Open Journal of Chemistry
Mini Tablets: A Short Review-Revision
Ezgi Ilhan, Timucin Ugurlu* and Oya Kerimoglu
Cite this as
Ilhan E, Ugurlu T, Kerimoglu O (2017) Mini Tablets: A Short Review-Revision. Peertechz J Med Chem Res 3(1): 012-022. DOI: 10.17352/ojc.000007Mini tablets are solid dosage forms with a diameter ≤ 3 mm and separated into subunits of conventional tablets. Production methods are similar to standard tablets, but the only difference is the use of multiple punches. They have advantageous for use in patients suffering from swallowing difficulty and receiving multiple drug treatment. They provide a more effective treatment by reducing the fluctuation in the drug’s release profile. At the same time, different release systems can be used together. In addition, mini tablets have a number of advantages over single unit dosage forms, and in recent years the prominence continues to increase. In the light of this information, the advantages and disadvantages of mini tablets, production equipment, formulation designs, different emission characteristics and evaluation criteria are emphasized in this compilation.
Introduction
Oral administration of medicines has an advantage for patient’s compliance. Most of the solid dosage forms administered orally are tablets. Tablets have many advantages over other dosage forms, such as ease of transportation, application and production, high patient compliance, accurate dosing, control of drug release and stability. However, the desired release profile, therapeutic effect or ease of use in pediatrics or geriatrics, difficulty in swallowing may not be achieved by conventional tablets. Drug delivery system ensure reaching the effect area of the administered drug and sufficient concentration of the drug at the site of action. Conventional tablets may not be sufficient for treatment due to fluctuation in the blood concentration of the drug. Repeated doses may lead to toxic concentrations. Single unit or multi-unit dosage forms with different release profiles have been developed in order to provide effective treatment by reducing fluctuation in the concentration. In single unit systems, the release of the drug is changed using matrix or membrane systems. In multi-unit systems such as pellets and mini tablets, the dose is divided into subunits and spread to the entire gastrointestinal tract. Mini tablets are systems designed to resolve the disadvantages of conventional solid dosage forms. This new approach is promising to overcome therapeutic obstacles such as swallowing difficulty, multiple dosing, as well as the development of dosage forms that allow successful treatment by combining different delivery systems.
Multiple unit dosage forms
The purpose of drug delivery systems is delivering the drug to a particular site and providing the desired drug concentration for effective treatment at that site. Conventional dosage forms cause fluctuations in the blood concentration of the drug, and the drug may lead to toxic concentrations in blood or may be inefficient. The main purposes of designing sustained or controlled drug delivery systems are reducing the frequency of dosing and increasing its efficiency by localizing the area of action of the drug to a specific region [1].
Oral controlled release drug delivery systems are divided into two classes:
• Single unit dosage forms, such as tablets, capsules,
• Multi-unit dosage forms, granules, pellets or mini tablets.
In multi-unit dosage forms, the dose is divided into subunits and each unit contains the drug. The total dose is the sum of the drug in the subunits and the dose is dependent on the functionality of the subunits. Multi unit dosage forms are useful when the selected ingredients exhibit additive or synergistic effects or the dose can be reduced according to a single unit dosage form. After administration, the dosage units are spread to the stomach and gastrointestinal tract and the risk of local irritation is reduced as a result of an equal drug release. Multi-unit dosage forms show a more reliable dissolution profile than single units, which means better bioavailability [2].
The properties sof multi-unit and single unit dosage forms are given comparatively at Table 1 [3].
Definition, properties and production equipment
Mini tablets are tablets with diameters ≤ 3 mm and have a wide application area (Figure 1). For ease of use, they are usually filled in capsules, or they can be compressed in larger tablets or filled into sachets [1,4]. Mini tablets are produced with multiple punches using eccentric or rotary tablet press machines. Thanks to easy production techniques, mini tablets can be produced in a certain size and dosage. The variability between series is also low [1,2]. Apart from productivity, the use of multiple punches in their production increases the amount of dust that can be consumed at a time. Thus, the fill time is shortening. In concequence of the short waiting time, the separation of the powders is prevented [4,5].
Benefits of multiple punches:
• Increase productivity,
• Does not require a different production equipment, only mold cost,
• Shorten the working time,
• No separate equipment is required to collect the products obtained.
• Cost is low due to all these features [6].
Multiple punches are often used as multi piece assemblies or as monoblocks. There are two varieties, the one internal cap fixing (Figure 2A) and the other one external cap fixing (Figure 2B). The internal fixing pins are immobilzed into the punch body. Mounting and disassembly of them are easy and they have fewer pins compared to external cap fixing. The risk of contamination of the product in multi piece punches is low. However, parts need to be separated before they are cleaned. Monoblock punches (Figure 2C) require less installation time and are easier to clean.
While multiple punches are resistant to breakage and abrasion, monoblocks are more resistant. However, the eroded edges of multiple punches can be replaced without having to change the punches. If these types of punches are not installed carefully, they can be eroded or damaged during use. They are also nondurable to non-axial stresses due to the high length / diameter ratio of the punch tips. For this reason, the length / diameter ratios and the speed of the device have to be adjusted well. Compared to conventional tablets, mini tablets need lower pressures. A single punch having 2-3 mm diameter is durable and can take up to 2-3 kN axial force. For this reason, the process must be started with low pressure values [4,6].
Advantages of the mini tablets
• Their production is easy. It is an alternative to pellets and granules due to its reproducible production and dimensional similarity.
• Provides a more uniform release kinetics. Thus, the risk of sudden increase in blood concentration is reduced.
• Formulation development is easy.
• İntra and inter individual variability is low. Because the size is too small, even if the pylor is closed, it can pass to the intestine.
• They can be easily coated thanks to shape and size uniformity.
• The risk of local irritation is reduced because they spread throughout the gastrointestinal tract.
• Drug loading capacity is high.
• Setting the release profile is easy [3,7].
