Open Journal of Chemistry
Alzheimer’s disease and its current treatments; Is there a possibility for a cure?
Ammar Ehab1, Mohamed Ibrahim2, Marel Magdi3, Mohamed Ayman4, Nourhan Zidan5, Abdelbaset Osman2, Sara Ashraf6, Mayar Mohamed1, Mirna Magdy1, Marina Hany4, Marise Adly3, Nourhan Kamel1, Amr Maher1, Ammar Yaser3, Yara Ahmed7, Amal Abdelkarim1, Marehan Ehab1, Rana Wael8 and Rania M Hathout9*
2Faculty of Pharmacy, El-Azhar University, Cairo, Egypt
3Faculty of Pharmacy, Helwan University, Cairo, Egypt
4Faculty of Pharmacy, Misr International University, Cairo, Egypt
5Faculty of Pharmacy, The Egyptian Russian University, Cairo, Egypt
6Faculty of Pharmacy, The British University, Cairo, Egypt
7Misr University for Science & Technology, Cairo, Egypt
8Faculty of Pharmacy, Cairo University, Cairo, Egypt
9Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
Cite this as
Ehab A, Ibrahim M, Magdi M, Ayman M, Hathout RM, et al. (2019) Alzheimer’s disease and its current treatments; Is there a possibility for a cure? Open Journal of Chemistry 5(1): 013-019. DOI: 10.17352/ojc.000012Alzheimer’s disease (AD) is an irreversible, progressive brain disorder that slowly destroys the memory and the thinking skills, and eventually the ability to carry out the simplest tasks. This has motivated lots of scientists to search for an ultimate treatment or cure for this serious disease. There are various causes & risk factors which cause AD and are the reasons for its progression. The drugs used in AD are usually a combination between different classes but never those of the same class such as Acetyl cholinesterase inhibitors which increase the availability of acetylcholine & NMDA receptors antagonists such as memantine. Most of the currently used drugs are mainly used to treat the symptoms, but lately some drugs have shown some promise in both treating & curing AD such as Aducanumab which is in the final phases of the clinical trials that resulted in the clearing of Amyloid plaques affecting cells communication. Since there are newly discovered causes of the disease, there are various other approaches in dealing with AD which will be discussed thoroughly in this review article.
Introduction
Dementia is a general term for brain disorders that effects the person’s ability to think or remember things. This is due to the damage to the brain cells which eventually causes the lack of communication between the brain’s cells leading to a decrease in these skills [1]. Dementia is referred to as senile dementia which is an incorrect term because memory loss isn not necessarily linked to the increasing age solely [1]. Alzheimer’s disease (AD) is the most common form of dementia (AD accounts for 60% to 80% of dementia causes). It is not a normal part of aging but its greatest known risk factor is old age, and the majority of people with Alzheimer’s are 65 and older, yet, some still have at age younger than 65 [1-3].
AD is a progressive chronic neurodegenerative disease which only worsens with time as it affects the brain parts responsible for thinking and memory by damaging the nerve cells. This damage starts a decade or more before thinking & memory problems appear. This is due to a few reasons some of which are I) accumulation of β-amyloid plaques which deposits in the spaces between nerve cells [2], II) formation of Tau protein causing neurofibrillary tangles that builds up inside the cell [4,5]. These reasons leads to III) loss of cortical neurons because they stop functioning, loses the communication between surrounding cells & eventually die causing the shrinking of the brain by time [1,3].
Understanding the pathology and main causes of any disease or condition is usually the main gate that proposes the curative approaches of that disease.
The maincauses of AD is not fully known yet but it is most probably due to the combination of genetic, environmental & lifestyle factors. The decreasing and increasing risk of developing AD may differ from one person to another [3,6]. The biggest risk factor is age. On the other hand, it was found that sex is not a risk factor. Genetic factors can cause the development of AD and the development of its symptoms at an early onset. Additionally, a correlation was proven between chromosome 21 & AD. Thereforem, children with downs syndrome might develop AD as well. Head injury is another factor which might cause AD. Other factors such as race & profession are a bit vague & inconclusive but there is an evidence to suggest that people with a high level of education have a lesser risk than those with a low level of education [6].
Since AD is a progressive disease, then it eventually affects the person’s ability to do normal everyday activities due to the decrease in the cognitive & behavioral function. One of the early signs is the short-term memory loss. While the long-term memory gets affected over the disease’s course. Other functions such as the non-memory aspects of cognition such as language, thinking, attention, & visuospatial abilities are decreased as well [5,7]. Ideomotor apraxia is also present as a symptom if there is a damage in the parietal cortex. Moreover, it was found that this disease has a direct relationship with the density of the neurofibrillary tangles in the superior parietal. Thus, it is associated with the loss of the ability to use the common objects and tools [8]. A recent study stated that getting a proper sleep helps in decreasing the amount of tau protein which helps in slowing down the progression of the disease [9].
