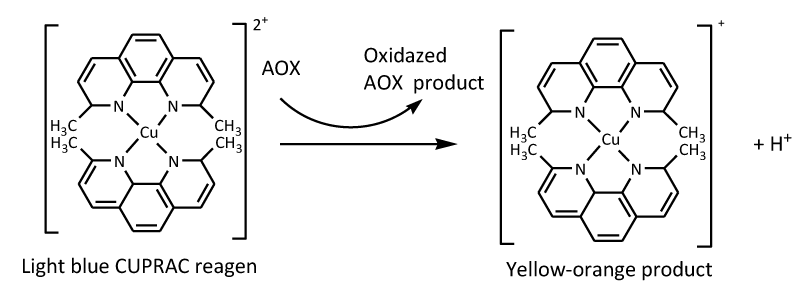
Another method used to measure the antioxidant activity of Libho fruits is the CUPRAC method. This method uses copper neocuproine (2,9-dimethyl-1,10-phenanthroline) chelate abbreviated (Cu(II)-Nc ) as a chromogenic oxidant [35,36]. An antioxidant reduces Cu (II) to Cu (I) in this test. The chromophore Neocuproine (Nc) reacts with CuCl2 to form a Cu(I)–Nc complex at pH 7 at a wavelength of 450 nm. The reaction of chromogenic reagents with n-electron reductant (AO) of antioxidant compounds produces a stable yellow-orange color within 30 minutes.
The chromogenic oxidizing reagent of the developed CUPRAC method, namely, Cu(II)-Nc, reacts with an n-electron reducing antioxidant (AOX) [37], as shown in Figure 4.
The data in Table 1 shows that the extract and fraction of Libho fruits have a very strong ability to reduce Cu (II) to Cu (I). The data in Table 1 shows that the ethyl acetate fraction has stronger antioxidant activity than methanol extract, chloroform fraction, n-hexane fraction and water fractions, with IC50 values of 5.05 ± 0.02 g/mL (ethyl acetate fraction), 6.09 ± 0.06 g/mL (methanol extract), 7.71 ± 0.05 g/mL (chloroform fraction), 9.01 ± 0.12 g/mL (n-hexane fraction), and 11.91 ± 0.3 (water fraction), respectively, Ascorbic acid was used as standard. This is in line with several studies showing that the ethyl acetate fraction has very strong antioxidant potential, including the ethyl acetate fraction of Nicotiana glauca leaves [38].
Determination of total phenolic and flavonoid contents
The flavonoid compounds in the fruits of Libho were measured by adding hydrochloric acid and aluminum powder. This addition aims to reduce the benzopyrene core in the flavonoid structure so that the color changes to orange and red [39]. Changes in color to red and orange indicate the formation of a complex between the hydroxyl group and the ketone or hydroxyl group in the ortho position. Meanwhile, the addition of sodium acetate to detect the presence of a 7-hydroxy group indicates a flavonol group [40]. Therefore, in this study, quercetin was used as a standard.
Based on the data, Table 2 shows that the flavonoid content at extract and fraction of Libho fruit with value, 43.08 ± 0.48 mg QE/100 mg sample (ethyl acetate fraction), 28.46 ±
0.08 mg QE/100 mg sample (methanol extract), 23.67 ± 0.42 mg QE/100 mg sample (chloroform fraction), 22.13 ± 0,47 mg QE/100 mg sample (n-hexane fraction) and 14.41 ± 0.42 mg QE/100 mg sample (water fraction), respectively.
Determination of phenolic content in Libho fruits using the Folin-Ciocalteau method. The principle of this method is the formation of complex compounds that are blue. The formation of this blue color occurs because the Folin-Ciocalteau reagent oxidizes the phenolic group (alkali salt) or the phenolic group reduces heteropoly acid (phosphomolybdate-phosphotungstic) into a molybdenum-tungsten complex. The reaction between phenol compounds and the Folin-Ciocalteau reagent can only occur in an alkaline environment. As a result, protons dissociate in phenolic compounds into phenolic ions. The greater the concentration of phenolic compounds in the sample, the more phenolic ions that will reduce heteropoly acid (phosphomolybdate phosphotungstic) to a molybdenum-tungsten complex so that the resulting blue color is more intense [40].