• Superiority to pellets:
• Pellets are usually bead-like structures filled into capsules or compressed in tablets.
• Pellets are produced by fluid bed granulation or extrusion-spheronization methods, while mini tablets are produced by simple tablet production methods. This saves time and money [8].
• The absence of solvent use in production increases the stability.
• Since the production methods of the mini tablets are easier, the tablets which have uniform size and dosage and do not differ from batch to batch can be produced [9].
• Superiority to granules:
• Mini tablets have a smooth surface, stable surface area and high mechanical resistance compared to granules. It can be easily coated and requires less coating material than granules [7, 10].
Formulation and production requirements
Unlike conventional tablets, mini tablets have important parameters such as cylindrical hole length and diameter as well as particle size, size distribution, surface properties, length-to-width ratio and compression properties (bulk and tapped density). In mini tablets, in addition to the particle characteristics (particle size, size distribution, surface properties) and compression characteristics (bulk and tapped density) of powder, the cylindrical hole length and diameter of dies are important parameters. In dies with large diameter size (4 mm), the bulk flow rate increases with increased lenght of die whereas in smaller dies (2 mm), it decreases with increased lenght. Variation in flow rate is due to the increase of the negative pressure gradient in the punch. In addition, environmental conditions (humidity, temperature, static load) must be taken into account during production [11]. The narrow diameter of the die requires an excellent powder fluidity and a narrow maximum particle size range to prevent occlusion. In a study by Mielck and Flemming (1995), [12], it is found that when D / dp 99 & gt; 3, the powder has the desired particle size, and when D / dp 99 & lt; 3, the powder flow may be disrupted (the minimum particle diameter (D) has been associated with the maximum particle size which represents 99% of the mixture).
Good flowability of powder is necessary to obtain tablets in uniform weight as well as uniform filling of die. The tablets must have a certain mechanical resistance in order to coat and fill in a capsule easily. This is made possible by the correct choice of formulation components such as filler, binder, lubricant. The dosage form which is improved with appropriate pharmaceutical and physico-chemical properties allows release of the drug at the desired time, as well as uniformity of weight and uniformity of tablets.
In addition to the content, the size of the tablets is also influential on mechanical resistance. Lennartz and Mielck, Tissen et al. 1998 [13], examined the effect of tablet size, content and applied pressure on tensile strength and capping tendency. Both studies have shown that even with high drug loading, the reduction of tablet size leads to higher mechanical resistance and a lower capping tendency. This is because the surface area / volume ratio of mini tablets is higher than conventional tablets. Thus, the higher amounts of powder mixture contacts the punch and die wall, and as a result, more homogeneous distribution of densities is achieved. Surface area to bond the particles each other is increased, and a protective shell on the tablet surface is formed. This situation may vary according to the properties of the active substance [13-15].
Formulation options of mini tablet dosage forms
• Compressed mini tablets
• Encapsulated mini tablets
• Biphasic drug delivery system prepared as mini tablet (1)
Mini tablets are usually used by filling with capsules or by tabletting (Figure 3).
Compressed mini tablets
In order to avoid the cost of hard gelatin capsules, mini tablets can be formulated as tablet. Uniform sizes, smooth shapes, smooth surfaces, low porosity and high mechanical resistance make them more uniform and reproducible tablets than pellets and granules. Depending on the properties of the external phase that provides the filling of the cavity (hydrophobic / hydrophilic polymer matrix used and the number of mini tablets), release profile can be changed. Bifasic drug delivery systems are developed using different release characteristics. In these systems, one phase initiates the rapid action by providing the immediate release while the other phase releases the long-term effect, ensuring continuity of efficacy and eliminating the need for recurrent doses of the drug [1, 16, 17].
Tablet coating
Coating of tablets is a separate formulation, is a separate production step and increases cost. For this reason, there are some requirements for a tablet coating.
These;
• Mask bad taste and smell,
• Change the color of the drug,
• İncrease physical and chemical stability,
• Control the release of the drug,
• Protecting the digestive enzyme in the gastrointestinal tract,
• İmprove the appearance of the drug,
• Make an identity
Coating is the last critical step in tablet production. The industry usually uses four coating processes:
• Sugar coating
• Film coating
• Coating with pressure
• Enteric coating
The selection of the coating process depends on the type of coating material, the strength of the core tablet to covering material and the application process [1]. Due to the high surface area / volume ratio in mini tablets, it may be difficult to control the release with matrix systems. Mohamed et al. (2015) [21], examined the effect of theophylline-containing mini matrix tablets and non-matrix tablets by coating films with ethyl cellulose at different ratios. The results of the study showed that release in mini matrix tablets containing high soluble active substance can be achieved with the appropriate amount of film coating.
Compressed mini tablets as a biphasic drug delivery system
To reduce the cost of the product, mini tablets can be compressed as a larger tablet instead of filled in a capsule. Dimensional uniformity maintains its form and shape thanks to smooth shapes, smooth surfaces, low porosities and high resistance to forces. Thus, it is more advantageous than pellets and granules [2]. In biphasic drug delivery systems, the rapid release period and the long release period of the drug are combined. The rapid release compartment provides a jump effect at the beginning, while the slow release compartment allows the drug effect to continue at a constant rate for a certain period of time. Also, the desired dosage regimen can be provided by changing the number of mini tablets providing extended release and the dosage of the drug in the immediate release component. Biphasic systems can be designed to be fast / slow as well as slow / fast [22, 23]. The relationship between the amount of powder that will surround the mini tablets and the weight of the mini tablets is important. It has been determined that the ratio between the amount of powder and the weight of the mini tablet should be at least 3/1. Fewer amounts of powder are insufficient to fill the gap between the mini tablets and fracture may appear on the tablets after compressing [2].
Mini tablets and modified drug delivery
There are different approaches to change the release of active substance from dosage forms. Examples include prolonged release, delayed release, pulsatile and bimodal release, and targeted drug release [4].
