Open Journal of Biological Sciences
Some aspects of physiological studies of two reef building corals in the red sea
Yahya AM Floos1* and Abdulmohsin AAl-Sofyani2
2Department of Marine Biology, Faculty of Marine Sciences, King Abdulaziz University, Saudi Arabia
Cite this as
AM Floos Y, AAl-Sofyani A (2023) Some aspects of physiological studies of two reef building corals in the red sea. Open J Biol Sci 8(1): 008-027. DOI: 10.17352/ojbs.000034Copyright
© 2023 AM Floos Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Some aspects of the physiology of the corals Seriatopora hystrix and Lobophyllia corymbosa described in this paper. At the study site, the lowest mean of seawater temperature is 27.42 °C and 27.17 °C at 5m and 10m depths respectively during winter, while the maximum mean of seawater temperature was 32.67 °C and 31.17 °C in 5 m and 10 m depths respectively during summer. L. corymbosa, had a lower number of zooxanthellae 0.05×105 , 0.43×105 , 0.06×105 and 0.46×105 mg-1 dry tissue weight than S. hystrix 0.19 ×105 , 5.1 ×105 , 0.27×105 and 9.59×105 mg-1 dry tissue weight in two season and two depths respectively. The mean respiration rate of whole colonies of S. hystrix was higher than that of L. corymbosa at the same depths both in summer and winter. The mean dark respiration rate decreases with increasing depths. The mean photosynthesis vs irradiance curves of S. hystrix and L. corymbosa were plotted to the hyperbolic tangent function (Chalker,1981) for summer and winter season. The growth rate was linear during each period of measurement during summer, the highest mean daily skeletal growth rate of S. hystrix was 2.3 ± 1.3 (20) mg.skel.d-1 in 10m depth and it was 1.6 ± 0.5 (21) mg .skel.d-1 at 5m depth. Whilst during winter, the lowest was 1.9 ± 0.96 (20) mg .skel. d-1 at 10m and also lowest (1.5 ± 0.7 (20) mg .skel. d-1) at 5m depth. The growth rate of the two species was lower in the winter than in summer.
Introduction
Coral reefs are one of the most interesting environments due to their enormous biodiversity. Corals, which are members of the phylum Coelenterata, class Anthozoa, sub-class Hexacorallia and order Scleractinia [1-3], are the dominant species. The Scleractinia has been divided into hermatypic and ahermatypic. The terms are usually used by biologists to distinguish between corals with or without symbiotic algae (zooxanthellae) respectively [4]. The majority of hermatypes is reef building and live usually in shallow water, whilst the ahermatypes are non-reef building, and are often found in deep water.
Hermatypic corals are restricted to tropical and sub-tropical seas where the temperature is not lower than 18 °C, with optimal reef development between 25° and 29 °C this is expressed in latitudinal patterns of coral reefs distribution and diversity [4-6]. Rising seawater temperature is one of the greatest threats to the persistence of coral reefs [7]. In 1998, an estimation of 16% of the world’s living corals were eliminated in a single warming event related to El Niño [8], during this event, sea temperatures warmed to 2-3 C above long-term average summer temperatures and resulted in a catastrophic “bleaching” event that caused significant mortality of many species of coral. The impacts of this thermal event decrease the percent living coral cover of shallow reef worldwide (Hoegh-guldberg 1999). Coral reefs are under high threats including ocean warming and human disturbance, which together lead to widespread coral mortality [9,10].
There are three main metabolic processes in corals (i) photosynthesis (CO2 consumption and O2 release), (ii) respiration (O2 consumption and CO2 release) and the (iii) biogeogenic process of calcification (deposition of CaCO3). These processes are interconnected in corals [11].
Zooxanthellae of scleractinian corals have been grouped into clades. In recent studies, clade “D” was found in P. verrucosa from eastern Pacific reefs [12], while clade “C” was found in P. damicornis [13]. The work of Baker, et al. [14]. suggests that members of clades D may have greater thermal tolerances than clade C in their zoanthid samples of Palythoa caribaeorum. Clade A and B inhabit shallow water colonies, while clade C zooxanthellae are found in deeper water colonies, or in shaded areas of the same individual coral colony [15,16].
The term bleaching refers to the disruption of the coral-algae symbiosis caused by the loss of photopigments or endosymbiotic dinoflagellates from the animal tissues [17]. During the phenomenon of bleaching in August 1998 some coral species such as P. damicornis had less resistance against bleaching than P. verrucosa due to the unusual high seawater temperature and calm sea condition [18,19]. Nakamura, et al. [20] reported that the dark respiration rate of the coral species Acropora pruinosa was higher in winter than in summer, especially in the temperature range from 20–30 °C. Moreover, Coles & Jokiel [21] showed very high response in metabolic rate with temperature change in corals Pocillopora damicornis, Montipora verrucosa, Porites compressa and Fungia scutaria over the range 19-31° C in Hawaii and Enewetak.
Through previous studies, it is clear to us that different species of corals have different autotrophic photosynthesis and heterotrophic feeding abilities [22] in addition to different tissue biomass and lipid concentrations [23].
Growth of scleractinian corals can be divided in two mechanisms: first, skeletal growth due to the deposition of an external skeleton of calcium carbonate by the synthesis of an organic matrix, a process called as calcification, and the second one is the tissue growth. According to the light-enhanced calcification theory [24,25], the symbiosis with zooxanthellae is helping the process of skeletal growth. According to this theory, calcification of the coral host is enhanced by photosynthesis of zooxanthellae [26-28]. Certainly, on average, calcification in light is found to be around three times higher than calcification in darkness [25]. However, the growth rate of hermatypic corals is influenced by external factors such as light quality and intensity, temperature, and sedimentation and by internal factors such as reproduction, genetics, growth form of colony, number or type of zooxanthellae [29,30].
Photosynthesis and calcification are spatially separated processes (photosynthesis occurs in the oral tissue layer and calcification in the aboral tissue layer), they do share a common pool of inorganic carbon inside the coelenteron of the coral host, accounting for the interaction between these two processes, the exact mechanisms of the enhancement of calcification by photosynthesis are still a matter of debate [25]. Some of the proposed mechanisms include that: (1) photosynthesis provides energy for the energy-demanding processes associated with calcification, such as calcium transport and organic matrix synthesis and (2) photosynthesis raises intracellular pH and intracellular saturation state of calcium carbonate, thereby favouring the precipitation of calcium carbonate [24,26].
The main objective of this study was, to compare the respiration, photosynthesis, growth and their sensitivity range of Seriatopora hystrix and Lobophyllia corymbosa to the environmental factors during two seasons (Summer and Winter). Results obtained this study will improve our knowledge on the ecology and physiology of Red Sea corals.
