Open Journal of Chemistry
Recent Structure Activity Relationship Studies of 1,4-Benzodiazepines
Noor ul Amin Mohsin1 and Muhammad Imran Qadir2*
2Institute of Molecular Biology & Biotechnology, Bahauddin Zakariya University, Multan, Pakistan
Cite this as
Mohsin NA, Qadir MI (2015) Recent Structure Activity Relationship Studies of 1,4-Benzodiazepines. Open J Chem 1(1): 008-012 DOI: 10.17352/ojc.000002Structure activity relationship studies of 1,4-benzodiazepines have been discussed especially with their effects as antianxiety and anticonvulsants. The currently available benzodiazepines are associated with various side effects. Nowadays the purpose of these studies is to minimize side effects with these drugs. A very little alteration is possible on the benzene ring while the modification can be done on the diazepine ring. It can adopt the different conformations and in some cases some aromatic and heterocyclic rings have been fused with this part in order to see the effect of these conformation blockers on the pharmacological activity. The structure activity studies are also linked to molecular modeling studies. This is important in adding some information for the interaction of these drugs with the receptors and how this interaction can be improved.
Introduction
Benzodiazepines (BDZs) are the bicyclic heterocyclic compounds in which benzene ring is fused to seven membered diazepine ring containing two nitrogen. There are different types of BDZs such as 1,2-benzodiazepines, 1,3-benzodiazepines, 1,4-benzodiazepines, 1,5-benzodiazepines, 2,3-benzodiazepines. 1,4-BDZs possess anxiolytic, anticonvulsant, muscle relaxant properties. They are widely used as a treatment of anxiety, insomnia, epilepsy, alcohol with drawl, for anaesthesia and about 500 million peoples have been treated with BDZs [1]. There are two types of BDZs receptors, central BDZs receptors and peripheral BDZs receptors. Physiological functions of peripheral BDZs receptors are different from the central BDZs receptors [2]. BDZs produce their effect by binding to the central BDZs receptors which are located at the post and presynaptic membranes [3]. Their binding to γ aminobutyric acid (GABAA) receptors increases the chloride ion conductance by inducing some conformational changes and causes inhibition of action potential [4]. BDZs enhance the effect of neurotransmitter GABA at their receptors.
Use of BDZs is increased from the last 15 and 20 years and now they are widely prescribed. Side effects associated with the use of these drugs such as drowsiness, tolerance, addiction and withdrawal potential causes problems [5]. The discovery of selective ligands for the central BDZs receptors, which have the anxiolytic activity without producing these side effects, is important. Docking of BDZs with the GABA receptors determine the binding analysis of drug with BDZs receptors [6]. The main objective of this is the discovery of product with narrow spectrum of activities.
In this paper some structure modifications studies carried out on different position of the 1,4-BDZs in the last two decades have been summarized. Studies were focused especially on their antianxiety, anticonvulsant and muscular relaxant effects.
Chemistry and biological activity
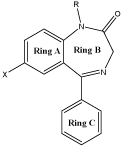
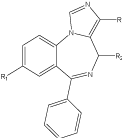
The discovery of BDZs dates back to 1950 and the first agent which was introduced in the market was chlordiazepoxide. Diazepam, the second member of this series was more active [7]. Introduction of substituent is very important in BDZs especially the position 7. The potency of these compounds also increases by adding the substituent’s in ring B and C [8]. Triazolo BDZs which contains triazole ring fused with diazepine nucleus are most potent agents in this class [9,10](Figure 1).
Furan and thiophene are the bioisosteres of the phenyl ring [11]. 5-thienyl and 5-furyl substituted BDZs were synthesized and evaluated for their affinity at the BDZs receptors in order to get information about the binding of lipophilic pocket and its effect on the biological activity. The 5-(2’-thienyl) BDZs showed high affinity for the BDZs receptors which displaced flunitrazepam from rat cortical membrane with high affinity and were anticonvulsant and muscle relaxant. When a bromine atom was substituted at position 4’, the compound was less active. The 5-(3’-thienyl) and 5-(2’-furyl) were less potent. The 2’-halo substituted phenyl was more potent than the 2’-thienyl and 2’-furfuryl [12] (Figure 2).
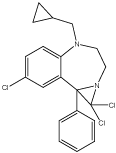
Azirino BDZs are more lipophilic than the BDZs. Some azirino compounds were active as anticonvulsant but failed to displace flumazenil. The activity was evaluated as inhibitor of binding of flumazenil to the cerebral receptors. The less activity may be related to steric hindrance of the azirine nucleus so that the phenyl group cannot adopt the proper conformation for binding or may be due to the less penetration into the central nervous system (CNS) by crossing blood brain barrier [13] (Figure 3).