Extended release mini tablets
In extended release formulations, the active ingredient is slowly released over a wide period of time from the dosage form. This is accomplished by altering the diffusion from the dosage form of the drug or by prolonging the time of transition through the gastrointestinal tract. In extended release tablets, release slowing is achieved by altering the dissolution and diffusion of the drug through barrier coating, matrix system or chemical interaction / reaction [24].
As with conventional tablets, the drug release profile is also greatly influenced by formulation parameters in mini tablets. Generally, polymers, gums or lipid excipients are used to provide extended release in tablets. Hydrophobic compounds exhibit Fickian release (diffusion) while compounds in the hydrophilic structure exhibit non-Fickian drug transport principle (diffusion + erosion). It is expected that drug release will be slow in all extended release mini tablets. Hydrophobic compounds such as microcrystalline gum, glyceryl behenate in the lipid structure, ethyl cellulose in the polymeric structure slow the release by increasing the hydrophobicity of the system. On the other hand, hydrophilic polymers such as hydroxypropyl methyl cellulose (HPMC) exhibit their effect by forming a resistant and less permeable hydrogel layer [26-28].
Apart from the effect of other excipients such as filler, disintegrant, lubricant etc., the controlled release agent on release should not be overlooked. For example, water solubility and diffusion support of hydrophilic compounds such as lactose or dispersing properties of compounds such as starch may accelerate the release of the drug. Water-insoluble compounds may delay the release of the drug as it will increase the hydrophobicity of the system. The difference in size between standard tablets and mini tablets affects the effectiveness of the release. As long as tablet size decreases, the rate of release increases due to increased surface area / volume ratio and reduced distance that the drug will diffuse [4].
The solubility of the drug is an important parameter for the release profile. Particularly weakly acidic and weakly basic drugs show pH-dependent solubility. The pH-dependent solubility causes the ionic or non-ionic ratio of the drug to change depending on the pH of the release medium and the gastrointestinal fluid. This changes the dissolution of the drug and the expected bioavailability can not be achieved. In extended release dosage forms, it is desirable that the solubility of the biopharmaceutical variant be independent of pH [29]. One of the approach to making the solubility of pH dependent substances to independent from pH is creating microenvironments and these are provided by pH modifying substances. Microenvironmental pH is also effective on the stability of compound. pH modifiers can control the dissolution profile of immediate release and extended release dosage forms. In immediate release dosage forms, combination with salt-formulated ingredients reduces the dissolution of the less soluble free form at the beginning of the dissolution. In extended release dosage forms, pH-independent release of weakly acidic or weak basic drugs is provided by using pH modifiers with organic acid or organic bases characteristics. For example, an acidic pH modifier may be used in combination with a basic drug to increase the solubility of the drug at high pH values and to ensure that the release is not affected [30].
Streubel et al., (2000) [31], used two separate polymer matrices, water-insoluble ethyl cellulose (EC), water-soluble and swellable hydroxypropyl methyl cellulose (HPMC), to make the dissolution of the poorly basic verapamil hydrochloride independent of pH. Firstly, hydroxypropyl methyl cellulose acetate succinate (HPMCAS) enteric polymer was added to the formulation, and secondly, drug-polymer systems were developed using organic acids such as fumaric acid, succinic acid. As a result, the combined use of organic acids with matrix systems ensured a stable release profile for both the hydrophobic and hydrophilic matrix system. However, the first approach failed to achieve a pH-independent release profile. Studies have shown that, besides the use of pH modifiers, the properties of the gel layer formed by the hydrophilic polymers are also effective in obtaining a pH-independent release profile [32,33]. The use of pH modifiers such as magnesium oxide and magnesium hydroxide gave successful results to make the solubility of weak acidic drugs prepared from the matrix system pH-independent [29].
Pulsatile and bimodal release
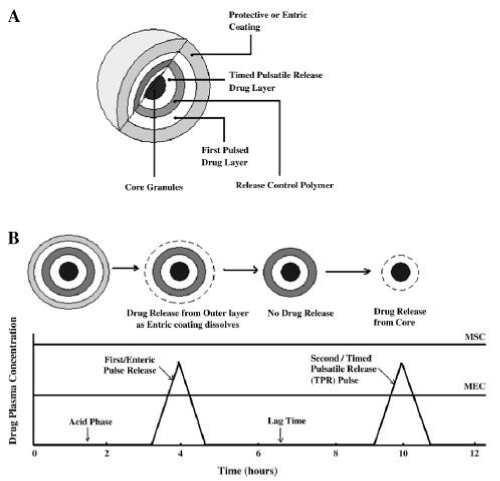
In view of physiological parameters such as heart rate, blood pressure, hormone, enzymes and concentration of plasma proteins, drug delivery systems may not show a steady release profile as planned. Irregularities in drug concentration may occur due to physiological parameters and circadian rhythms in pathological conditions. Different drug delivery systems have been designed to avoid this [34]. Pulsatile drug release is delayed release within a programmed time period to meet the chronotherapeutic need. These systems are time-controlled systems and site-specific systems. While site-specific systems are provided by environmental factors such as pH, enzymes, time-controlled drug delivery is provided by the drug delivery system [35]. Pulsatile drug release may be useful in the treatment of diseases that require chronotherapy, such as bronchial asthma, angina pectoris, and sleep disorders. After oral administration, intestinal areas such as colon can be released. Pulsatile release is achieved by coating a tablet with controlled releasing polymer. When the drug is compared to an aqueous medium, the coating acts as a protective layer. The release occurs at a defined time, depending on the physicochemical properties of the drug. Pulsatile release coatings may be rupturable, erodible, permeable and semi-permeable film coating (Figure 4). Tablets are often coated by spray coating, but pressure coating or dipping coating methods can also be used [36, 37].