AD progress through several stages with an increase in symptoms severity & complications. At the mild stage, the patient suffers loss of memory accompanied by mood and personality changes. This stage gives an early indication of AD. At the moderate stage, the memory loss is increased with difficulty in recognizing relatives, in language and in organizing thoughts and the patient might be found wandering away from home. At the severe stage, the patient becomes completely dependent on others for their care and he might suffer from weight loss, seizures, and inability to talk and becomes more susceptible to infections [10].
Methods of Treatment
There are a lot of drug classes that are approved for treatment of Alzheimer’s disease. The most important class of these drugs are called the Acetylcholine esterase inhibitors (AchEI) because the cholinergic system plays an important role in the regulation of learning and memory processes. Also, because several studies has shown that that both acetyl cholinesterase and butyl cholinesterase (BuChE) play an important role in Aβ-aggregation “amyloid beta” during the early stages of amyloid plaque formation therefore by inhibiting AChE & BuChe an increase of the Ach in the brain region occurs and hence a reduction in plaque formation results. BuChE is an enzyme which is closely related to AchE. It leads to the hydrolysis of Ach and is present mainly in the peripherals including the plasma so by inhibiting BuChE many side effects may appear. Therefore, scientists have been developing selective AchEI in order to decrease these side effects [11-15]. Tacrine, donepezil, rivastigmine and galantamine are clinically employed AChE inhibitors for the management of AD and although they have a limited efficacy yet, they are effective. Therefore, it was important to further develop them to be more potent & highly effective. This is done by the modification of the main template moieties of these AchEI [11,16].
AchEI
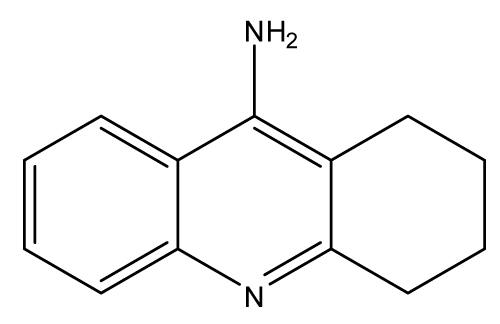
Tacrine: In 1961 tacrine (IC50 = 167 nM) was the first approved drug for the treatment of AD because it was a reversible inhibitor of AChE and BuChE. It is considered to be a nootropic agent which helps to facilitate learning and to prevent cognitive defects associated with dementias, it is also a parasympathomimetic agent as it increases the availability of Ach, and of course as stated before; it is an AchEI that causes an increase in Ach available to be used by the undamaged neurons left in the brain to maintain a normal function. It has a great efficacy in delaying the deterioration of the Alzheimer’s symptoms and retards its progressive nature.
Tacrine itself is a very toxic and hazardous agent that if it comes in contact with any body tissue it will cause severe irritation and damage. It is also considered to be a carcinogenic agent. It was quickly withdrawn from the market due to its main and serious side effect; hepatotoxicity which shows in the form of an acute liver failure. This was shown as an increase in most of the liver enzymes after few weeks from starting the therapy such as ALT (Alanine Aminotransferase) therefore patients who have to take tacrine should have their ALT level monitored. Another reason for its withdrawal is that other AchEIs do not cause the rise of the ALT and rarely affect the liver so they were more preferable to tacrine [17-22].
Tough all of the AchEI have some common cholinergic adverse effects yet, tacrine as stated before have a specific adverse effect which is the acute liver failure besides the other usual adverse effects such as nausea, vomiting, anorexia, restlessness, tremors, myalgia, arthralgia, rash and excessive sweating. Some of the less frequent adverse effects are hypotension, bradycardia, syncope, ataxia and confusion. The cholinomimetic effects might increase the gastric acid secretions which increases the risk of gastric ulcerations. Some of the hematologic adverse effects are agranulocytosis but it rarely shows. As for pregnant women, tacrine should only be given in pregnancy when its benefit outweighs it risk (Figure 1) [23-41].
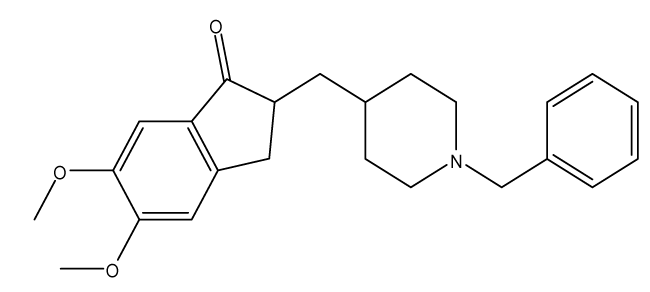
Donepezil: Donepezil (IC50 = 5.7 nM) was the second FDA (Food and Drug Agency) approved drug for the treatment of AD and is considered to be safe & well tolerated with a suitable effectiveness [11]. It is a centrally acting reversible acetyl cholinesterase inhibitor and is mainly used to increase the cortical acetylcholine. Donepezil’s efficacy is very obvious at early stages but its effect decreases as the disease progresses and fewer cholinergic neurons remain functionally intact which also happens to all of the AchEI. And like tacrine and other AchEI; it is only used for the symptoms treatment and not for the curing of AD. Donepezil also reduces sedation associated with opioid treatment of cancer pain, and improves neurocognitive function in patients who have received radiation therapy for primary brain tumors or for brain metastases [28].