Table 2 shows that the phenolic content in Libho fruit with value, 28 ± 0.05 mg GAE/100 mg sample (ethyl acetate fraction), 26.05 ± 0.19 mg GAE/100 mg sample (chloroform fraction), 23.32 ± 0.11 mg GAE/100 mg sample (methanol extract), 23 ± 0.19 mg GAE/100 mg sample (n-hexane fraction) and 7.6 ± 0.12 mg GAE/100 mg sample (water fraction), respectively.
The high content of phenolic and flavonoids in an ordinary sample correlates with the antioxidant activity of natural compounds. This is due to the presence of hydroxy groups in flavonoids and phenolics. The hydroxy group contributes to scavenging radicals or reducing radicals because the hydroxy group compounds in phenols and flavonoids have redox properties and high hydrogen mobility capabilities in their molecular structure [41-43]. In addition, the presence of hydroxyl groups, and o-hydroxy groups in phenolic and flavonoid compounds, greatly influences free radical scavenging. In addition, polyphenolic compounds such as flavonoids, an ortho-dihydroxy structure in ring B, 2,3 double bonds in conjugation with 4-oxo in ring C, hydroxy groups at positions 3 and 5 on ring A, or the angle between the rings in the compound structure causes the antioxidant effect [44-47].
The correlation between the phenolic and flavonoid content in the sample shows that the phenolic and flavonoid levels simultaneously provide a very strong relationship in ABTS cation radical scavenging. Based on the analysis results using SPSS 16.0, partially flavonoid compounds did not significantly influence the stabilization of ABTS cation radicals. As indicated by the significance value of 0.934 > 0.05. Meanwhile, the total phenolic content partially contributed to the radical stabilization of ABTS. This contribution is shown by the statistical analysis results, where the significance value is 0.024 < 0.05. Meanwhile, the simultaneous contribution of phenolic compounds and flavonoids in stabilizing ABTS radicals, based on the results of data analysis, showed that phenolic compounds and flavonoids simultaneously gave the strongest contributions to stabilizing ABTS cation radicals with a value of R2 = 0.959. This indicates that the contribution of phenolic compounds and flavonoids simultaneously in stabilizing ABTS radicals contributes 95.9%. While other compounds besides phenolic and flavonoid influenced 4.1%. Meanwhile, phenolic compounds and flavonoids did not show a significant correlation in reducing CUPRAC radicals. This is indicated by the results of statistical analysis either partially or simultaneously. Partially, the correlation between phenolic compounds in reducing CUPRAC radicals was 0.46 > 0.05, and the correlation between flavonoid compounds in reducing CUPRAC radicals was 0.33 > 0.05. while simultaneously, the correlation of phenolic compounds and flavonoids in reducing CUPRAC radicals was 0.82 > 0.05.
Conclusion
Extracts and fractions of Libho (Ficus Septica Burm. F) fruits showed antiradical activity using the ABTS and CUPRAC radical methods. Among the samples evaluated, the n-hexane fraction of Libho fruits showed very strong antioxidant activity and very high phenolic and flavonoid content Libho fruits can be developed as a source of antioxidants from natural ingredients.
Gratitude is due to the Rector of Universitas Halu Oleh, who has funded this research through DIPA Halu Oleo University in the Basic Research Program, 2020
- Rahman MM, Islam MB, Biswas M, Khurshid Alam AH. in vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015 Oct 30;8:621. doi: 10.1186/s13104-015-1618-6. PMID: 26518275; PMCID: PMC4627625.
- Sukweenadhi J, Yunita O, Setiawan F, Siagian MT, Danduru AP, Avanti C. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas. 2020; 21(5): 2062-2067. https://doi.org/10.13057/biodiv/d210532
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015 Jan;30(1):11-26. doi: 10.1007/s12291-014-0446-0. Epub 2014 Jul 15. PMID: 25646037; PMCID: PMC4310837.