In addition, pulsatile release systems can also be used in high metabolised with first pass effect or pharmacologically tolerated drugs as they can show multiple release profiles. For example, multiple release of antibiotics provides an effective treatment and increases patient compliance. Administration of the drug in divided doses prevents bacteria from becoming resistant and improving biological tolerance [38]. In addition, pulsatile release inhibits the interaction of the dosage forms with the gastrointestinal tract [39]. Multiple release in pulsatile systems is achieved by coating the drug core with functional polymers. (Figure 5). These systems can be multi unit or single unit.
Bimodal drug delivery systems have different release characteristics within a single unit. Systems such as rapid release / prolonged release, extended release / delayed release may be combined to increase therapeutic efficacy and patient compliance. Bimodal or combined release can be provided by single-unit systems such as layered tablets, as well as by multi unit systems such as pellets and mini tablets. In zero order release systems, the release rate of the drug is independent of blood concentration and is considered to be the ideal system for keeping the amount of drug in the plasma constant. In these systems, the absorption of the drug is assumed to be rapid and uniform throughout the entire gastrointestinal tract. However, the absorption of most drugs is partially slower at the stomach, faster at the proximal part of the gut, and too slow at the distal part of the gut. For this reason, the rate of release from the dosage form of the drug should be increased or decreased in certain regions to achieve a constant drug blood concentration. Thus, it can be considered that the release rate in varying proportions is more favorable than the zero-order constant release. Bimodal systems provide such a volatile release. It consists of initial rapid release and a constant and slow release period followed by a second rapid release phase. That is, with a sigmoidal release profile [40, 41].
Floating mini tablets targeted to the gastrointestinal system
Floating mini tablets in the stomach: For extended release systems, it is important not only releasing the drug for a sufficient period of time, but also to remaining in the gastrointestinal tract for a sufficient period of time. Following oral administration of drugs, bioavailability may not be sufficient due to absorption in the gastrointestinal tract. His is due to the fact that the drug is not stable at the intestinal pH and that absorption at the onset of the small intestine is limited. Floating systems in the stomach increase the absorption of the drug by prolonging the duration of the drug’s retention. It is also an advantageous system for drugs that do not dissolve in the intestinal pH or that are effective locally on the stomach, and reduce side effects of drugs that cause local irritation. They are not affected by stomach contents and gastric emptying time [42-44]. For the preparation of a floating system of a drug, it should be locally effective in the stomach, be absorbed largely from the stomach, has low dissolution at alkaline pH, and narrow absorption window. The density of these systems may be lower because they are lower than the aqueous environment of the gastrointestinal tract. These systems may flow in the stomach because the density of them should be lower than the aqueous environment of the gastrointestinal tract and should be less than 1 g / ml so that it can go on the surface. However, the density of all the excipients used is not so low. Different systems have been developed to overcome this problem. Floating systems are divided into two types, these are effervescent and non-effervescent systems and they can be single unit or multi unit. With multi unit floating systems, the fluctuation in absorption and release of the drug can be reduced [44-46].
In non-effervescent systems, polymers such as polysaccharides, hydrocolloids, or gel-forming or high-swelling substances or matrix-forming polymers are used. The result of mixing the drug with a gel-forming agent is that the system in contact with the stomach fluid swells while preserving the shape integrity. The air that enters the swollen polymer allows the system to float, as well as the controlled release of the drug. The external fluid enters the dosage form by swelling the system and allows the drug to dissolve. The dissolved drug then diffuses through the hydrated gel layer [45]. An intragastric floating system has been developed by Harrigan RM (1997) [47], to prevent irritation of the undissolved part of drugs. His system consists of micro-holes on the top and bottom surface and a chamber that allows the system to float. While the swimming chamber allows the system to stay above the gastric fluid, micro-holes allow the gastric fluid to enter the system, dissolving the drug and making the dissolved drug diffuse.
Iannuccelli et al., (1998) [48,49], designed multi unit floating systems with air compartments. Each unit comprises an air compartment separated by a calcium alginate core and calcium alginate or calcium alginate / polyvinyl alcohol (PVA) membrane. The flow of the system depends on the presence of the air chamber and the porosity of the membrane. He porous structure is provided by the addition of a water-soluble material to the PVA composition, thereby preventing shrinkage of the system. It has been observed that the ability of the system to float increases with increasing molecular weight and quantity of PVA.
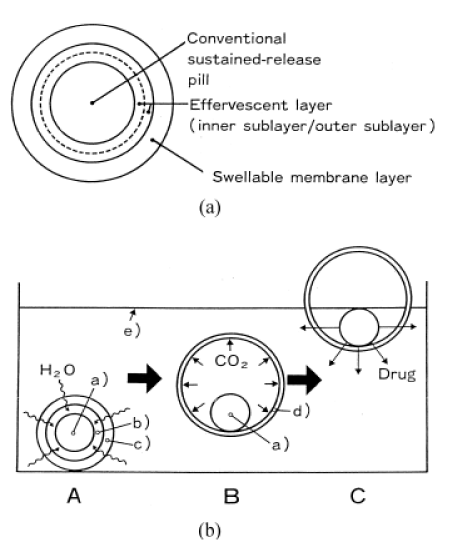
Floating effervescent systems include an effervescent component such as citric acid and sodium bicarbonate, or a liquid space that can evaporate at body temperature in the matrix, apart from a swollen polymer. When the system compared to gastric fluid, carbon dioxide is released and this released gas is trapped by the hydrocolloid polymer. (Figure. 6). Carbondioxide-forming layer could be mixed with the matrix systems for single-layered tablets, as well as using it in the bilayer formulations where one layer forms the controlled-release formulation and the other is a polymer mixed with the effervescent component [50].