It also has cholinergic adverse effects, the most common of them include diarrhea, loss of appetite, muscle cramps, bone fracture, nausea, insomnia, headache, blurred vision, vertigo, dyspnea, syncope, pruritus, eczema rash, nocturia, dehydration, unusual tiredness or weakness, and vomiting, while in the cases of overdose it might lead to increased sweating, salivation, hypotension, slow heartbeat, troubled breathing, and seizures. The haptic involvement is very rare and it is only used in the case of pregnancy and nursing mother if the benefits outweighs the risks as it is category C (Figure 2) [42].
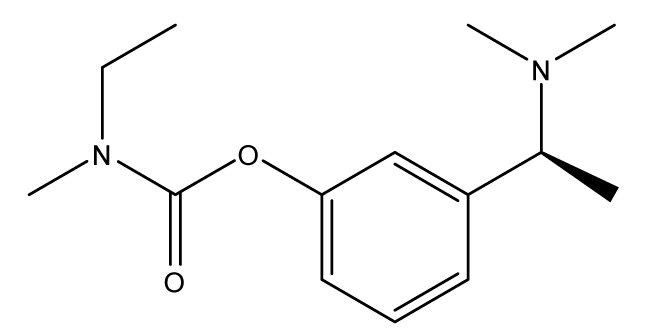
Rivastigmine: Rivastigmine (IC50 = 4.150 nM) is the third drug used in AD treatment. It belongs to a new generation of cholinesterase inhibitors with a carbamate moiety which is able to react irreversibly with the active sites of the AchE enzyme. It has ten-fold affinity to AchE of the brain more than BuChE of the peripherals. It seems to be useful and beneficial for patients with mild to moderate Alzheimer’s disease [32].
It has many adverse effects related to its very high volume of distribution (Vd). It causes dermatologic effects such as hyperhydrosis while as for the CVS (cardio-vascular system), it causes hypertension amd bradycardia. Regarding the gastrointestinal effects it causes nausea, vomiting, dyspepsia and abdominal pain. Additionally, it has neronal effects suh as tremors and dizziness and also causes psychiatric effects such as insomnia, anxiety, nightmares and other associated effects such as application site erythema becuase it is usually applied in the form of a transdermal patch. It can only be used in pregnancy if the benefit outweighs the risk although the drug is class B (Figure 3) [43,44].
Galantamine: Galantamine (IC50 = 800 nM) is an alkaloid isolated from different species of the Amaryllidaceae family which is also considered a competitive and a reversible inhibitor of AChE and exhibits significant improvement of cognitive performances in AD due to the increased availability of the Ach in the brain making it a good nootropic agent for the cognitive deficits associated with dementia. Galantamine have a unique dual mechanisms which includes the inhibition of the AchEI together wih the allosteric modulation of nicotinic acetylcholine receptors (nAChR) and hence its effect is accompanied by an increased release of Ach. Its use is common because it is less toxic than tacrine & physostigmine despite the fact that it is less potent. Galantamine is usually well tolerated and exhibits modest but consistent cognitive and clinical benefits of continuous treatment (Figure 4) [29].
Its action is considered of the same mode of Tacrine and Donepezil which makes it appropriate in the early stages of AD. Galantamine can also cause cholinergic adverse effects but it has an additional side effect which is its related allergic reaction which is usually reported as a difficulty in breathing, swelling of the face, tongue and the lips. It displays serious drug-drug interactions with the other anticholinergic drugs that might decrease its effect such as atropine and Parkinson’s drugs. If Galantamine is used with donepezil then the side effects will be augmented dramatically therefore it is not favorable to use both of them together. Galantamine is a category C drug so it is not used in pregnancy unless the benefits outweighs the risks [43].
Others: Xanthostigmine derivatives are a new class of potent AChE inhibitors which inhibits the amyloid pro-aggregatory function [11,36].
Aminobenzoic acid derivatives such as meta-aminobenzoic acid derivatives showed anticholinesterase activity with an IC50 value in the range of 33.4–357 nM [11,37]. Also, pyrrolo-isoxazole derivatives emerged as AChE inhibitors. These drugs are currently under investigation [11].
Coumarins are a naturally occurring phytochemicals in many species of plants with a wide range of biological activities. Studies have also shown that naturally occurring as well as the chemically synthesized coumarin analogues exhibit a potent AChE inhibitory activity [38]. The studies have also documented that it had an affect on amnesia and loss of memory. It has been well established that coumarins primarily interact with PAS (Peripheral anionic site) of AChE. Furthermore, they also inhibit Monoamine oxidase (MAO). However, amongst its side effects are the loss of appetite, nausea, diarrhea or blurred vision occuring at first usage period when the patient’s body adjusts to the medication. It may also cause urine coloration; orange-red in color [38,39].