Materials and methods
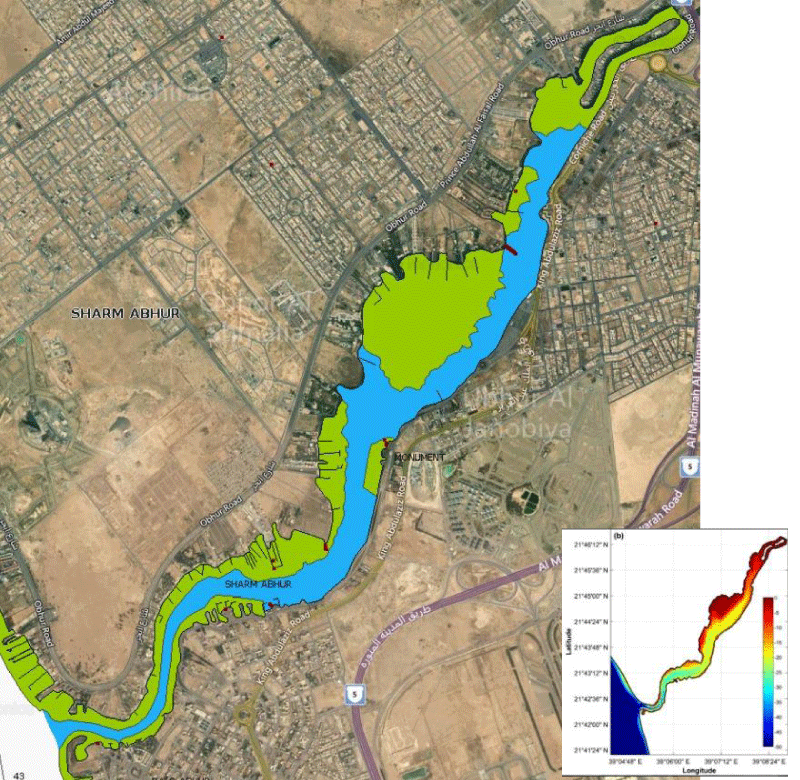
The study was carried out in the onshore laboratory of the Faculty of Marine Sciences, King Abdulaziz University, Jeddah, which is located adjacent to the fringing reefs of an inlet, Obhur creek, away from the north of the city Creek (Figure 1). The study site was located at the mouth of the creek on its northern shore, at the water depths of 5 m and 10 m. Specimens of S. hystrix and L. corymbosa were collected at this depths.
Measurement of environmental parameters
Water temperature was measured in situ by using two maximum and minimum thermometers attached to the reef in a shaded location. They were read at monthly intervals from July 2009 to Jun 2010. Underwater down-dwelling UV temperature loggers (HOBO) launch devices (model UA-002-64) were setup in the lab using the HOBOware® Pro software. The sampling intervals were of 15 min and the total recording light is one day in summer 2010 and during winter 2010. The recording time start at 06:00 pm to 06:00 am measuring photosynthetically Active Radiation (P.A.R) between 400 and 700 nm.
Specimen preparation
All determinations were made using branch tips or “nubbins” [32-34]. Samples from L. corymbosa & S. hystrix were collected from the study area at 5m and 10m water depths. Samples were transported in shaded buckets to the laboratory. The nubbins from each collection depth were collected and transported to the laboratory in shaded buckets. Any debris or fouling organism that had settled on the tiles bearing the nubbins were carefully scraped off. The nubbins were then placed in the running seawater tank overnight. These were attached to pre-weighted 3×3 cm acrylic tiles, using a small quantity of cyanoacrylate adhesive. Similar-sized pieces of L. corymbosa, with more irregular growth form, were made in to nubbins in the same way. They were then placed on racks and returned to the reef to recover for at least once in a week.
Dark respiration
Respiration oxygen uptake of nubbins was measured in darkness using respirometer cell immediately before the commencement of a photosynthesis run. Respiration rate was linear with production of O2 over the 100% to 90% saturation levels that were used for the determination. Dark respiration values were added to the net photosynthesis values to obtain gross photosynthesis.
Respiration rate of Zooxanthellae were determined on freshly isolated sample, from nubbins that had been maintained in darkness for 12 h. Coral tissue was removed with a water pick, and the slurry was centrifuged at 2000 x g for 1 min. Further separation was achieved with a hand-held potter homogenizer, after which the suspension was centrifuged, washed with seawater thrice and re-suspended in 1 ml of filtered seawater. The rate of oxygen uptake was measured in darkness in an RC 300 respirometer cell (Strathkelvin instrument) with a 1302 paleographic oxygen electrode. Zooxanthellae concentrations within the cell were measured on a subsample using haemacytometer
Photosynthesis
Nubbins were placed in water-jacketed clear acrylic closed respirometer cell with a water volume of 128 ml. Oxygen flux was detected by a polarographic oxygen electrode (Strathkelvin Instruments, 1302) connected to a oxygen meter. Light was provided by a bank of overhead fluorescent lambs whose output was varied between 25 and 300 µE m-2 sec-1 by mean of a variable transformer. Net oxygen production rates were normalized to dry weight of coral tissue, mean values derived at each irradiance value and best fit curves for the plots of net ( P v I) were obtained using a hyperbolic tangent function curve-fitting program [35].
Growth
Nubbins were placed on racks at 5 m and 10 m in the experimental sites on the fringing reef of S . hystrix and L. corymbosa , after taking the buoyant weight using a precise 120A balance [36]. They were returned to the laboratory for reweighing after intervals of approximately seven days. Growth rates were determined in summer 2009 and winter 2010 and were expressed in mg skeleton d-1.