Mucoadhesive mini tablets
It is possible to obtain local and systemic effect by using mucoadhesive systems. They allow the drug to remain in the area of action for a long time, thereby providing the local effect due to increase the duration of the absorption in the absorption zone [51, 52]. Mucoadhesive polymers can adhere to the surface of the gastric mucosa, thus allowing the drug to remain in this area for a longer period of time, thereby increasing bioavailability [53]. The use of thiolated polymers as a mucoadhesive agent has gained importance in recent years. The thiolated polymers have higher mucoadhesive power. They increase bioavailability through penetration enhancing effects [54, 55].
Guggi et al., (2003) [56], prepared mucoadhesive mini tablets containig peptide-structured calcitonin compound and targeted to the stomach. Thiolated chitosan is used as a mucoadhesive polymer and glutathione is used as a penetration enhancer. Chitosan-pepstatin conjugate acts as a peptide-protecting agent. With this system, the peptides are administered orally to show the pharmacological effect.
Colon targeted mini tablets
Targeting of drugs to cola increases the rate of treatment especially for local bowel diseases such as Chron’s disease, irritable bowel syndrome, and ulcerative colitis. The specificity and local effect of drugs on a particular site reduces systemic side effects. The enzyme activity at the end is low. This allows protein and peptide structured drugs to be successfully used with colon targeted systems. Targeting of drugs to colon can be achieved in different ways. For example, coating with enteric polymer showing pH dependent dissolution provides colon targeting. In areas with low pH, such as in the proximal part of the stomach and small intestine, the polymer will not dissolve, dissolve in the proximal part of the small intestine and in the stomach, as a result the drug will be released [57,58]. Colon targeting can also be achieved by microbially triggering the onset of action by biodegradable polymers sensitive to colonic microflora enzymes, by prodrugs susceptible to enzymatic transformations, or by providing delayed release with time-controlled swelling polymers, other than enteric coatings with pH sensitive polymers [59]. When single-unit drug delivery systems are targeted to the colon, problems may arise such as an unexpected disintegration of the system and loss of drug along the gastrointestinal tract. Colon targeting systems may be single unit or multi unit such as mini tablet, microparticle, pellet, granule [58]s. 5-Aminosalicylic acid, water-soluble dextrin and Nutriose® containing mini tablets that sensitive to enzymes secreted by colon bacteria were prepared, and as a result it was found that this system prevented the drug from being released in the acidic medium and continued to release in the colon for 8 hours [60].
Evaluation of mini tablets characteristics
Evaluation of powder mixture:
• Bulk density
• Tapped density
• Measurement of the compressibility of the powders (Carr’s index, Hausner ratio)
Bulk Density: The bulk density of a powder also includes the inter-particle gap. It is therefore also dependent on the density of the powder particles and the arrangement of the interstices between the particles in the powder bed. Care must be taken when measuring the bulk density, because even a small amount of dust mass shaking can cause a change in density. In the American Pharmacopoeia, the bulk density of a specific weight is explained in detail using a graded cylinder (Method 1), a volumetric method (Method 2) and a measurement in a container (Method 3). The bulk density is expressed in g/ml or g/cm3. If the weight of powder is represented by M, initial volume of the powder in a particular weight is represented by VO, bulk density is expressed as;
M/ V0
And the average of the three should be taken by making three separate measurements.
Tapped density: The compacted density is the increased bulk density obtained after the mechanical compacting of the powder mass. After the initial powder volume has been determined, the powder mass is compressed until smaller changes are made. If the weight of powder is represented by M, the compressed final volume of the powder is represented by Vf, Tapped density is expressed as;
M/ VF
And the average of the three should be taken by making three separate measurements (61).
Measurement of the compressibility of the powders
The interaction between the particles affects the flow of the powder as well as the properties of the batch. For this reason, the comparison of the bulk and tapped densities gives information about the interaction between the particles and the flow of the powder. This comparison is made using the Compressibility Index (Carr’s Index) and the Hausner Ratio. The Compressibility Index and Hausner’s Ratio are calculated using the following formulas:
Compressibility index: 100(V0- VF)/ V0
Hausner Ratio: V0/ VF
Alternatively, instead of the volume values in the formulas, densities can be used. The evaluation criteria are indicated in Table 2 [62].
Mini Tablets control
Weight Variation: According to the European Pharmacopoeia, 20 of the randomly selected dosage forms are individually weighed and the average weight is determined. Up to two of these determined weights may vary in percentage from the mean weight in the table (Table 3), but none should vary by more than twice that percentage [63].
Uniformity of tablets: To ensure consistency of dosage units, each unit on the shelf should contain active substance in the vicinity of the label value and within a narrow range. Dosage units are dosage forms containing a dose or a portion of an active substance in each dosage unit. The uniformity of the tablets may be indicated by two methods: weight variation or content uniformity. Unless otherwise indicated in the monograph, it is administered individually for each active ingredient in dosage forms containing a single active agent and two or more active agents. The content uniformity test of the drug in the dosage unit is performed to determine if the individual contents are within limits. Content Uniformity (CU) and weight variability (MV) are applied as given in the table (Table 4) [64].
Friability: This test is conducted under certain conditions to establish evidence of lamination, fracture of the uncoated tablets, resistance to mechanical impact, extent of damage to the surface, or mechanical resistance. The tablets are weighed to give a total weight of 6.5 g for tablets having a unit weight of 650 mg or less. In tablets with a unit weight of more than 650 mg, 10 tablets are weighed. Dust of tablets is removed. The device will rotate a total of 100 rotation for 4 minutes and 25 rotations after the tablets are inserted. Then the tablets are removed the dust again. This test is usually done once. If obviously cracked and broken tablets are present, the sample test is considered to have not passed. If the weight loss is more than expected, the test is repeated twice and the results are expressed as the average of the three. Reductions that do not exceed 1% by weight are generally acceptable [65].
