Open Journal of Chemistry
Biodegradable masterbatch blends: The implications on thermal conversion and recycling stream of polyethylene
João Vitor Ferreira Duque1-3, Márcio Ferreira Martins3* and Marcos Tadeu D’Azeredo Orlando3
2Institut de Mécanique des Fluides de Toulouse (IMFT) - Université de Toulouse, CNRS-INPT-UPS, Toulouse, France
3Laboratory of Combustion and Combustible Matter (LCC), PPGEM, Federal University of Espírito Santo, Vitória, Brazil
Cite this as
Ferreira Duque JV, Martins MF, D’Azeredo Orlando MT (2020) Biodegradable masterbatch blends: The implications on thermal conversion and recycling stream of polyethylene. Open Journal of Chemistry 6(1): 008-010. DOI: 10.17352/ojc.000016In this case report, we are dealing with the implications of blending fossil LDPE with biodegradable masterbatches on the thermal conversion and recycling stream of Low-Density Polyethylene (LDPE). Two types of commercial masterbatch are evaluated against a pure LDPE. The thermal degradation study was carried out by using thermogravimetric analysis guided by X-ray diffraction, ultimate and proximate analyses. When compared with the LDPE pyrolysis, the conversion of the blend of thermoplastic starch and Polyethylene happens in a wider temperature range, involving multi-step reactions. In contrast, the pyrolysis of iron-based-oxo-biodegradable masterbatch leaves behind 30 wt.% of an unconverted mass. Even though the blend of organic additives with fossil polyethylene might aid the biodegradability of plastics disposed of in the environment, some drawbacks should be accounted for the plastic life cycle.
Introduction
During the past two decades, significant advances have been made in the development of biodegradable polymeric materials for many applications such as in packaging and hygiene products [1,2]. Still, they accumulate in the environment as these materials are not readily biodegradable and because of their resistance to microbial degradation [3]. Among the many classes of polymers, are the blend of conventional plastic with different commercial biodegradable masterbatches [4,5] and the compostable plastics fully derived from renewable agricultural resources [6]. The blends intend to confer suitable performance during service life and short degradation times during subsequent disposal, and the compostables are supposed to break down to basic elemental components with the aid of microorganisms.
If on the one hand, the biodegradable masterbatch can be blended with a wide range of polyolefin materials to tailor a product with properties perfectly matched to the application, on the other, they break the material recycling cycle since they actually only fragment the plastics. The fragmented plastics arises the migration, via rivers to the ocean, of the often called “micro-plastics” [7]. Also, some conventional masterbatch enters the market as a mixture (iron, stearate, oxides, etc.) to design a polymer labeled biodegradable as well and their fragments are spread on the environment. In this case report, we are dealing with the implications of blending fossil LDPE with biodegradable masterbatches on pyrolysis. Two types of commercial masterbatch are evaluated against a pure fossil LDPE.
Material and methods
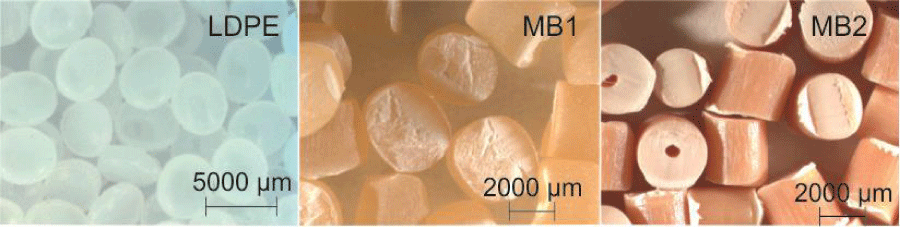
The materials used were samples of Low-Density Polyethylene (LDPE), button type geometry with an average diameter of 4 mm, and two types of approximately equal length and radius cylindrical geometry masterbatches. All these samples are commercial and commonly used for the local manufacturers for making packaging. The first masterbatch is a blend of thermoplastic starch (66 wt.%) with LDPE (MB1). The second one is, so-called by the manufacturer, an environmentally friendly iron-based/oxo-biodegradable additive (MB2) in which the mixture proportions are not revealed. The LDPE has no pigmentation and the masterbatches are pigmented (Figure 1).
The mutual physical properties provided in the materials data sheet are Melt Flow Rate (ASTM D1238) and Density (ASTM D792), 1.4 g /10 min and 0.921 g/cm 3 for LDPE, 1.2 g/10 min and 1.18 g/cm3 for MB1, and 20-25 g/10min and 1.02 g/cm3 for MB2 respectively.
The thermal degradation study was carried out by using thermogravimetric analysis (TGA). X-ray diffraction (XRD), ultimate and proximate analyses were applied to investigate if the samples could have a certain degree of differentiation either on composition and chemical structure arrangement. The full configuration of the experiments is detailed in Table 1 [3].
Results
The LDPE sample is a high-quality polymer, its proximate and ultimate analysis indicated only the presence of carbon and hydrogen, 85.7 wt.% and 14.3 wt.% respectively. The blended sample (MB1) contains 57.2 wt.% of C, 9.8 wt.% of H, and 33.0 wt.% of other elements, in which 98.35 wt.% are volatile material. The environmental friendly masterbatch, MB2, has 83.24 wt.% of volatile material distributes in 63.6 wt.% of C, 9.6 wt.% of H, and 26.8 wt.% of other elements.