Flavonoid derivatives which are polyphenolic compounds such as curcumin also exhibit a wide range of pharmacological properties including AChE inhibition. In several studies it revealed an anti-amnestic and restoration of memory effects with slowing of the neurodegeneration in AD [40]. Recently, they have also shown interactions and inhibition effects with the amyloid plaques [45].
NMDA blockers
Another class is the N-methyl-D-aspartate antagonists (NMDA). NMDA receptor is a type of glutamate receptor which is excited by either Glycine or Glutamate, upon excitation the ion gated channels open leading to the influx of calcium ions into the post-synaptic neurons triggering pathways important for synaptic plasticity, however some theories say that the overstimulation of the NMDA receptor causes neurodegeneration and synaptic dysfunction due to excitotoxicity, so memantine is used which acts as uncompetitive NMDA antagonist with moderate binding affinity therefore it partially closes the receptor preventing pathological influx of calcium ions and allowing physiological signals important for learning and memory processes [46,47].
Memantine is considered to be a very effective drug in the case of treating AD. This was proven by various studies some of which had a total of 2433 patients to test the drug. Memantine monotherapy did indeed significantly improve their cognitive function, daily living activities, and behavioral disturbances [47].
Nevertheless, memantine is not usually used solely but it is used in combination with one of the AchEI such as donepezil for an increased efficacy and decreased side effects due to the reduction in their doses when combined together. This combination gives a better effect due to the dual therapy by inhibiting the AchE and by also the blocking of the NMDA receptor which is responsible for the excitotoxicty that usually leads to the neurons damage and sometimes their destruction [48].
Its side effects are manifested in the form of pain, abnormal crying, leg pain, fever, increased appetite. Additionally, the adverse drug reactions include: dizziness, confusion, headache, hallucinations, and tiredness. Some less common side effects include: vomiting, anxiety, hypertonia, cystitis, and increased libido. It must be first known if the AD patient had any drug allergies or not because memantine might cause an allergic reaction so a sensitivity test should be done before administration. As for pregnant & nursing women memantine is not recommended unless it was clearly necessary although it is category B [49]. It is contraindicated in the cases of renal failure, renal impairment, urinary tract infection, and hepatic diseases [50].
Drugs for curing AD under clinical trials
The traditional treatment of AD included only AchEI in order to increase the Ach levels in the brain. This was followed by the use of the NMDA blocker memantine in order to prevent any excitotoxicity. Neverthelesss, after discovering the presence of the amyloid plaques scientists started to consider its combating in order to prevent its deposition & decrease its formation. One of the drugs in this category that has proven promising results, was aducanumab. Aducanumab is a high-affinity, fully human IgG1 monoclonal antibody acting against a conformational epitope found on Aβ [53]. Therefore, it selectively targets aggregated forms of Aβ including soluble oligomers and insoluble fibrils. Aducanumab was shown to reduce Aβ plaques and slow decline in clinical measures in patients with prodromal or mild AD with acceptable safety and tolerability.
Due to their clearance, there was possible cognitive benefits in the AD patients. However, the main side effect was the amyloid-related imaging abnormalities (ARIA) that was a side effect associated with the removal of Aβ, which was reported to occur in a dose-dependent manner and occurred more often in ApoE E4 carriers than non-carriers [52]. ARIA-E refers to cerebral edema, involving the breakdown of the tight endothelial junctions of the blood-brain barrier and the subsequent accumulation of fluid [53], while ARIA-H refers to cerebral microhaemorrhages, small hemorrhages on the brain, often accompanied by hemosiderosis. mH is usually seen as small, round and low intensity lesions and are small hemosiderin deposits [54]. As stated before, ARIA is a safety finding which is dose-dependent so if the dose of aducanumab decreases, it decreases as well and vice versa. Some other minor side effects are headache, diarrhea, and dizziness which unlike ARIA are not dose-dependent. Aducanumab is currently in phase III clinical trials and is still under sinvestigation [55].
Gantenerumab is another human IgG1 antibody acts centrally to disassemble and degrade amyloid plaques by recruiting microglia and activating phagocytosis. It preferentially interacts with aggregated brain Aβ, both parenchymal and vascular. It is still in the phase III clinical trials. It also safe & well-tolerated but there is a concern about the ARIA finding as there was cases who showed vasogenic edema on MRI scans in brain areas with the most amyloid reduction [56].