Dissolution test
This test is used to determine the dissolution rate of the active substance from solid dosage forms such as tablets and capsules. Dosage forms are checked for compliance with the dissolution requirements specified in the monograph. In the American Pharmacopoeia, four different mechanisms for dissolution have been identified. Along with the use of the ‘basket system’ (Apparatus 1) and the ‘pallet system’ (Apparatus 2), there are also mechanisms of ‘iner-out cylinder’ (Apparatus 3) and ‘continuous flow cell’ (Apparatus 4) in drug release monographs. It should be selected from the informations mentioned in monograph. In enteric coated dosage forms, the rules under the delayed release heading apply unless otherwise indicated in the monograph.
For the preparation to be tested for dissolution, the following substances should be specified:
• The device to be used
• The content of the dissolution medium
• Rotation speed
• Sampling method, duration and amount
• Analysis method
• The amount of active substance required to dissolve in the specified time
This information is monograft and the conditions for capsules or soft gelatin capsules are the same (66, 67).
Disintegration test
Using the disintegration test it determines whether the tablets or capsules are dispersed within the prescribed time when they are placed in the liquid medium under the test conditions described below.
Disintegration may be accepted to have occurred under the following conditions:
• If no residue is left,
• If a residue is present, this residue is not composed of a solid, non-moistened soft structure, or,
• If only coating residues are present, or if only capsule shell particles are present, or if a disk is used, there are capsule shell particles adhering to the bottom of the disk [68].
Conclusion
Compared to single unit dosage forms, mini tablets are good alternative to granules and pellets. However, production parameters must be carefully assessed to ensure a good flow, correct and complete filling of the die and damage to the equipment. They can be made into tablets or they can be filled with capsules or used as a sachet, which is advantageous both in terms of ease of production and cost. They increase patient compliance by allowing coexistence of drugs with each other and by combining drugs with different release kinetics. They are suitable for most of drug molecule. Especially in geriatric and pediatric patient groups, there is a very high potential for achieving success in treatment. Studies have shown that mini tablets adapt to a multitude of modified release patterns such as extended, delayed, pulsatile, bimodal release and colon targeting. As discussed in the review, mini tablets have become an interesting topic for researchers because of their numerous advantages.
- Keerthi ML, Kiran RS, Rao VUM, Sannapu A, Dutt AG, et al. (2014) Pharmaceutical Mini-Tablets, its Advantages, Formulation Possibilities and General Evaluation Aspects: A Review. Int. J. Pharm. Sci. Rev. Res. 28: 214-221. Link: https://goo.gl/4d27rY
- Lopes CM, Lobo JMS, Pinto JF, Costa P (2006) Compressed mini-tablets as a biphasic delivery system. International Journal of Pharmaceutics. 323: 93-100. Link: https://goo.gl/B55kaq
- Swarbrlck J (1994) Drugs and Pharmaceutical Sciences, A Series of Textbooks and Monographs. ln: Ghebre-Sellassie I, ed. Multi particulate oral drug delivery, New Jersey, p: 461-463.
- Aleksovski A, Dreu R, Gasperlin M, Planinsek O (2014) Mini-tablets: a contemporary system for oral drug delivery in targeted patient groups. Expert Opin. Drug Deliv. 12. Link: https://goo.gl/MBEzWi
- Hayakawa Y, Uchida S, Namiki N (2016) Evaluation of the ease of taking mini-tablets compared with other tablet formulations in healthy volunteers. European Journal of Pharmaceutical Sciences. 84: 157-161. Link: https://goo.gl/drd77w
- Multiple Tip Tooling: durability, Productivity, Longevity, I Holland Ltd. Nottingham. Eri?im link: Link: https://goo.gl/Bm7gp8
- Dey NS, Majumdar S, Rao MEB (2008) Multiparticulate Drug Delivery Systems for Controlled Release. Tropical Journal of Pharmaceutical Research. 7: 1067-1075. Link: https://goo.gl/NKSPSL
- Schmidt C, Kleine B (1999) Influence of the Granulation Step on Pellets Prepared by Extrusion/Spheronization. Chem. Pharm. Bull. 47: 405-412. Link: https://goo.gl/aHScL1
- Swati G, Sushma S (2014) Multiple Unit System: An Approach Towards Gastroretention. Journal of Biological & Scientific Opinion. 2: 188-195. Link: https://goo.gl/mkuntu
- Gaber DM, Nafee N, Abdallah OY (2015) Mini-tablets versus pellets as promising multiparticulate modified release delivery systems for highly soluble drugs. International Journal of Pharmaceutics. 488: 86-94. Link: https://goo.gl/A5yySX
- Kachrimanis K, Petrides M, Malamataris S (2005) Flow rate of some pharmaceutical diluents through die-orifices relevant to mini-tableting. International Journal of Pharmaceutics. 303: 72-80. Link: https://goo.gl/6N4fTp
- Flemming J, Mielck JB (1995) Requirements for the Production of Microtablets: Suitability of Direct-Compression Excipients Estimated from Powder Characteristics and Flow Rates. Drug Development and Industrial Pharmacy. 21: 2239-2251. Link: https://goo.gl/fGKLTn
- Lennartz P, Mielck JB (1998) Minitabletting: improving the compactability of paracetamol powder mixtures. International Journal of Pharmaceutics. 173: 75-85. Link: https://goo.gl/NWd3ya
- Tissen C, Woertz K, Breitkreutz J, Kleinebudde P (2011) Development of mini-tablets with 1 mm and 2 mm diameter. International Journal of Pharmaceutics. 416: 164-170. Link: https://goo.gl/asR1Q6
- Bodea M, Tomuta I, Leucuta S (2010) Identification of critical formulation variables for obtaining metoprolol tartrate mini-tablets. Farmacia. 58: 719-727. Link: https://goo.gl/Eo282z
- Mahajan KV, Akarte1 AM, Sapate MK , Baviskar DT, Jain DK. (2013) Designing and evaluation of compressed mini-tablets of ramipril as a biphasic delivery system. Indo American Journal of Pharmaceutical Research. 3: 7277-2787. Link: https://goo.gl/qU4Kyn
- Lopes CM, Lobo JMS, Pinto JF, Costa P (2006) Directly compressed mini matrix tablets containing ibuprofen: preparation and evaluation of sustained release. Drug. Dev. Ind. Pharm. 32: 95-106. Link: https://goo.gl/zmsqjM
- Teehsen N, Rao V, Hadi MA (2013) Design and characterization of twice daily mini-tablets formulation of pregabalin. International Journal of Pharmacy and Pharmaceutical Sciences. 5: 168-175. Link: https://goo.gl/xuU9D7
- Aleksovski A, Luštrik M, Šibanc R, Dreu R (2015) Design and evaluation of a specific, bi-phase extended release system based on differently coated mini-tablets. European Journal of Pharmaceutical Sciences. 75: 114-122. Link: https://goo.gl/mvRUJC
- Stawarski T, Sieradzki E, Galecka E, Binek K (2016) Kinetics study on ketoprofen release from mini tablets and multi-compartment systems. Acta Pol. Pharm. 73: 731-737. Link: https://goo.gl/s6m57s
- Mohamed FA, Roberts M, Seton L, Ford JL, Levina M, et al. (2015) Film-coated matrix mini-tablets for the extended release of a water-soluble drug. Drug Dev. Ind. Pharm. 41: 623-30. Link: https://goo.gl/troH6z
- Patel HP, Karwa P, Patel NJ (2011) A novel approach to sustained Zolpidem tartrate release: Compressed mini-tablets. International Journal of Pharmaceutical Sciences Review and Research. 7: 53-58.