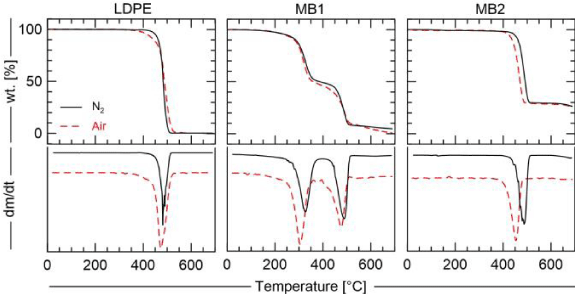
TGA and DTG results under inert and oxidative atmospheres are summarized in Fig. 2. Under both atmospheres, the LDPE presents similar thermochemical behavior, one conversion step between 325 °C and 525 °C where the mass is totally converted. The supposed biodegradable sample, MB2, presented one conversion step between 375 °C and 525 °C leaving about 30 wt.% of an unconverted matter. Another reaction (inorganic one) has the potential to occur greater than 650 0C. The blended sample, MB1, has a more complex conversion chemical pathway. Under N2 two main mass losses converting almost 97 wt.% of the total mass, and a third slow rate reaction starting about 500 °C are observed. Still for MB1, when undergoing oxidation, the two main blocks of compounds released in pyrolysis are oxidized. The first block can be approximated to one oxidation reaction step, and for the second block first occurs less strong oxidation that is partially covered by the main one. The last mass loss is observed starting as well as about 500 °C (Figure 2).
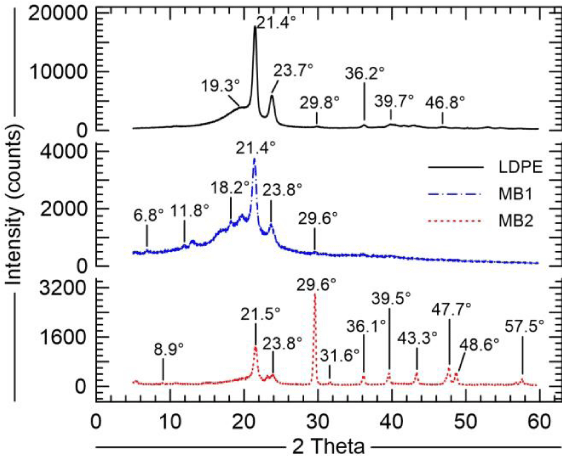
Figure 3 shows the X-ray powder diffraction patterns for all samples. The diffractograms of MB1 and MB2 showing the diffraction peaks characteristic of the orthorhombic cell of polyethylene are very similar to that of LDPE. This confirms the presence of the main compound forming the LDPE in the masterbatch samples. The essential difference of the observations is the amorphous wide peak, centered at around 19.5 degrees, which is not observed in the MB2 sample. Also, the MB2, according to the manufacture, is an iron-based/oxo-biodegradable additive a strong peak centered at around 29 degrees and successive small ones are observed. The average crystallinity was 48% for LDPE. For the masterbatches, as they are blends, another methodology different from the used for pure LDPE should be applied to allow evaluating their degree of crystallinity.
The apparent relative reduction in the crystallinity for MB1 and MB2 may be explained on the basis of the proportion of LDPE in the blends, just 34 wt.% of LDPE in MB1, and for MB2, as shown in TG analysis, the proportion of the inorganic matter was important suggesting less than 1/3 in wt. basis of LDPE in MB2.
Final remarks
The former results reveal some implications. The ash content observed on proximate and TGA analysis is significant if we think the catalytic effect on the thermal conversion that such not converted components can promote at temperatures high as 700 °C. This is an important consideration since the samples, MB1 and MB2, are supposed to be used as a mixture with pure or recycled polyethylenes, and even if the MB2 should be mixed no more than 2 wt.% in the mixture. Note that, small quantities of some oxides in ash, or even ingredients widely used in masterbatch mixtures such as glycerol, sorbitol might have a catalytic effect on thermochemical conversion.
When compared with the LDPE pyrolysis, the conversion of the blend of thermoplastic starch and Polyethylene (MB1) happens in a wider temperature range, involving multi-step reactions, which suggest an increase in the overall pyrolysis enthalpy. In contrast, the pyrolysis of iron-based-oxo-biodegradable masterbatch (MB2) leaves behind a large amount of mass not converted material suggesting the presence of an important amount of inorganic material in its composition. Even though the blend of organic additives with fossil polyethylene might aid the biodegradability of plastics disposed of in the environment, some drawbacks should be accounted for the plastic life cycle: -the impact of this blends in the plastic recycling stream; - and the intensive use of agriculture for bioplastics manufacturing.
Looking ahead, if the main goal of this blends brings only plastic fragments, the process of collecting the debris becomes unviable breaking down the recycling cycle. Concerning, the intensive use of agriculture, even if this industry is toward the use of agriculture residues, it might induce competition with the biomass to energy industry.
- Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Progress in Polymer Science 32: 762-798. Link: https://bit.ly/2UTEfUU
- Laycock (2017) Lifetime prediction of biodegradable polymers Progress in Polymer Science 71: 144-189. Link: https://bit.ly/2UFx9EC
- Duque (2020) The influence of the recycling stress history on LDPE waste pyrolysis. Polymer Testing 86: 106460. Link: https://bit.ly/2xOEWaa
- Chenetal (2018) Optimizationof thepreparationprocess of biodegradable masterbatches and characterization of their rheological and application properties. Polymer Testing 70: 526-532. Link: https://bit.ly/2V1z6ug
- Peres AM, Pires RR, Orefice RL (2016) Evaluation of the effect of reprocessing on the structure and properties of low density polyethylene/thermoplastic starch blends. Carbohydrate polymers 136: 210-215. Link: https://bit.ly/2wTBbAu
- Lorcks J (1998) Properties and applications of compostable starch-based plastic material. Polymer degradation and stability 59: 245-249. Link: https://bit.ly/39AwB7q
- Haider (2019) Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angewandte Chemie International Edition 58: 50-62. Link: https://bit.ly/3dVWQs6
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