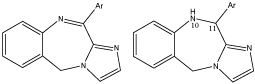
11-aryl-5H-imidazo [2,1-c][1,4] benzodiazepines and their 10,11 dihydro derivatives were prepared to investigate the effect of additional aromatic ring in the BDZs. The most active compound was with the 11(2’-thienyl) ring as determined for their potency to displace flumazenil from the binding site in rat brain. When the diazepine ring is reduced between positions 10-11, the activity of the compounds decreases because the reduced compound showed less affinity for the central GABA receptors as determined by radioligand binding assay [14] (Figure 4).
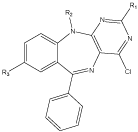
4-chloro-pyrimido [4,5-b][1,4] benzodiazepines were synthesized by a new method. The starting compound was the 4,6-dichloro-5-nitropyrimidines, amines and different carboxylic acids. By this method the final compound were obtained in a good yield. Although these compounds were not evaluated for their tranquilizer activity but they contain a 1,4-BDZs core and they can be interesting candidate for the antianxiety and anticonvulsant activity [15] (Figure 5).
Triazole ring system of alprazolam was modified by some aromatic ring such as pyridine, 4- florophenyl, 6-methoxy-2-naphthyl, 2-bromo-5-methoxy phenyl. Some of these compounds showed excellent anticonvulsive activity when compared with diazepam. These compounds also need further evaluation in order to clear their therapeutic profile [16] (Figure 6).
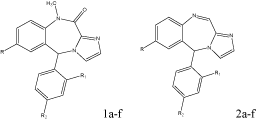
Four different series of 5-aryl-imidazo [2,1-c] [1,4] benzodiazepine were synthesized and evaluated for their central (flumazenil) and peripheral (PK11195) BDZs receptors (PBR) affinity. All compounds were found to be inactive at the central BDZs receptors. For the peripheral receptors compound 1c and 2 b-c were active. 1c, a member of methylated carboxamide derivatives was highly active which indicates the methyl group at position 10 increases the activity. The introduction of chlorine at position 7 significantly increases the activity as compared to the unsubstituted analogues indicating that it might interact with the lipophilic core of PBR. In the oxidized derivatives the activity is associated with the presence of unsaturation between positions 10-11 [17] (Figure 7).
a b c d e f
R Cl Cl Cl H H H
R1 H H Cl H H Cl
R2 H Cl H H Cl H
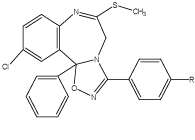
Another approach of fusion of heterocyclic ring with the BDZs nucleus was the synthesis of 1-[(p-substituted)phenyl]-3a-[(o-and p-substituted)-phenyl]-5-chloro-9-methylthio-10,3a-dihydro-[1,2,4]-oxadiazolo [2,3-b][1,4] benzodiazepines. These compounds are prepared by the reaction of 2-methylthio-5-[(o- p-substituted)-phenyl]-3H-7-chloro-[1,4] benzodiazepine with the benzonitrile oxide amoyl chloride and the resultant mixture were purified by the column chromatography. These compounds also showed the potential for antianxiety, anticonvulsant activity [18] (Figure 8).
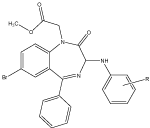
3-arylamine derivatives of 1,4-benzodiazepine-2-one were prepared by the reaction of 1-methoxycarbonyl methyl-7-bromo-5-phenyl-1,2-dihydro-3H-1,4-benzoidazepines-2-one with the substituted anilines. These compounds were prepared on the basis of quantitative structure activity relationship (QSAR) studies. Affinity of the compounds for the central and peripheral BDZs receptors was determined by radio ligand method by the ability of investigated compounds to displace competitively radioligand central BDZs receptor antagonist (Flumazenil) and peripheral BDZs receptors antagonists (Pk 11195) from their binding sites on BDZs receptors. Ortho substituted nitro aniline was more active than the para and Meta nitro aniline and nitro group was determinant of biological activity [20] (Figure 10).
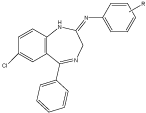
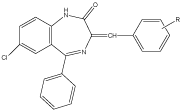
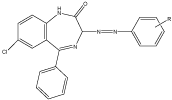
The introduction of substituted aniline (by imine linkage) at the position 2 of the BDZs showed increased neurotoxicity particularly when aniline is substituted at the para position with halogens atom, the most active in this series was compound with fluoro atom. The biological activity was determined by rota rod animal model and ethanol potentiation animal model [21] (Figure 11).
The introduction of benzylidene at the position 3 of the diazepine ring also produced the potent compounds especially when the benzylidene is substituted at para position with the electron withdrawing and lipophilic atom such as halogens. The compounds were evaluated for antianxiety activity via elevated plus maze apparatus [22] (Figure 12).