- Maggi L, Machiste EO, Torre ML, Conte U (1999) Formulation of biphasic release tablets containing slightly soluble drugs. European Journal of Pharmaceutics and Biopharmaceutics. 48: 37-42. Link: https://goo.gl/KkEWXP
- Allen LV, Popovich NG, Ansel HC (2011) Ansels drug delivery system, Ninth Edition. In: Troy DB, ed. Philadelphia .Link: https://goo.gl/AfH6SN
- Rosiaux Y, Jannin V, Hughes S, Marchaud D (2014) Solid lipid excipients - Matrix agents for sustained drug delivery. Journal of Controlled Release. 188: 18-30. Link: https://goo.gl/xkAH5T
- Mohamed FA, Roberts M, Seton L, Ford JL, Levina M (2012) Production of extended release mini-tablets using directly compressible grades of HPMC. Drug Dev. Ind. Pharm. 39: 1690-1697. Link: https://goo.gl/xS7Lbp
- De Brabander C, Vervaet C, Fiermans L, Remon JP (2000) Matrix mini-tablets based on starch:microcrystalline wax mixtures. Int J Pharm. 199: 195-203. Link: https://goo.gl/32zrnC
- Roberts M, Vellucci D, Mostafa S, Miolane C, Marchaud D (2012) Development and evaluation of sustained-release Compritol® 888 ATO matrix mini-tablets. Drug Dev Ind Pharm. 38: 1068-1076. Link: https://goo.gl/xxzjPZ
- Riis T, Bauer-Brandl A, Wagner T, Kranz H (2007) pH-independent drug release of an extremely poorly soluble weakly acidic drug from multiparticulate extended release formulations. European Journal of Pharmaceutics and Biopharmaceutics. 65: 78-84. Link: https://goo.gl/1jnz2S
- Badawy SI, Hussain MA (2007) Microenvironmental pH modulation in solid dosage forms. Journal of Pharmaceutical Sciences. 96: 948-59. Link: https://goo.gl/KWaZod
- Streubel A, Siepmann J, Dashevsky A, Bodmeier R (2000) pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. Journal of Controlled Release. 67: 101-110. Link: https://goo.gl/6Lk8m9
- Varma MVS, Kaushal AM, Garg S (2015) Influence of micro-environmental pH on the gel layer behavior and release of a basic drug from various hydrophilic matrices. Journal of Controlled Release. 103: 499-510. Link: https://goo.gl/rqJa8G
- Rao VM, Engh K, Qiu Y (2003) Design of pH-independent controlled release matrix tablets for acidic drugs. International Journal of Pharmaceutics. 252: 81-86. Link: https://goo.gl/GPNLvp
- Stubbe BG, De Smedt SC, Demeester J (2004) "Programmed polymeric devices"for pulsed drug delivery. Pharm. Res. 21: 1732-1740. Link: https://goo.gl/rLhmLJ
- Bussemer T, Otto I, Bodmeier R (2001) Pulsatile drug-delivery systems. Crit. Rev.Ther. Drug Carrier Syst. 18:433-458. Link: https://goo.gl/2NwuiW
- Maroni A, Zema L, Loreti G, Palugan L, Gazzaniga A (2013) Film coatings for oral pulsatile release. International Journal of Pharmaceutics. 457: 362-371. Link: https://goo.gl/wiDnzm
- Roy P, Shahiwala A (2009) Multiparticulate formulation approach to pulsatile drug delivery: Current perspectives. Journal of Controlled Release. 134: 74-80. Link: https://goo.gl/ZtR2Bo
- Saigal N, Baboota S, Ahuja A, Ali J (2009) Multiple-pulse drug delivery systems: setting a new paradigm for infectious disease therapy. Expert Opinion on Drug Delivery. 6: 441-452. Link: https://goo.gl/xLQbTA
- Sawada T, Sako K, Yoshihara K, Nakamura K, Yokohama S, et al. (2004) Timed-release formulation to avoid drug-drug interaction between diltiazem and midazolam. Journal of Pharmaceutical Sciences. 92: 790-797. Link: https://goo.gl/HT2QaG
- Streubel A, Siepmann J, Peppas NA, Bodmeier R (2000) Bimodal drug release achieved with multi-layer matrix tablets: transport mechanisms and device design. Journal of Controlled Release. 69: 455-468. Link: https://goo.gl/Jsi6jT
- Abdul S, Poddar SS (2004) A flexible technology for modified release of drugs: multi layered tablets. Journal of Controlled Release. 97: 393-405. Link: https://goo.gl/xDu3Dq
- Baumgartner S, Kristl J, Vrecer F, Vodopivec P, Zorko B (2000) Optimisation of floating matrix tablets and evaluation of their gastric residence time. International Journal of Pharmaceutics. 195: 125-135. Link: https://goo.gl/bDyqgx
- Jiménez-Martinez I, Quirino-Barreda T, Villafuerte-Robles L (2008) Sustained delivery of captopril from floating matrix tablets. International Journal of Pharmaceutics. 362: 37-43. Link: https://goo.gl/bdk4c2
- Tadros MI (2010) Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. European Journal of Pharmaceutics and Biopharmaceutics. 74: 332-9. Link: https://goo.gl/9n6MLe