Results
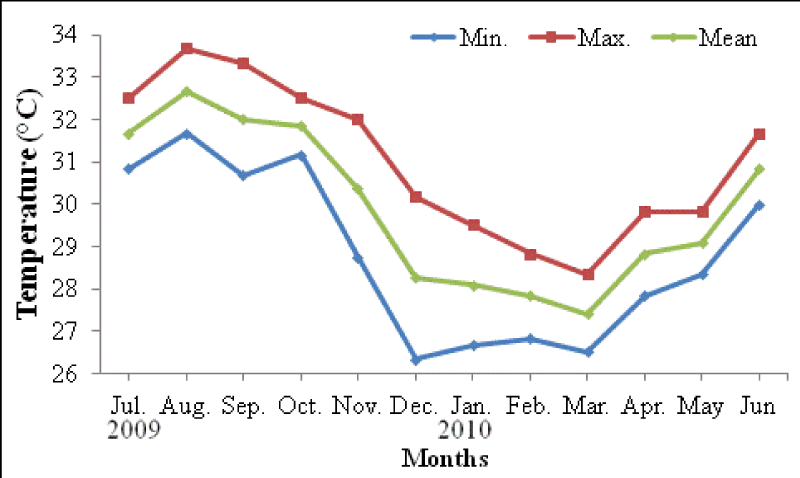
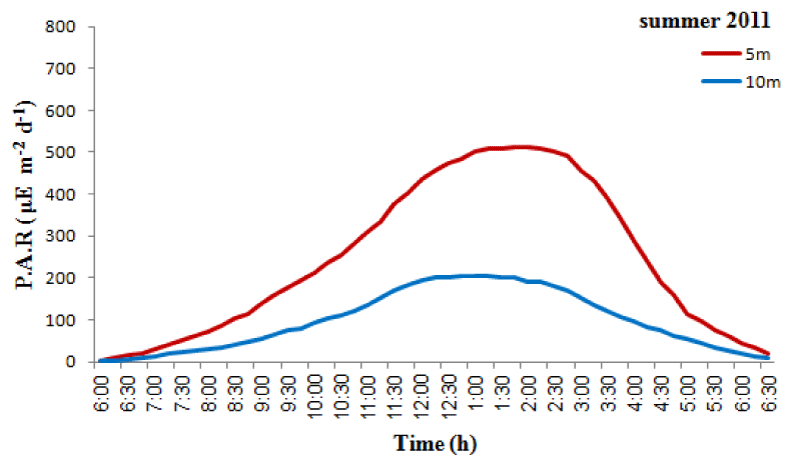
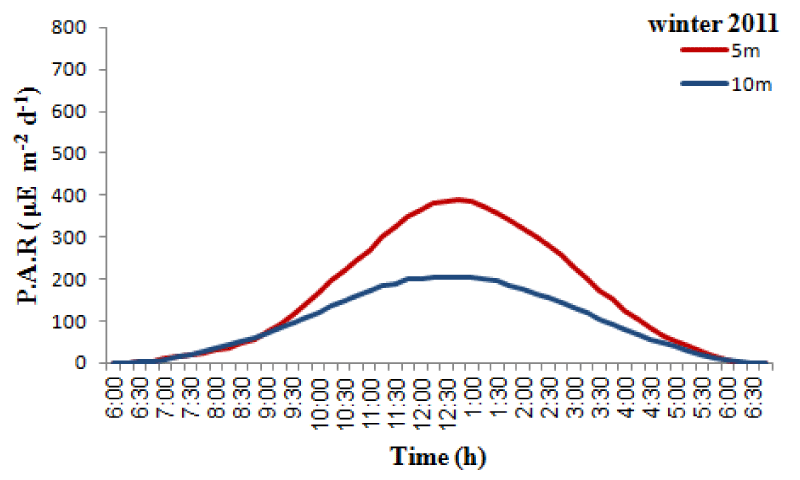
The monthly maximum and minimum mean temperatures of the study sites are given in Figures 2,3. The lowest temperature observed was 27.42 ± 0.80 °C and in March 2010 and the highest observed temperature was 32.67 ± 0.29 °C during August 2009. The mean difference between the maximum and minimum readings was 2.5 °C. The daily maximum variation of light intensity of photosynthetically active radiation (P.A.R) and the duration are shown in the Figures 4,5. The P.A.R of daily light curve was maximum in the summer (August 2010) with the value of 512 μE s-1 m-2 and minimum in the winter (February 2010) with a value of (389 μE s-1 m-2).
Skeleton and Biomass Characteristics of S. hystrix and L. corymbosa are shown in (Table 1). The mean density of the skeleton of both species was identical at 2.5 ± 0.16 (10) g.cm-3 and 2.75 ± 0.05 (10) g.cm-3 respectively. The mean surface area (cm2 g-1 skeleton) was higher in L. corymbosa 4.73 ± 2.31 (10) cm2 g-1 skeleton than S. hystrix 3.58 ± 1.2 (10) cm2 g-1 skeleton with no significant difference. The mean dry tissue weight g-1skeleton in summer at 5m depth was higher in L. corymbosa 39.6 ± 24.9 (10) mg.d.t.g-1 skeleton than S. hystrix 24.9 ± 16.4 (10) mg.d.t.g-1 skeleton and was significantly different (t - test p ≤ 0.03). While in summer at 10m depth was higher in L. corymbosa 45.49 ± 14.00 (10) mg.d.t.g-1 skeleton than S. hystrix 24.9 ± 19.6 (10) mg.d.t.g-1 skeleton and was significantly different (t - test p ≤ 0.01). On the other hand the mean dry tissue weight per g. skeleton in winter at 5m depth was higher in L. corymbosa 36.9 ± 4.9 (10) mg.d.t.g-1 skeleton than S. hystrix 13.3 ± 4.9 (10) mg.d.t.g-1 skeleton and was significantly different (t - test p ≤ 0.001). While in winter at 10m depth was higher in L. corymbosa 44.1 ± 15.00 (10) mg.d.t.g-1 skeleton than S. hystrix 11.7 ± 5.8 (10) mg.d.t.g-1 skeleton and was significantly different (t-test P ≤ 0.0001) relative biomass between the two species and two season. The values remain significantly different when they were expressed on a surface area basis, i.e. 9.17 ± 3.97 (10) mg.d.t.cm-2 higher in L. corymbosa than S. hystrix 7.81 ± 5.82 (10) mg.d.t.cm-2 in summer at 5m depth and was not significantly difference. While in summer at 10m depth, it was higher in L. corymbosa 11.05 ± 4.91 (10) mg.d.t.cm-2 than S. hystrix 7.7 ± 6.6 (10) mg.d.t.cm-2 and was no significantly different. While in winter at 5m depth it was higher in L. corymbosa 8.16 ± 2.43 (10) than S. hystrix 4.14 ± 2.0 (10) mg.d.t.cm-2 and was significantly different (t - test p ≤ 0.001). However, in winter at 10m depth it was higher in L. corymbosa 11.57 ± 7.26 (10) than S. hystrix 3.32 ± 1.6 (10) mg.d.t.cm-2 and is significantly different (t - test p ≤ 0.003) between the two species and two season.
There was a significant seasonal difference in the numbers of zooxanthellae of both the species at two different depths on the basis of biomass and surface area (t - test p < 0.005). L. corymbosa, had a lower number of zooxanthellae 0.05×105 , 0.43×105 , 0.06×105 and 0.46×105 mg-1 dry tissue weight than S. hystrix 0.19 ×105 , 5.1 ×105 , 0.27×105 and 9.59×105 mg-1 dry tissue weight in two season and two depths respectively. S. hystrix had higher number of zooxanthellae in 5m depth both in summer and winter respectively (1.2×105, 19.09×105 cm-2) and in 10m depth it was 1.6×105 and 25.7×105 cm-2 in summer and winter than the L. corymbosa. At 5m depth, the observed zooxanthellae of L. corymbosa was 0.50×105 in summer and 3.51×105 cm-2 in winter and it was 0.58×105 in summer and 4.42×105 cm-2 in winter respectively
Dark respiratio
- Colony: The colony respiration mean values of both species were estimated using tissue biomass and surface area studies and are given in (Table 2) and (Figures 6-9).