The diazonium salts derived aromatic group were attached at the position 3 and tested for their anticonvulsant activity. The compounds exhibited good anticonvulsant activity when aromatic ring was substituted with chloro, floro and nitro groups as determined by the maximal electro shock model and inhibition of pentylenetetrazole induced convulsions. This group seems to be affecting the conformation of diazepine ring which is significant for binding with the GABA receptors [23] (Figure 13).
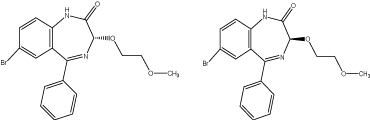
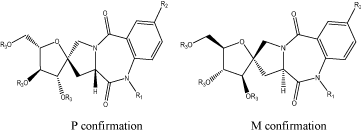
The alkoxy were attached at the position 3 and the resulting compounds were separated into two enantiomers by chiral chromatography. The activity of S enantiomer was found greater than R enantiomer for the cerebral benzodiazepine receptors (CBR) and peripheral benzodiazepine receptors (PBR) of rat. The selectivity of compounds for the CBR was greater than the PBR [24] (Figure 14).
Sugar based pyrrole moiety was connected to the BDZs and evaluated for their GABAA receptor affinity as an inhibitor of binding of [3H]Flunitrazepam. Different structure variation studies showed that N methylation and halogen substituent are important for the GABAA receptor binding. Pyrrole ring adopt a rigid conformation around the BDZs and in some cases increases the binding of these compounds. D and L fructose based pyrrole BDZs have different binding capacity. The biological activities of these compounds were less than the traditional GABA receptors ligand but they added some information regarding the conformations of BDZs while binding to the receptors [25] (Figure 15).
3-substituted 6-phenyl-4H-imidazo [1,5-a][1,4]-benzodiazepine are the compound containing the substituent at position 3 such as esters, amides and nitriles. The active compound in this series was having the ester at this position as determined by their activity to displace Flumazenil from their binding sites. Upon in vivo evaluation this compound showed prominent effect without the side effects. Molecular dynamics studies of the complex of BDZs with the receptors showed that binding is stable and smaller groups were active at this position while the larger bulkier groups showed less binding. The additional imidazo ring in these derivatives is involved in π - π interactions while the phenyl ring increase the hydrophobic interactions [26] (Figure 16).
Conclusion
Lot of compounds in the series of 1,4-BDZs have been synthesized with the substituent’s at almost all the positions. The diazepine ring seems to be most flexible for the various structural modification. Very little alteration is possible in benzene ring. At present structure activity relationship studies of BDZs are also associated with the molecular modeling and docking studies. Enantiomeric separation and QSAR studies of the drugs are also the subject of investigation. These information help in more clear understanding of the physiochemical properties of these drugs and their interaction with receptors at molecular level. The results of these techniques have started to appear as some new compounds exhibited their anxiolytic activity without producing undesirable effects. So in future it might be possible to get some BDZs which are active at the target receptors and without the side effects associated with the currently available BDZs.
- Bernardy NC (2013) The role of benzodiazepine in the treatment of post-traumatic stress disorders. PTSD. Research Quarterly 23: 9.
- Marangos PJ, Patel J, Boulenger JP, Rosenberg RC (1982) Characterization of peripheral-type benzodiazepine binding sites in brain using [3H] Ro 5-4864. Mol Pharmac 22: 26-32 .
- Burt DR, Kamatchi GL (1991) GABA a receptor subtypes: from pharmacology to molecular biology. FASEB J 5: 2916-2923 .
- Macdonald, RL, Olsen, RW (1994) GABA(A) receptor channels. Annu Rev Neurosci 17: 569–602 .
- O’Brien CP (1996) Drug addiction and drug abuse. In: Brunton L, Chabner B, Knollman B, Goodman and Gilman’s the pharmacological basis of therapeutics. 9th ed editors. McGraw-Hill Professional New York: 570.
- Reddy BR, Reddy DLP (2013) Structural analysis and binding modes of benzodiazepines with modelled GABAA receptor subunit gamma-2. Int J eng res app 3: 451-456 .
- Sternbach LH (1979) The benzodiazepine story. J Med Chem 22: 1-7.
- Sternbach LH, Randall LO, Banziger R, Lehr H (1968) Structure activity relationships in 1,4 benzodiazepine series. In: Berger A, Decker M, Drug affecting central nervous system. editors Inc. New York: 237-264.
- Meguro K, Kuwada Y (1970) Synthesis and structures of 7-chloro-2-hyrazino-5-phenyl-3H-1,4-benzodiazepines and some isomeric 1,4,5-benzotriazocines. Tetrahedron lett 11(47): 4039-4042.
- Hester JB, Duchamp DJ, Chidester CG (1971) A synthetic approach to new 1,4 benzodiazepine derivatives. Tetrahedron lett 20: 1609-1612 .