- Mahdi-Pour B, Jothy SL, Latha LY, Chen Y, Sasidharan S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac J Trop Biomed. 2012 Dec;2(12):960-5. doi: 10.1016/S2221-1691(13)60007-6. PMID: 23593576; PMCID: PMC3621472.
- Hamid K, Saha MR, Urmi KF, Habib MR, Rahman MM. Screening of different parts of the plant Pandanus odorus for its antioxidant activity. International Journal of Applied Biology and Pharmaceutical Technology. 2010; 1(3):1364-1368.
- Bukharov AF, Barakat ISA, Hammouri MK, Habib I, Popkova MA, Budnikova NV. Antioxidant vitamins and their effect on immune system. Journal of Physics: Conference Series. 2021; 1853:1-2. https://doi.org/10.1088/1742- 6596/1853/1/012065
- Parcheta M, Świsłocka R, Orzechowska S, Akimowicz M, Choińska R, Lewandowski W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials (Basel). 2021 Apr 15;14(8):1984. doi: 10.3390/ma14081984. PMID: 33921014; PMCID: PMC8071393.
- Wu YY, Li W, Xu Y, Jin EH, Tu YY. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J Zhejiang Univ Sci B. 2011 Sep;12(9):744-51. doi: 10.1631/jzus.B1100041. PMID: 21887850; PMCID: PMC3167908.
- Muflihah YM, Gollavelli G, Ling YC. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants (Basel). 2021 Sep 27;10(10):1530. doi: 10.3390/antiox10101530. PMID: 34679665; PMCID: PMC8533117.
- Ramesh P, Subramani A. Effect of antimicrobial activity of Eupatorium odoratum against clinical microbes. International Journal of Scientific Research in Biological Sciences. 2018; 5(5): 30-35.
- Akhtar N, Nasir A, Parichener H. A Review of Globally Important Plant Origin Antioxidants and their Folk Utility for Various Diseases A Review of Globally Important Plant Origin Antioxidants and their Folk Utility for. Journal of Bioresource Management. 2021; 8(4):144-154.
- Ifeanyi N, Chinanu A, Upabi F, Ukogo C, Ifeoma. In-Vitro Antibacterial Effect of Crude Extract of Chromolaena Odorata Leaves on Wound Isolates. IOSR Journal of Pharmacy and Biological Sciences. 2016; 11(6):49-52. https://doi.org/10.9790/3008-1106024952.
- Aneklaphakij C, Saigo T, Watanabe M, Naake T, Fernie AR, Bunsupa S, Satitpatipan V, Tohge T. Diversity of Chemical Structures and Biosynthesis of Polyphenols in Nut-Bearing Species. Front Plant Sci. 2021 Apr 6;12:642581. doi: 10.3389/fpls.2021.642581. PMID: 33889165; PMCID: PMC8056029.
- Rajasree RS, Ittiyavirah SP, Naseef PP, Kuruniyan MS. An Evaluation of the Antioxidant Activity of a Methanolic Extract of Cucumis melo L. Fruit (F1 Hybrid). Separations. 2021; 8(123):1-15. https://doi.org/10.3390/separations8080123
- Utami NE, Salni, Verawaty M. Antioxidant activity leaves katimaha (Kleinhovia hospita L.). BIOVALENTIA: Biological Research Journal. 2022; 8(1): 22–31.
- Putri DA, Fatmawati S. A New Flavanone as a Potent Antioxidant Isolated from Chromolaena odorata L. Leaves. Evid Based Complement Alternat Med. 2019 Jun 18;2019:1453612. doi: 10.1155/2019/1453612. PMID: 31316568; PMCID: PMC6604423.
- Maulida PA, Putri DA, Fatmawati S. Free Radical Scavenging Activity of Chromolaena odorata L. Leaves. IPTEK The Journal for Technology and Science. 2019; 30(3):73-75.
- Wojdyło A, Oszmian´ski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry. 2007; 105:942-949. https://doi.org/10.1016/j.foodchem.2007.04.038
- Hayati EK, Ningsih R, Latifah. Antioxidant Activity of Flavonoid from Rhizome Kaemferia galanga L . Extract. ALCHEMY: Journal of Chemistry. 2015; 4(2):127-137.