- Species variations: The mean respiration rate of whole colonies of S. hystrix was higher than that of L. corymbosa at the same depths both in summer 2009 and winter 2010. The difference was significant in all cases (Table 3). The difference significant was found when making similar comparisons on the rate of respiration of freshly isolated zooxanthellae at (t - test p ≤ 0.05).
- Seasonal variations
Seriatopora hystrix: In S. hystrix, the average rate of dark respiration was higher 4.56 ± 2.29 (10) µl O2 mg-1.d.t h-1 during summer (32 °C) than winter (30 °C) season (3.164 ± 1.32 (11) µl O2 mg-1.d.t h-1) at 5m depth. But the variation was not significant at this 5 m depth (Tables 3,4). The surface area value was lower (16.97 ± 8.54 (10) µl O2 cm-2 h-1) during summer (32 °C) than the winter (30 °C) season (22.01 ± 9.194 µlO2 cm-2 h-1). with no significant difference at the 5 m depth (Table 2 ). It was also higher in summer (31 °C) at 10 m depth 2.477 ± 1.143 (10) µl O2 mg-1.d.t h-1, than the winter (29 °C), 1.432 ± 0.553 (10) µl O2 mg-1.d.t h-1. The difference was significant at the 10m depth (t - test p ≤ 0.01). The surface area comparison studies revealed that it was lower (8.09 ± 3.728 (10) µl O2 cm-2 h-1) during summer (31 °C) than the (9.958 ± 3.842 (10) µl O2 cm-2 h-1) winter (29 °C). The surface area difference was not significant in the 5m and 10m depths (Table 2).
Lobophyllia corymbosa: The mean rate of the dark respiration was higher 1.035 ± 0.469 (10) µl O2 mg-1 d. t. wt. h-1 during summer (32 °C) than winter (30 °C) 0.844 ± 0.3823 (11) µl O2 mg-1 d. t. wt. h-1, at 5m depth. The difference was no significant at depth (Table 2). On basis of surface area, the values show higher in summer (32 °C) 8.082 ± 3.67 (10) µl O2 cm-2. h-1, and winter (30 °C), 5.871 ± 2.135 (10) µl O2 cm-2. h-1, at the same depth, with no significant difference (Table 2). The mean rate of dark respiration on biomass and basis of surface area were considerably lower, 0.618 ± 0.113 (14) µl O2 mg-1 d.t.wt.h-1, (5.761 ± 1.054 (10) µl O2 cm-2. h-1 ), during summer (31 °C) than 0.471 ± 0.091 (14) µl O2 mg-1 d. t. wt. h-1, (3.167 ± 2.128(11) µl O2 cm-2 h-1 ), during winter (29 °C) at 10m (Table 2).
- Depth variations
Seriatopora hystrix: The mean rate of dark respiration was significantly higher at 5m depth, 4.563 (summer 32 °C ) and 3.164 (winter 30 °C) µl O2 mg-1 d. t. wt. h-1 than at 10m 2.477 (summer 31 °C) and 1.432 (winter 29 °C) µl O2 mg-1 d. t. wt. h-1(t - test p ≤ 0.005 summer and t -test p ≤ 0.001 in winter).
Lobophyllia corymbosa: The mean value of oxygen consumption decreases with increasing depth 1.035 ± 0.469 (10) during summer at 5m depth and 0.618 ± 0.113 (14) µl O2 mg-1 d. t. wt. h-1 at 10m and increasing with decreases depth 0.844 ± 0.3823 (11) and 0.471 ± 0.0.91 (14) at 5m and 10m during winter respectively. There was a significant difference during summer whilst during winter there was no significant difference.
- Zooxanthellae
The mean rate of dark respiration of zooxanthellae on the basis of tissue biomass and the surface area is shown in (Table 2).
a) Seasonal variation
Seriatopora hystrix: On the basis of tissue biomass, the average rate of respiration was lower 0.0675 ± 0.030 (10) µl O2 mg-1 d. t. wt. h-1 in summer (32 °C) than the winter (30 °C) 0.779 ± 0.274 (10) µl O2 mg-1 d. t. wt. h-1 at 5m depth. This results were significantly varied at 5m depth (Tables 3,4). In the case of surface area, the results were lower and significantly varied in summer (32 °C) 0.467 ± 0.212 (10) µl O2 cm-2 h-1 than in winter (30 °C) 2.88 ± 1.014 (10) µl O2 cm-2 h-1, at 5m depth (Table 2). In the case of 10m depth, the value was 0.0569 ± 0.0196 µl O2 mg-1 d. t. wt. h-1 (10) in summer (31 °C) and 0.1918 ± 0.098 (11) µl O2 mg-1 d. t. wt. h-1 in winter (29 °C) respectively. The difference in the value was apparently significant (Tables 3,4). In addition, the surface area was lower (0.39 ± 1.357 (10) µl O2 cm-2 h-1) in summer (31 °C) season than (4.669 ± 2.39 (12) µl O2 cm-2 h-1) the winter (29 °C) at 10m depth. The surface area variation was a significantly different at 10m depth (Table 2).
Lobophyllia corymbosa: The average rate of zooxanthellae respiration was lower (0.0865 ± 0.015 (10) µl O2 mg-1 d. t. wt. h-1) in summer (32 °C) and higher (0.2537 ± 0.16 (10) µl O2 mg-1 d. t. wt. h-1) in winter (30 °C) at 5m depth. The seasonal difference of the zooxanthellae was significantly varied (Table 2). The surface area of value was significantly lower 0.724 ± 0.130 (10) µl O2 cm-2 h-1 in summer (32 °C) and higher 1.982 ± 1.275 (12) µl O2 cm-2 h-1 in winter (30 °C) in the 5m depth (Table 2 ). Similarly at 10m depth, the respiration value was lower 0.0305 ± 0.0119 (10) µl O2 mg-1 d. t. wt. h-1 in summer (31 °C) and higher 0.1264 ± 0.0342 (10) µl O2 mg-1 d. t. wt. h-1 in winter (29 °C). This was also significantly varied as shown in the Table 3,4. In 10m depth, the surface area was lower 0.303 ± 0.114 (11) µl O2 cm-2 h-1 in the summer (31 °C) higher 1.177 ± 0.325 (10) µl O2 cm-2 h-1 in the winter (29 °C). There was significant difference observed between the summer and winter season (Table 2).