- Burger A (1991) Isosterism and bioisosterism in drug design. Prog Drug Res 37: 287-371 .
- Zhang W, Liu R, Huang Q, Zhang P, Koehler KF, (1995) Synthesis of 5-thienyl- and 5-furyl-substituted benzodiazepines: Probes of the pharmacophore for benzodiazepine receptor agonists. J Med Chem 30: 483-96 .
- Desarro GB, Chirnirri A, Zappala M, Guisti P, Lipartiti M, et al. (1996) Azirino [1,2-d] [1,4]benzodiazepines derivatives and related 1,4 benzodiazepines as anticonvulsant agents in DBA2 Mice. Gen Pharmac 27: 1155-1162 .
- Castellano S, Zorzin L, Florio E, Frausin F, Stefancich G (2001) Synthesis of 11-aryl-5H-imidazo [2,1-c] [1,4] benzodiazepines and their benzodiazepine and A1 adenosine binding activity. II Farmaco 56: 771-778 .
- Yang J, Che X, Dang Q, Wei Z, Gao S, et al. (2005) Synthesis of tricyclic 4-chloro-pyrimido[4,5-b] [1,4] benzodiazepines. Org lett 7: 1541-1543 .
- Naryana B, Raj KKV, Ashalatha BV, Kumari NS (2006) Synthesis of some new substituted triazolo[4,3a] [1,4] benzodiazepines derivatives as a potent anticonvulsants. Eu Jour Med Chem 41: 417-422 .
- Rizzetto E, Castellano S, Florio C, Vadori M, Stefancich G (2006) 5-Aryl-imidazo [2,1-c][1,4]benzodiazepine derivatives as tricyclic constrained analogues of diazepam and Ro5-4864. Synthesis and binding properties at peripheral and central benzodiazepine receptors. Pharmazie 61: 505–510 .
- Cortes E, Ramirez EP, Cortes OGM (2007) 1-[(p-substituted)phenyl]-3a-[(o- and p-substituted)-phenyl]-5-chloro-9-methylthio-10,3a-dihydro-[1,2,4]-oxadiazolo[2,3-b][1,4]benzodiazepines. J Heterocyclic Chem 44: 189-192 .
- Anzini M, Braile C, Valenti S, Capelli A, Vomero S, et al. (2008) Ethyl 8-fluoro-6-(3-nitrophenyl)-4H-imidazo [1,5-a] [1,4] benzodiazepine 3-carboxylate as a novel, highly potent and safe antianxiety agent. J Med Chem 51: 4730–4743 .
- Burenkova NA, Pavlovsky VI, Oleinich IA, Boyko IA, Makan S Yu, et al. (2009) Synthesis and selectivity of 1-methoxycarbonyl methyl-7-bromo-5-phenyl-1,2-dihydro-3H-1,4-benzoidazepines -2-one binding for CNS benzodiazepine receptors. Ukr Bioorg Acta 1: 8-15 .
- Rashid M, Ahmed B, Mishra R (2010) Synthesis and psychotropic activity of some substituted aniline derivatives of 7-chloro-5-phenyl-1,3-dihydro-1H, 3H-1,4-bezodiazepines-2-ones. Int J Pharm Sci 2: 616-622.
- Rashid M, Hussian A, Mishra R, Ahmed B (2010) Synthesis and antianxiety activity of some 7-chloro-5- phenyl-1,3-dihydro-1H,3H-1,4 benzodiazepine-2-one derivatives in mice. Der Pharmacia lett 2: 253-256 .
- Rashid M, Mishra R, Hussain A, Ahmed B (2010) Synthesis of some novel C3 substituted new diazo -[1,4] -bezodiazepines-2-one derivatives as potent anticonvulsants. Chem Sci J 5: 1-9 .
- Andronati S, Semenishyna E, pavlovsky V, Simonov Y, Makan S, et al. (2010) Synthesis, structure and affinity of novel 3-alkoxy-1,2-dihydro-3H-1,4-benzodiazepine-2-ones for CNS central and peripheral benzodiazepine receptors. Eur J Med Chem 45: 1346-1351 .
- Araujo AC, Rauter AP, Nicotra F, Airoldi C, Costa B, et al. (2011) Sugar based enantiomeric and conformationally constrained pyrollo [2,1 c] [1,4] benzodiazepines as potential GABA ligands. J Med Chem 54: 1266-1275 .
- Anzini M, Valenti S, Braile C, Cappelli A, Vomero S, et al. (2011) New insight into the Central Benzodiazepine receptor ligand Interactions: Design, synthesis, biological evaluation and molecular modeling of 3-Substituted 6-Phenyl-4H-imidazo[1,5-a]-[1,4]benzodiazepines and related Compounds. J Med Chem 54: 5694-5711 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