- Riyanto MS, Rohman A. Antioxidant activities of rambutan (Nephelium lappaceum L) peel in vitro. Food Research. 2018; 2(1):119-123. https://doi.org/10.26656/fr.2017.2(1).150
- Olszowy M, Dawidowicz AL. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds ? Chemical Papers. 2018; 72(2):393-400. https://doi.org/10.1007/s11696-017-0288-3
- Apak R, Güçlü K, Ozyürek M, Esin Karademir S, Erçağ E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr. 2006 Aug-Sep;57(5-6):292-304. doi: 10.1080/09637480600798132. PMID: 17135020.
- Diniyah N, Alam B, Lee S. Antioxidant potential of non-oil seed legumes of Indonesian ’ s ethnobotanical extracts. Arabian Journal of Chemistry. 2020; 13(5):5208-5217. https://doi.org/10.1016/j.arabjc.2020.02.019
- Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants (Basel). 2019 Apr 11;8(4):96. doi: 10.3390/plants8040096. PMID: 30978964; PMCID: PMC6524357.
- Ruslin S, Zubaydah WOS, Sartinah A, Buton S, Yamin. Antiradical activity, total phenolic, and total flavonoids extract and fractions of pumpkin (Cucurbita moshata Duch) leaves. Food Research. 2021; 5(2):348-353. https://doi.org/0.26656/fr.2017.5(2).529
- Ruslin S, Andriani M, Manangkara R, Mistriyani M, Yamin. in vitro antioxidant activity test and determination of phenolic and flavonoid content of Moringa oleifera pulp and seeds. Food Research. 2021; 59-65. https://doi.org/10.26656/fr.2017.5(4).033
- Bag GC, Devi PG, Bhaigyabati T. Assessment of Total Flavonoid Content and Antioxidant Activity of Methanolic Rhizome Extract of Three Hedychium Species of Manipur Valley. Int. J. Pharm. Sci. Rev. Res. 2015; 30(28):154-159.
- Sembiring EN, Elya B, Sauriasari R. Phytochemical Screening , Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc ( L .) Roxb. Pharmacognosy Journal. 2018; 10(1):123-127. https://doi.org/10.5530/pj.2018.1.22
- Zubaydah WOS, Sahumena MH, Fatimah WON, Sabarudin AM, Yamin. Determination of antiradical activity and phenolic and flavonoid contents of extracts and fractions of jackfruit ( Artocarpus heterophyllus Lamk ) seeds. Food Research.2021; 5(3):36-43. https://doi.org/10.26656/fr.2017.5(3).563
- Xiao F, Xu T, Lu B, Liu R. Guidelines for antioxidant assays for food components. Food Frontiers. 2020; 60-69. https://doi.org/10.1002/fft2.10
- Wahyuni, Diantini A, Ghozali M, Subarnas A, Julaeha E, Amalia R, Sahidin I. Phytochemical Screening, Toxicity Activity and Antioxidant Capacity of Ethanolic Extract of Etlingera alba Rhizome. Pak J Biol Sci. 2021 Jan;24(7):807-814. doi: 10.3923/pjbs.2021.807.814. PMID: 34486300.
- Hidayati MD, Ersam T, Shimizu K, Fatmawati S. Antioxidant Activity of Syzygium polynthum Extracts. Indonesian Journal of Chemistry. 2017; 17(1):49-53. https://doi.org/10.22146/ijc.23545
- Kyriazis ID, Skaperda ZOI, Tekos F, Makri S, Vardakas P, Vassi E, Patouna A, Terizi K, Angelakis C, Kouretas D.. Methodology for the biofunctional assessment of honey ( Review ). International Journal of Functional Nutrition. 2021; 2(5):1-11. https://doi.org/10.3892/ijfn.2021.15
- Zhou J, Yang Q, Zhu X, Lin T, Hao D, Xu J. Antioxidant activities of Clerodendrum cyrtophyllum Turcz leaf extracts and their major components. PLoS One. 2020 Jun 23;15(6):e0234435. doi: 10.1371/journal.pone.0234435. PMID: 32574221; PMCID: PMC7310832.