(b) Depth variations
Seriatopora hystrix: The mean dark respiration rate decreases with increasing depths. In summer, the value was higher 0.0675 µl O2 mg-1 d. t. wt. h-1 at 10m depth and lower 0.0569 µl O2 mg-1 d. t. wt. h-1 at 5m depth, but it was not significantly different. In winter at 5m depth, the dark respiration rate was higher 0.779 µl O2 mg-1 d. t. wt. h-1 and lower 0.192 µl O2 mg-1 d. t. wt. h-1 at 10 depth. There was a significant difference between 10 m depth.
Lobophyllia corymbosa: The zooxanthellae of this species showed the same pattern of respiration rates which decrease with increasing depths, the rates was lower at 5m, during both season 0.0865 and 0.2537, and higher at 10m, 0.0305 and 0.1264 µl O2 mg-1 d. t. wt. h-1 during summer and winter respectively. There was a significant difference between the two depths during summer but no significant difference in winter.
Photosynthesis
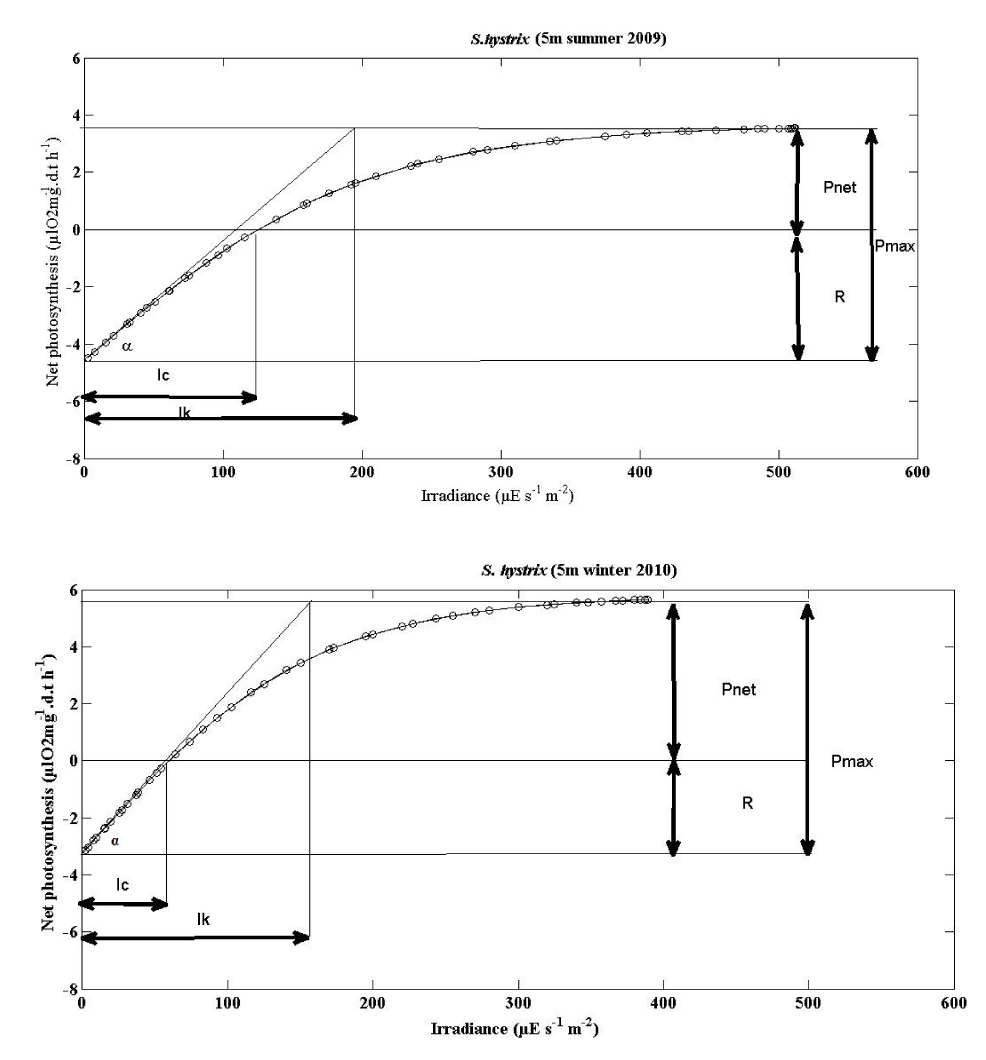
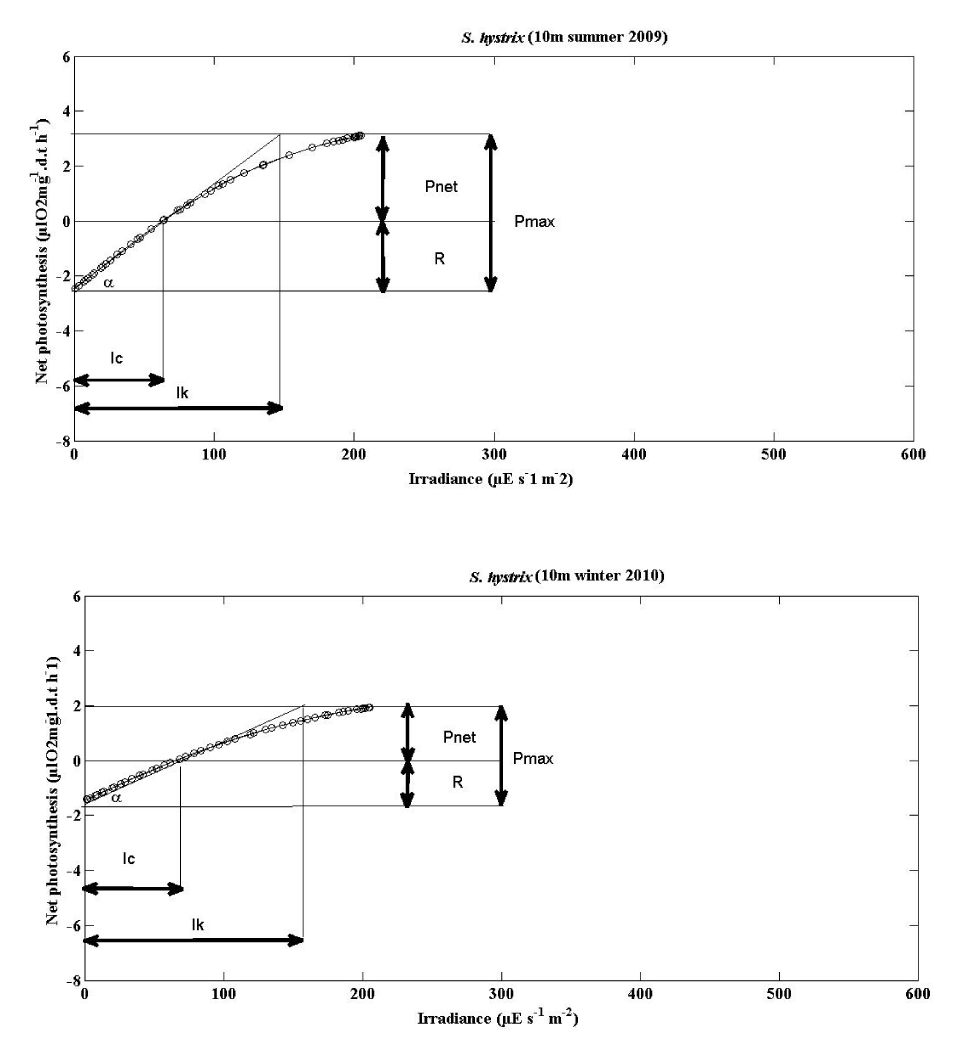
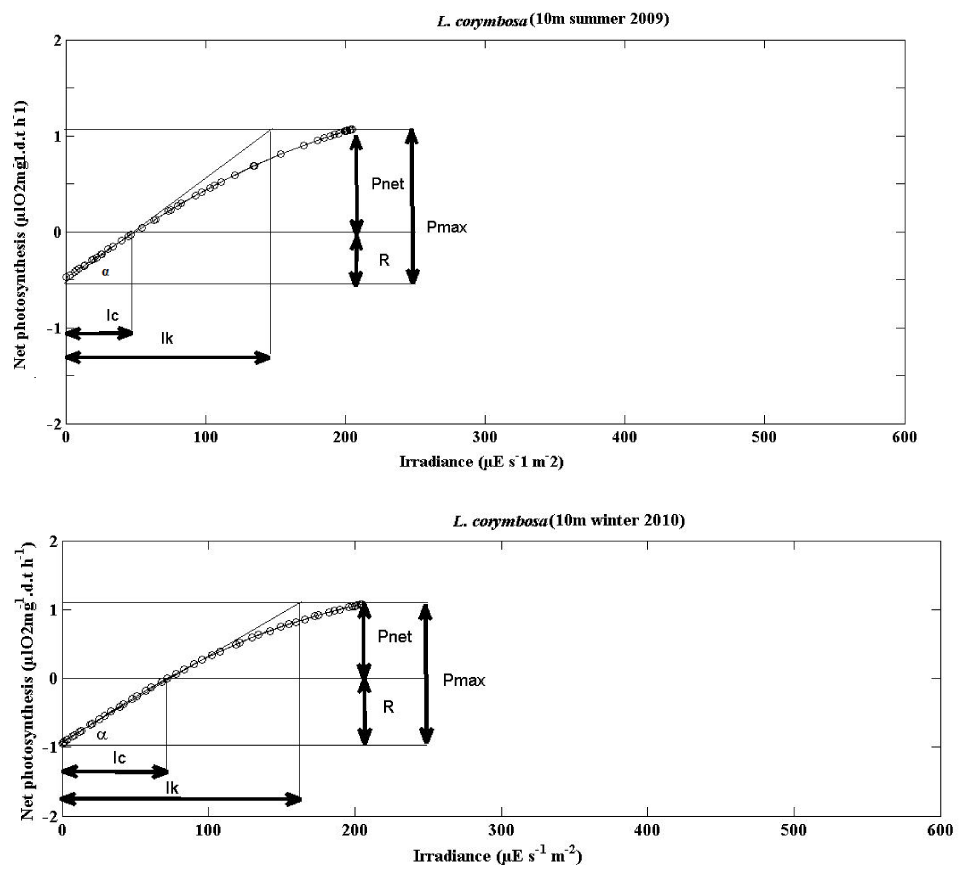
The mean photosynthesis vs irradiance curves of S. hystrix and L. corymbosa were plotted to the hyperbolic tangent function [35] for summer 2009 and winter 2010 season (5m and 10m depths). They are shown in the Figures 6-9. The photosynthesis characteristics of these curves are summarized in the Table 4.
(a) Species Variations
S. hystrix had a maximum gross photosynthesis (Pgmax) in the winter season ranging from 4.145 ± 2.402 (10) µl O2 mg-1 d. t. wt. h-1 at 10m depth to 8.712 ± 4.2114 (11) µl O2 mg-1 d. t. wt. h-1 at 5m depth. In the Summer season it was ranging from 6.4339 ± 2.892 (10) µl O2 mg-1 d. t. wt. h-1) at 10m depth to 8.1965 ± 5.144 (10) µl O2 mg-1 d. t. wt. h-1 at 5m depth. In the case of L. corymbosa, the maximum gross photosynthesis of the winter season was in the range of (0.9515 ± 0.222 (10) µl O2 mg-1 d. t. wt. h-1at 5m