Others
It was found that oxidative stress and metal ions contribute in the progression of Alzheimer’s disease and hence it is more effective to use a drug that targets areas than to use those that target single pathogenic contributor. Accordingly, these potential multi-target inhibitors were designed based on the beta carboline core structure. A variety of β-carbolines with an extended aromatic ring system were synthesized and tested with the aim of identifying potential multitarget agents, that can interfere with Aβ self-assembly and cholinesterase activity while exhibiting promising antioxidant properties for AD treatment. Based on data analysis, a compound emerged as a potential lead compound for further structure activity relationship studies. This molecule exhibited moderate to high activity in a range of assays suggesting that further modification of its basic ring system could yield a truly efficient candidate to develop effective drugs for disease management [51].
Future drug treatments
There are other future drug treatments which include the following: anti-amyloid therapy which aims at the amyloid precursor protein (APP), this precursor can be broken down by gamma or beta-secretase enzymes which prevent the formation of amyloid plaques. Examples on Beta-secretase enzyme are AZD3293 and MK-8931 which are in Phase II/III clinical trials and are to be completed in2019 [52,58]. While an example on Gamma-secretase enzyme is semagacestat which was in the Phase III clinical trials but was discontinued because of no improvement in cognition in the study group and worsening cognition at higher doses [52,58,59]. Last drug was related to the NSAIDs class which is Tarenflurbil has been shown to reduce levels of Aβ by modulating the gamma-secretase enzyme, but demonstrated no improvement in cognition or function [60]. As another approach the immunisation therapy through active immunisation against Aβ with the agent AN 1792 was soufht of then halted because of reported cases of meningoencephalitis which was eventually stopped at phase III especially that there were no evidence for slowing progression of AD [61]. In the same context, monoclonal antibodies such as Bapineuzumab that did not demonstrate any treatment effect [62] and solanezumab which had a very small benefit [63]. Lastly, aducanumab is still being clinically trialed as mentioned previously. Tau-targeted therapy strategies that are currently in clinical trials include agents to prevent hyperphosphorylation, as well as those targeting microtubule stability and aggregation [64]. Exploiting the different targeting approaches and nanomedicine would open new horizons for the currently used drugs [66-69].
Authors Opinion
Most of the demonstrated drugs are used in the treatment of the symptoms caused by AD and decreasing its progression of neurodegeneration. Starting from the oldest agents to the newest, the AchEI such as the tacrine causes severe hepatotoxicity which requires continuous monitoring of the liver function tests that is why it was withdrawn from the market. Other AchEI are more preferred such as donepezil, rivastigmine, and galanthamine. Efficiency was found approximately similar between these agents so the choice should be according to cost, individual patient condition, patient tolerance and physician experience. Small portion of patients may experience acute worsening of cognition, or agitation on the starting of the drugs, if such signs was observed the drugs must be stopped immediately. Donepezil have the least side effects of these AchEI while galanthamine have the most side effects. As for memantine it have various side effects which might affect the compliance of the patient. While the rest of the drugs are still under study, Aducanumab seems to be a promising cure for the future making it a perfect choice. And although there are still newly discovered approaches for AD treatment yet they still need more research such as anti-amyloid therapy, immunisation and tau-targeted therapy. Accordingly, in conclusion it can be said that the current regimen of memantine plus donepezil are perfectly recommended for the treatment and slowing of progression of AD due to their reported dual mechanism accompanied by lower incident of side effects when given together and the associated higher efficiency rate.
The authors should like to acknowledge Menna Ahmed, Mariam Gabra, Maryam Khaled and Nadine Hamed for their splendid work and passion throughout the phase (DM moderators 19’), Sandy Osama for her guidance (DM moderator 17’) and Hani Aldahol for his guidance as well (Clinical moderator 18’).
- Alzheimer's Disease AD (2019) U.S. National Library of Medicine. Medline Plus. Link: https://bit.ly/2KEhwGw
- Paul MM, Levine H (2010) Alzheimers Disease and the Amyloid-β Peptide. J Alzheimers Dis 19: 311–323. Link: https://bit.ly/2IUO0wm
- Alzheimer's Disease Fact Sheet. U.S. Department of Health and Human Services, National Institute on Aging. Link: https://bit.ly/2Guzxso
- Mandelkow EM, Mandelkow E (2012) Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harb Perspect Med 2: a006247. Link: https://bit.ly/2x6zCLU
- Wint D, Tavee J, Sweeney P (2014) Alzheimer's Disease. Cleveland Clinic, Link: https://bit.ly/2KuIseX
- (2019) Who Is Affected? Alzheimer Europe. Link: https://bit.ly/2L3EIk8
- Quental, Natália Bezerra Mota I2013) Visuospatial Function in Early Alzheimer’s Disease -The Use of the Visual Object and Space Perception (VOSP) Battery. PLoS ONE 8: 7.