- Singh BN, Kim KH (2000) Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. Journal of Controlled Release. Journal of Controlled Release. 63: 235-59. Link: https://goo.gl/MKdMD2
- Sungthongjeen S, Paeratakul O, Limmatvapirat S, Puttipipatkhachorn S (2006) Preparation and in vitro evaluation of a multiple-unit floating drug delivery system based on gas formation technique. International Journal of Pharmaceutics. 324: 136-43. Link: https://goo.gl/ja5pNN
- Harrigan RM (1977) Drug delivery device for preventing contact of undissolved drug with stomach lining. US4055178. Link: https://goo.gl/iV5YsQ
- Iannucelli V, Coppi G, Sansone R, Ferolla G (1998) Air compartment multiple-unit system for prolonged gastric residence. Part II. In vivo evaluation. International Journal of Bioapharmaceutis.174: 55-62. Link: https://goo.gl/3k8qhK
- Iannuccelli V, Coppi G, Bernabei MT, Cameroni R (1998) Air compartment multiple-unit system for prolonged gastric residence. Part I. Formulation study. International Journal of Biopharmaceutics.174: 47-54. Link: https://goo.gl/3yUcr7
- Ichikawa M, Watanabe S, Miyake Y (1991) A new multiple-unit oral floating dosage system. I: Preparation and in vitro evaluation of floating and sustained-release characteristics. Journal of Pharmaceutical Sciences. 80: 1062-1066. Link: https://goo.gl/66Ax8D
- Bruschi ML, Freitus O (2005) Oral bioadhesive drug delivery system. Drug Dev Ind Pharm. 31: 293-331. Link: https://goo.gl/3tJjuu
- Ahuja A, Khar RK, Ali J (1997) Mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 23: 489-515. Link: https://goo.gl/SYnnx2
- Jiao Y, Pang X, Lui M, Zhang B, Li L, Zhai G (2016) Recent progresses in bioadhesive microspheres via transmucosal administration. Colloids Surf B Biointerfaces. 140: 361-72. Link: https://goo.gl/v91HGi
- Guggi D, Marschütz MK, Bernkop-Schnürch A (2004) Matrix tablets based on thiolated poly(acrylic acid): pH-dependent variation in disintegration and mucoadhesion. International Journal of Pharmaceutics. 274: 97-105. Link: https://goo.gl/BUfdLR
- Bernkop-Schnürch A, Guggi D, Pinter Y (2004) Thiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. Journal of Controlled Release. 94: 177-86. Link: https://goo.gl/2WQqZi
- Guggi D, Krauland AH, Bernkop-Schnürch A (2003) Systemic peptide delivery via the stomach: in vivo evaluation of an oral dosage form for salmon calcitonin. Journal of Controlled Release. 92: 125-35. Link: https://goo.gl/Dcc9Ru
- Nykänen P, Lempää S, Aaltonen ML, Jürjenson H, Veski P, et al. (2001) Citric acid as excipient in multiple-unit enteric-coated tablets for targeting drugs on the colon. International Journal of Pharmaceutics. 229: 155-62. Link: https://goo.gl/pqAMiH
- Talaei F, Atyabi F, Azhdarzadeh M, Dinarvand R, Saadatzadeh A (2013) Overcoming therapeutic obstacles in inflammatory bowel diseases: a comprehensive review on novel drug delivery strategies. European Journal of Pharmaceutical Sciences. 49: 712-722. Link: https://goo.gl/EVb8LC
- Xiao B, Merlin D (2012) Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv. 9(: 1393-1407. Link: https://goo.gl/6HnY5E
- Krenzlin S, Siepmann F, Wils D, Guerin-Deremaux L, Flament MP, et al. (2011) Non-coated multiparticulate matrix systems for colon targeting. Drug Dev Ind Pharm. 37: 1150-1159. Link: https://goo.gl/puhzPv
- The United States Pharmacopoeia 34, The National Formulary 29, United States Pharmacopoeial convention Inc (2010) Twinbrook Parkway, Rockville, 616: 241-243.
- The United States Pharmacopoeia 34, The National Formulary 29, United States Pharmacopoeial convention Inc (2010) Twinbrook Parkway, Rockville, 1163, 723-726.
- European Pharmacopoeia Ninth Edition, Volume I, Council of Europe, Strasbourg Cedex, France, 2016, 311-312.
- European Pharmacopoeia Ninth Edition, Volume I, Council of Europe, Strasbourg Cedex, France, 2016, 372-375.
- European Pharmacopoeia Ninth Edition, Volume I, Council of Europe, Strasbourg Cedex, France, 2016, 312-313.
- European Pharmacopoeia Ninth Edition, Volume I, Council of Europe, Strasbourg Cedex, France, 2016, 302-309.
- The United States Pharmacopoeia 34, The National Formulary 29, United States Pharmacopoeial convention Inc (2010) Twinbrook Parkway, Rockville, 711, 278-284.
- European Pharmacopoeia Ninth Edition, Volume I, Council of Europe, Strasbourg Cedex, France, 2016, 299-301.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