depth, to 2.379 ± 1.1915 (14), µl O2 mg-1 d. t. wt. h-1 at 10m depth. In summer season, it was ranging from 2.067 ± 0.4127 (10) µl O2 mg-1 d. t. wt. h-1) at 10m depth to 2.594 ± 0.094 (10) µl O2 mg-1 d. t. wt. h-1) at 5m depth. The difference was significant between both species at each depth during the summer (t - test p ≤ 0.005), but not significant during winter (t - test p < 0.001).
(b) Seasonal variation
Seriatopora hystrix: The mean maximum gross photosynthesis (Pgmax), rates during summer were slightly lower 8.1965 ± 5.144 (10) µl O2 mg-1 d. t. wt. h-1 than the gross photosynthesis 8.7129 ± 4.211 (11) µl O2 mg-1 d. t. wt. h-1 of the winter season at 5m depth. They were not significantly different. In the case of 10m depth, the values were higher in summer 6.4339 ± 2.89 (10) µl O2 mg-1 d. t. wt. h-1 than in winter 4.145 ± 2.40 (10) µl O2 mg-1 d. t. wt. h-1 but they were not significantly different.
Lobophyllia corymbosa: The mean maximum gross photosynthesis (Pgmax) was higher 2.594 ± 0.094 (10) µl O2 mg-1 d. t. wt. h-1 during summer while compared with the winter season (0.9515 ± 0.222 (11) µl O2 mg-1 d. t. wt. h-1) at 5m depth. It was significantly higher during winter than the summer.
At 10m depth, the average maximum gross photosynthesis rate in summer, 2.067 ± 0.4127 (10) µl O2 mg-1 d. t. wt. h-1 was not significantly different from the winter value 2.379 ± 1.1915 (10) µl O2 mg-1 d. t. wt. h-1.
b) Depth variations
Seriatopora hystrix: The mean maximum gross photosynthesis (Pgmax) was significantly higher in summer at 5m depth, 8.1965 ± 5.1444 (10) when compared with 10m depth 6.4339 ± 2.892 (10) µl O2 mg-1 d. t. wt. h-1. Whilst the gross photosynthesis (Pgmax) at 5m depth during winter was maximum (8.7129 ± 4.2114 (11) µl O2 mg-1 d. t. wt. h-1), and was not significantly different from 4.145 ± 2.402 (10) µl O2 mg-1 d. t. wt. h-1 at 10m depth.