- Panteleimon G (1998) Pathologic Correlates of Apraxia in Alzheimer Disease. Archives of Neurology 55: 689-695. Link: https://bit.ly/2MZBTn1
- Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, et al. (2019) The Sleep-Wake Cycle Regulates Brain Interstitial Fluid Tau in Mice and CSF Tau in Humans. Science 363: 880-884. Link: https://bit.ly/2MZk6Mv
- (2017) What Are the Signs of Alzheimer's Disease? U.S. Department of Health and Human Services. National Institute on Aging. Link: https://bit.ly/2hvbcDR
- Anand, Preet, SinghB (2013) A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Archives of Pharmacal Research 36: 375-399. Link: https://bit.ly/2WQWQzV
- Savini L, Campiani G, Gaeta A, Pellerano C, Fattorusso C, et al (2001) Novel and Potent Tacrine-Related Hetero- and Homobivalent Ligands for Acetylcholinesterase and Butyrylcholinesterase. Bioorganic & Medicinal Chemistry Letters 11: 1779-1782. Link: https://bit.ly/2WVfvPW
- Savini L, Gaeta A, Fattorusso C, Catalanotti B, Campiani G, et al. (2003) Specific Targeting of Acetylcholinesterase and Butyrylcholinesterase Recognition Sites. Rational Design of Novel, Selective, and Highly Potent Cholinesterase Inhibitors. J Med Chem 46: 1-4. Link: https://bit.ly/2IrATn4
- Campiani G, Fattorusso C, Butini S, Gaeta A, Agnusdei M, et al. (2005) Development of Molecular Probes for the Identification of Extra Interaction Sites in the Mid-Gorge and Peripheral Sites of Butyrylcholinesterase (BuChE). Rational Design of Novel, Selective, and Highly Potent BuChE Inhibitors. J Med Chem 48: 1919-1929. Link: https://bit.ly/2FmlqD1
- Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, et al. (2002) Acetylcholinesterase Knockouts Establish Central Cholinergic Pathways and Can Use Butyrylcholinesterase to Hydrolyze Acetylcholine. Neuroscience 110: 627-639. Link: https://bit.ly/2L4qKhZ
- Anand P, Singh B (2012) Synthesis and Evaluation of Novel 4-[(3H,3aH,6aH)-3-Phenyl)-4,6-Dioxo-2-Phenyldihydro-2H-Pyrrolo[3,4-d]Isoxazol-5(3H,6H,6aH)-Yl]Benzoic Acid Derivatives as Potent Acetylcholinesterase Inhibitors and Anti-Amnestic Agents. Bioorg Med Chem 20: 521-530. Link: https://bit.ly/2N1QNsO
- Knapp, Margaret J (1994) A 30-Week Randomized Controlled Trial of High-Dose Tacrine in Patients With Alzheimers Disease. JAMA 271: 985-991. Link: https://bit.ly/2RmJJpa
- León R, Marco-Contelles J, García AG, Villarroya M (2005) Synthesis, Acetylcholinesterase Inhibition and Neuroprotective Activity of New Tacrine Analogues. Bioorg Med Chem 13: 1167-1175. Link: https://bit.ly/31NwItN
- Muñoz-Ruiz P, Rubio L, García-Palomero E, Dorronsoro I, del Monte-Millán M, et al. (2005) Design, Synthesis, and Biological Evaluation of Dual Binding Site Acetylcholinesterase Inhibitors: New Disease-Modifying Agents for Alzheimers Disease. J Med Chem 48: 7223-7233. Link: https://bit.ly/2WSWYPm
- Gunasingh MJ (2008) The Neuroprotective Role of Melatonin against Amyloidβpeptide Injected Mice. Free Radical Research 42: 661-673. Link: https://bit.ly/2FmLkqb
- Gunasingh MJ (2008) The Neuroprotective Role of Melatonin against Amyloidβpeptide Injected Mice. Free Radical Research 42: 661–673. Link: https://bit.ly/2FmLkqb
- Tacrine (2019) National Center for Biotechnology Information. U.S. National Library of Medicine. Link: https://bit.ly/2MXLtqo
- Donepezil () National Center for Biotechnology Information. U.S. National Library of Medicine. Link: https://bit.ly/2Rp1OD0
- Kryger G, Silman I, Sussman JL (1999) Structure of Acetylcholinesterase Complexed with E2020 (Aricept®): Implications for the Design of New Anti-Alzheimer Drugs. Structure 7: 297-307. Link: https://bit.ly/2L3rPH0
- Villalobos A, Blake JF, Biggers CK, Butler TW, Chapin DS, et al. (1994) Novel Benzisoxazole Derivatives as Potent and Selective Inhibitors of Acetylcholinesterase. J Med Chem 37: 2721-2734. Link: https://bit.ly/2WV6LJR