- Rafi M, Meitary N, Septaningsih DA, Bintang M, Rafi M. Phytochemical Profile and Antioxidant Activity of Guazuma ulmifolia Leaves Extracts Using Different Solvent Extraction. Indonesian Journal of Pharmacy. 2020; 31(3):171-180. https://doi.org/10.22146/ijp.598
- Tihauan B, Marinas I, Bleotu C, Dolete G, Onisei T, Serbancea F. Original paper Evaluation of cytotoxicity , nutritional and anti- oxidative status of lyophilized plant extracts used in dietary supplements. Romanian Biotechnological Letters. 2021; 26(6):2396-2405. https://doi.org/10.25083/rbl/26.2/2396.2405
- Munteanu IG, Apetrei C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int J Mol Sci. 2021 Mar 25;22(7):3380. doi: 10.3390/ijms22073380. PMID: 33806141; PMCID: PMC8037236.
- Trifa W, Akkal S, Lefahal M. Preliminary screening of Nicotiana glauca extracts for determination of antioxidant activity by different methods. Current Issues in Pharmacy and Medical Sciences. 2020; 33(1):32-37. https://doi.org/10.2478/cipms-2020- 0007
- Nur S, Mubarak F, Jannah C, Winarni DA, Rahman DA, Hamdayani LA, Sami FJ. Total phenolic and flavonoid compounds, antioxidant and toxicity profile of extract and fractions of paku atai tuber (Angiopteris ferox Copel). Food Research. 2019; 3(6): 734-740.
- Rahman NF, Megawati N, Handayani, Suares CAM. Total Phenolic and Flavonoid Contents and Antioxidant Activity of Kembang Bulan Leaves (Tithonia diversifolia (Hemsley) A. Gray). Indonesian Journal of Pharmaceutical Science and Technology. 2021; 1(1):57-65.
- Arina NB, Rohman A. The phenolic contents and antiradical activity of Indonesian. International Food Research Journal. 2013; 20(3):1119-1124.
- Lewoyehu M, Amare M. Comparative evaluation of analytical methods for determining the antioxidant activities of honey: A review. Cogent Food & Agriculture. 2019; 5(1):1-24. https://doi.org/10.1080/23311932.2019.1685059
- Chen Z, Liu Q, Zhao Z, Bai B, Sun Z, Cai L, Fu Y, Ma Y, Wang Q, Xi G. Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one to scavenge free radicals. RSC Adv. 2021 Oct 25;11(55):34456-34461. doi: 10.1039/d1ra06317k. PMID: 35494787; PMCID: PMC9042674.
- Aadesariya MK, Ram VR, Dave PN. Evaluation of antioxidant activities by use of various extracts from abutilon pannosum and grewia tenax in the kachchh region. MOJ Food Processing & Technology. 2017; 5(1):216-230. https://doi.org/10.15406/mojfpt.2017.05.00116
- Rusmana D, Wahyudianingsih R, Elisabeth M, Maesaroh B, Widowati W. Antioxidant Activity of Phyllanthus niruri Extract, Rutin and Quercetin. The Indonesian Biomedical Journal. 2017; 9(2):84-90. https://doi.org/10.18585/inabj.v9i2.281
- Fan M, Chen G, Zhang Y, Nahar L, Sarker SD, Hu G, Guo M. Antioxidant and Anti-Proliferative Properties of Hagenia abyssinica Roots and Their Potentially Active Components. Antioxidants (Basel). 2020 Feb 6;9(2):143. doi: 10.3390/antiox9020143. PMID: 32041310; PMCID: PMC7070924.
- Antão AR, Bangay G, Domínguez-Martín EM, Díaz-Lanza AM, Ríjo P. Plectranthus ecklonii Benth: A Comprehensive Review Into its Phytochemistry and Exerted Biological Activities. Front Pharmacol. 2021 Nov 30;12:768268. doi: 10.3389/fphar.2021.768268. PMID: 34916943; PMCID: PMC8670309.
- Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity / activity ( IUPAC Technical Report )*. Pure and Applied Chemistry. 2013; 85(5):957-998. https://doi.org/10.1351/PAC- REP-12-07-15
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