Lobophyllia corymbosa: The gross photosynthesis was maximum (2.594 ± 0.094 (10) µl O2 mg-1 d. t. wt. h-1) 5 m depth in summer and minimum (2.067 ± 0.4127 (10) µl O2 mg-1 d. t. wt. h-1) during summer at 10m depth respectively. They were not significantly different in summer. Whilst in winter season, the minimum gross photosynthesis (0.9515 ± 0.222 (11) µl O2 mg-1 d. t. wt. h-1) was observed in the 5m depth and was significantly different than the maximum (2.379 ± 1.1915 (14) µl O2 mg-1 d. t. wt. h-1) observed at 10 m depth.
- Characteristics of the (P v I) curve
The mean parameters of the P v I curve for two species were shown in (Tables 5,6) and (Figures 6-9).
- The initial slope (a)
Seriatopora hystrix: The mean values for α indicated that the zooxanthellae of S. hystrix could slightly utilize the light during the winter 0.0724 ± 0.0614 (11) at 5m depth and also at 10m depth 0.0231 ± 0.0133 (10). But in summer it utilized more light 0.0455 ± 0.0294 (10) at 5 m depth and also more (0.042 ± 0.0163 (10) µl O2 mg-1.d.t h-1 / μE s-1 m-2) at 10m depth.
The differences were not significantly different in seasons. During the summer and winter season, the α curve was also slightly higher at 5m depth than the 10m depth. The α values were not significantly different according to the depths.
Lobophyllia corymbosa: During summer, the mean values of α were significantly lower at 5m depth, 0.0099 ± 0.0045 (10) µl O2 mg-1.d.t h-1 / μE s-1 m-2 and higher 0.0111 ± 0.0041 (10) µl O2 mg-1.d.t h-1 / μE s-1 m-2 at 10 m depth. Where as in winter, it was lower 0.0053 ± 0.0023 (10) at 5 m and higher 0.0135 ± 0.0059 (10) at 10 m depth. The value was increased with depth both in summer and winter hence no significant difference.
- IK
Seriatopora hystrix: During summer, the values of IK, was higher 198.5 ± 78.728 (10) μE s-1 m-2 at 5m and lower 154.3 ± 25.829 (10) μE s-1 m-2 at 10m depth without significant difference. Whereas in winter, they were lower 158.273 ± 74.096 (11) μE s-1 m-2 at 5 m and higher 180.7 ± 30.423 (10) μE s-1 m-2 at 10m. These values were not significantly varied.
Lobophyllia corymbsa: During the summer season the light intensity was higher which was sufficient to saturate photosynthesis (291.1 ± 113.588 (10) μE s-1 m-2 at 5m depth and 250.4 ± 106.39 (10) μE s-1 m-2) at 10m water depth. In winter it was lower (202.727 ± 75.39 (10) and 180.357 ± 58.5 (14) μE s-1 m-2 at 5m and 10 m depths respectively. In summer, these values were similar in the depths whereas in winter they were significantly different.
- IC
Seriatopora hystrix: During summer, IC were higher with the range of 114.6 ± 31.63 (10) μE s-1 m-2 at 5m depth whereas lower with the range of 65.8 ± 14.868 (10) μE s-1 m-2 at 10m depth. Similarly, higher in winter season (66.364 ± 40.418 (10) μE s-1 m-2) at 5m depth and lower in winter season (77.5 ± 29.83 (10) μE s-1 m-2) at 10m depth. During summer, the IC values increased according to the depth, however in winter, it was decreased with increasing depth. The S. hystrix IC values were significantly different acording to the depth.
Lobophyllia corymbosa: In the case of L. corymbosa, the IC values were higher in summer (110.00 ± 19.488 (10) μE s-1 m-2) at 5m depth and lower in 10m depth (50.7 ± 12.641 (10) μE s-1 m-2) but the values were not significantly different. During winter, higher (83.00 ± 17.312 (10) μE s-1 m-2) at 5m depth and lower in 10 m depth (70.857 ± 17.399 (10) μE s-1 m-2).
The values were shown the similar pattern of S. hystrix like increased with increasing depth during the summer, and decreased with increasing depth in winter.
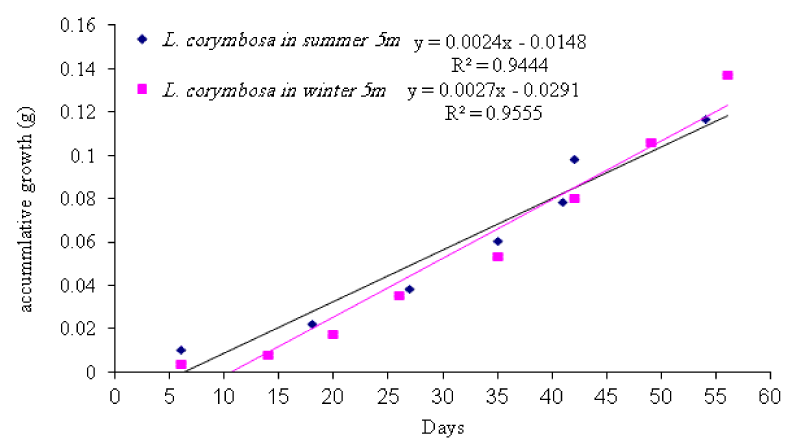
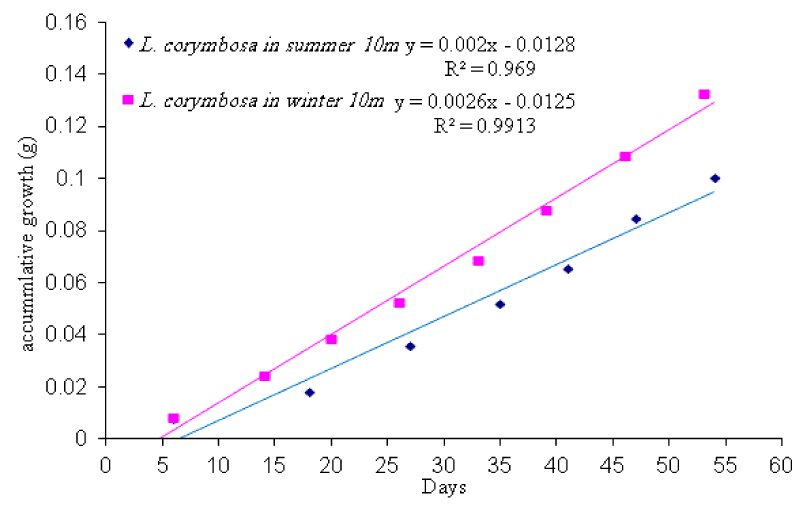
- Seasonal variation in daily growth rate
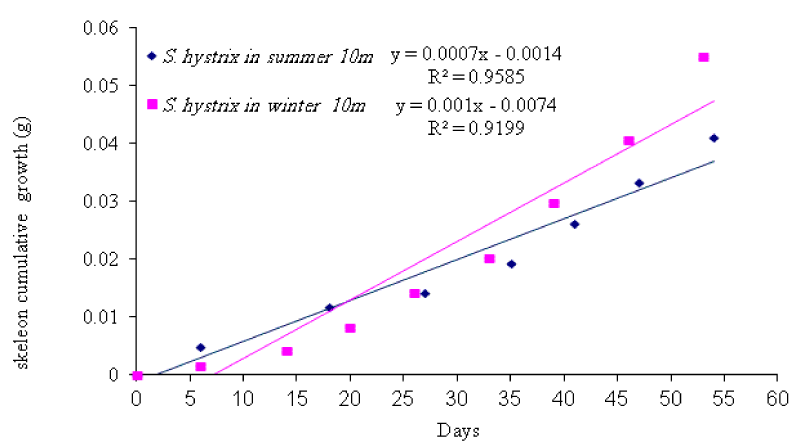
Seriatopora hystrix: The growth rate was linear during each period of measurement (Figures 10,11). During summer 2009, the highest mean daily skeletal growth rate of S. hystrix was 2.3 ± 1.3 (20) mg .skel.d-1 in 10m depth and it was 1.6 ± 0.5 (21) mg .skel.d-1 at 5m depth. Whilst during winter 2010, the lowest was 1.9 ± 0.96 (20) mg .skel. d-1 at 10m and also lowest (1.5 ± 0.7 (20) mg .skel. d-1) at 5m depth. The statistical comparison of the mean daily growth rate of skeleton indicated that, there were not significantly different seasonally (Table 7).