- Villalobos A, Butler TW, Chapin DS, Chen YL, DeMattos SB, et al. (1995) 5,7-Dihydro-3-[2-[1-(Phenylmethyl)-4-Piperidinyl]Ethyl]-6H- Pyrrolo [3,2-f]-1,2-Benzisoxazol-6-One: a Potent and Centrally-Selective Inhibitor of Acetylcholinesterase with an Improved Margin of Safety. J Med Chem 38: 2802-2808. Link: https://bit.ly/2ITEABh
- Meng FC, Mao F, Shan WJ, Qin F, Huang L, et al. (2012) Design, Synthesis, and Evaluation of Indanone Derivatives as Acetylcholinesterase Inhibitors and Metal-Chelating Agents. Bioorg Med Chem Lett 22: 4462-4466. Link:
- Donepezil (2019) National Center for Biotechnology Information. U.S. National Library of Medicine. Link: https://bit.ly/2Rp1OD0
- Harvey, Alan L (1995) The Pharmacology of Galanthamine and Its Analogues. Pharmacology & Therapeutics 68: 113-128. Link: https://bit.ly/2IrBy82
- Guillou C, Mary A, Renko DZ, Gras E, Thal C (2000) Potent Acetylcholinesterase Inhibitors: Design, Synthesis and Structure–Activity Relationships of Alkylene Linked Bis-Galanthamine and Galanthamine–Galanthaminium Salts. Bioorganic & Medicinal Chemistry Letters 10: 637-639. Link: https://bit.ly/2WUBEZY
- Jia P, Sheng R, Zhang J, Fang L, He Q, et al. (2009) Design, Synthesis and Evaluation of Galanthamine Derivatives as Acetylcholinesterase Inhibitors. Eur J Med Chem 44: 772-784. Link: https://bit.ly/2Rm9xlc
- Gottwald MD, Rozanski RI (1999) Rivastigmine, a Brain-Region Selective Acetylcholinesterase Inhibitor for Treating Alzheimer’s Disease: Review and Current Status. Expert Opin Investig Drugs 8: 1673-1682. Link: https://bit.ly/2x2UCmz
- Bolognesi ML, Bartolini M, Cavalli A, Andrisano V, Rosini M, et al. (2004) Design, Synthesis, and Biological Evaluation of Conformationally Restricted Rivastigmine Analogues. J Med Chem 47: 5945-5952. Link: https://bit.ly/2Xmwz0J
- Lin, Gialih (1999) Molecular Recognition by Acetylcholinesterase at the Peripheral Anionic Site: Structure–Activity Relationships for Inhibitions by Aryl Carbamates. Bioorg Med Chem 7: 2683-2689. Link: https://bit.ly/2RmdBSu
- Lin, Gialih (2005) A Rate Determining Step Change in the Pre-Steady State of Acetylcholinesterase Inhibitions by 1, n-Alkane-Di-N-Butylcarbamates. Bioorg Med Chem Lett 15: 951-955. Link: https://bit.ly/2Ktuw5e
- Belluti, Federica (2005) Cholinesterase Inhibitors: Xanthostigmine Derivatives Blocking the Acetylcholinesterase-Induced β-Amyloid Aggregation. J Med Chem 48: 4444-4456. Link: https://bit.ly/2Fo0z29
- Trujillo-Ferrara, José (2003) Synthesis, Anticholinesterase Activity and Structure–Activity Relationships of m-Aminobenzoic Acid Derivatives. Bioorganic & Medicinal Chemistry Letters 13: 1825-1827. Link: https://bit.ly/2WUFQxD
- Changwong N, Sabphon C, Ingkaninan K, Sawasdee P (2011) Acetyl- and Butyryl-Cholinesterase Inhibitory Activities of Mansorins and Mansonones. Phytotherapy Research 26: 392-396. Link: https://bit.ly/2x3iLcZ
- Wu, Chi-Rei (2007) Psoralen and Isopsoralen, Two Coumarins of Psoraleae Fructus, Can Alleviate Scopolamine-Induced Amnesia in Rats. Planta Medica 73: 275-278. Link: https://bit.ly/31JmhqN
- Richetti SK, Blank M, Capiotti KM, Piato AL, Bogo MR, et al. (2011) Quercetin and Rutin Prevent Scopolamine-Induced Memory Impairment in Zebrafish. Behav Brain Res 217: 10-15. Link: https://bit.ly/2IsiBCo
- Drugs.com (2019) Tacrine Side Effects. Drugs.com. Link: https://bit.ly/2WUR940
- Drugs.com (2019) Donepezil Side Effects. Drugs.com. Link: https://bit.ly/31MkWzx
- Drugs.com (2019) Galantamine Uses, Side Effects & Warnings. Drugs.com. Link: https://bit.ly/2WUkVuI
- Drugs.com (2019) Rivastigmine Side Effects. Drugs.com. Link: https://bit.ly/2MYtqjV
- Hathout RM, El-Ahmady SH, Metwally AA (2018) Curcumin or bisdemethoxycurcumin for nose-to-brain treatment of Alzheimer disease? A bio/chemo-informatics case study. Nat Prod Res 32: 2873-2881. Link: https://bit.ly/2Fm550Z
- Hunt DL, Castillo PE (2012) Synaptic Plasticity of NMDA Receptors: Mechanisms and Functional Implications. Neurobiology 22: 496-508. Link: https://bit.ly/2L3Bu06
- Matsunaga, Shinji (2015) Memantine Monotherapy for Alzhei mer’s Disease: A Systematic Review and Meta-Analysis. Plos One 10: e01234289. Link: https://bit.ly/2L02Rbm
- Deardorff, William James, George Grossberg (2016) A Fixed-Dose Combination of Memantine Extended-Release and Donepezil in the Treatment of Moderate-to-Severe Alzheimer’s Disease. Drug Des Devel Ther 10: 3267-3279. Link: https://bit.ly/2RmTLXm
- Drugbank ca (2019) Memantine – Drug Bank. Link: https://bit.ly/2WVxNR8
- Drugs.com (2019) Memantine Pregnancy and Breastfeeding Warnings. Drugs.com. Link: https://bit.ly/2WZIZfW
- Horton, William (2017) Synthesis and Application of β-Carbolines as Novel Multi-Functional Anti-Alzheimer’s Disease Agents. Bioorganic & Medicinal Chemistry Letters 27: 232-236. Link: https://bit.ly/2ZEBc3Z
- (2019) Who should not take Memantine HCL? Webmd.com. Link: https://wb.md/2x7oiPH
- Budd Haeberlein S, O'Gorman J, Chiao P, Bussière T, Von Rosenstiel P, et al. (2017) Clinical Development of Aducanumab, an Anti-Aβ Human Monoclonal Antibody Being Investigated for the Treatment of Early Alzheimer's Disease. J Prev Alzheimers Dis 4: 255-263. Link: https://bit.ly/2FmRnuT
- Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, et al. (2011) Amyloid-Related Imaging Abnormalities in Amyloid-Modifying Therapeutic Trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dementia 7: 367-385. Link: https://bit.ly/2RoWLm8
- Alzforum.org (2019) Aducanumab. ALZFORUM. Link: https://bit.ly/31JqzyB
- Sharma R, Frank Gaillard AP (2019) Cerebral microhemorrhage. Radiopaedia Link: https://bit.ly/2L32bC2
- Alzforum.org (2019) Gantenerumab. ALZFORUM. Link: https://bit.ly/2ZBxjga s
- Vassar R (2014) BACE1 Inhibitor Drugs in Clinical Trials for Alzheimer’s Disease. Alzheimers Res Ther 6: 89 Link: https://bit.ly/2FlDq0i
- Henley, David B (2009) Development of Semagacestat (LY450139), a Functional γ-Secretase Inhibitor, for the Treatment of Alzheimers Disease. Expert Opin Pharmacother 10: 1657-1664. Link: https://bit.ly/2Y0rZ5x
- Doody, Rachelle S (2013) A Phase 3 Trial of Semagacestat for Treatment of Alzheimers Disease. New England Journal of Medicine 369: 341-350. Link: https://bit.ly/2KuRc4L
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, et al. (2009) Effect of Tarenflurbil on Cognitive Decline and Activities of Daily Living in Patients With Mild Alzheimer DiseaseA Randomized Controlled Trial. JAMA 302: 2557-2564. Link: https://bit.ly/2XskyXH
- Loeffler, David A (2013) Intravenous Immunoglobulin and Alzheimer’s Disease: What Now? J Neuroinflammation 10: 70. Link: https://bit.ly/2N9gE2e
- Salloway, Stephen (2014) Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimers Disease. N Engl J Med 370: 322-333. Link: https://bit.ly/31IKlu1
- Siemers, Eric R (2016) Phase 3 Solanezumab Trials: Secondary Outcomes in Mild Alzheimers Disease Patients. Alzheimers Dementia 12: 110-120. Link: https://bit.ly/2WTIuPg
- Wischik, Claude M (2014) Tau-Aggregation Inhibitor Therapy for Alzheimers Disease. Biochemical Pharmacology 88: 529-539. Link: https://bit.ly/2MYwcFR
- Metwally AA, El-Ahmady SH, Hathout RM (2016) Selecting optimum protein nano-carriers for natural polyphenols using chemoinformatics tools. Phytomedicine 23: 1764-1770. Link: https://bit.ly/2Xk9v2V
- Hathout RM, Omran MK (2014) Gelatin-based particulate systems in ocular drug delivery. Pharm Dev Technol 21: 379-386. Link: https://bit.ly/2IXzN1o
- Abozeid S (2016) Silencing of the metastasis-linked gene, AEG-1, using siRNA-loaded cholamine surface-modified gelatin nanoparticles in the breast carcinoma cell line MCF-7. Colloids Surf B Biointerfaces 145: 607-616. Link: https://bit.ly/31G22u9
- Farid M () Silencing of the scavenger receptor (Class B-Type 1) gene using siRNA-loaded chitosan nanoparticles in a HepG2 cell model. Colloids Surf B Biointerfaces 123: 930-937. Link: https://bit.ly/2XhELQ7
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