Lobophyllia corymbosa: The mean growth rate of this species was also observed linear during the period of the study (Figures 12,13). L. corymbosa also showed higher seasonal variation in mean daily growth rate (7.27 ± 3.068 (17) mg .skel. d-1) at 5m depth (Tables 3) in summer. Whereas in winter, the growth rate was 5.637 ± 2.56 (27) mg. skel. d-1 at 5m with no significant difference. In the case of 10m depth, the mean growth rate was higher in summer 6.179 ± 2.95 (15) mg .skel. d-1 than the winter 4.78 ± 1.869 (21) mg .skel. d-1. The statistical comparison of mean growth rate revealed that there were not significant varied at difference depths (5m and 10m) and different seasons like summer 2009 and winter 2010 (Table 7).
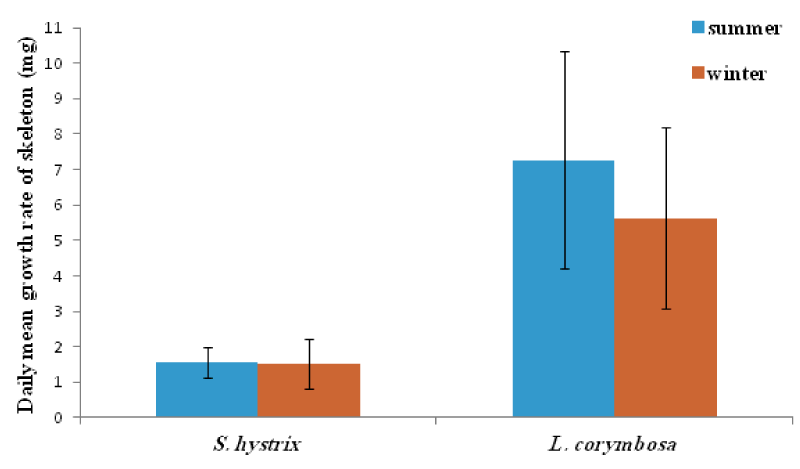
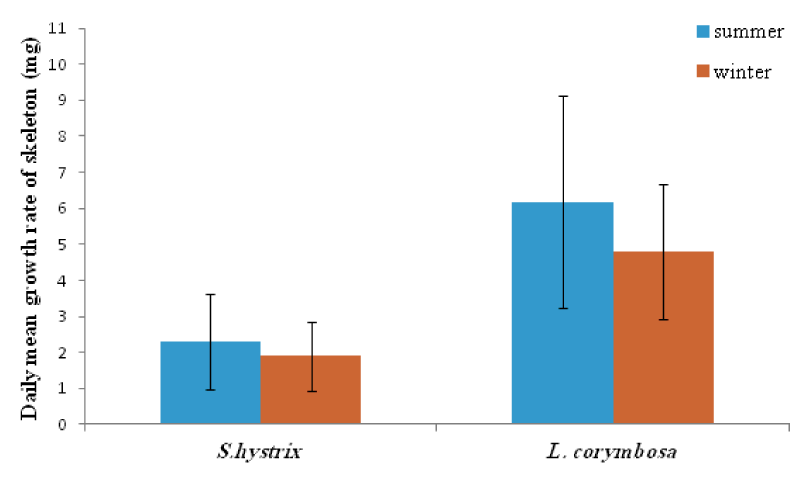
- Species variation in daily growth rate
In general, the L. corymbosa had higher mean daily skeleton growth rate than S. hystrix compared at 5m and 10m depths at both summer 2009 and winter 2010 seasons (Figures 14,15 ). But there was no significant difference in growth rate between the two species (Table 7).
Discussion
Environmental measurements
Temperature: The annual mean seawater temperature variations at Sharm Ubhur were lowest in March 2010 at 5 m and 10 m depths 27.42 °C and 27.17 °C respectively. However, the maximum mean seawater temperature were observed in August (2009) at 5 m and 10 m depths 32.67 °C and 31.17 °C respectively. Floos [37] observed the lowest seawater temperatures from February to March 2005 24.5 -24.75 °C at Sharm Ubhur at 3 m depth and the highest seawater temperature from July to August 2005 (30.25 to 33.00 °C). Similarly Al- Sofyani [34] recorded lowest temperatures in the months of February and March 1990 (25.5 °C ( and the highest temperatures in the months of July to October 1990 (30 °C to 31 °C ( respectively. In addition Fadlallah [38] recorded similar temperatures at Sharm Ubhur and adjacent Yanbu. He further described that the coldest season begins in February and the warmest season starts at September. The highest seawater temperature of Jeddah was recorded as 35 °C in July 1985 and the lowest as 22.5 °C during December 1985 [39] as well as lowest in February and March (1986) [39] . Similarly, the minimum temperature of 25.8 °C was observed in January 1981 and maximum of 34.8 °C in August 1981 were reported by Behiry, et al. [40] from the coastal waters of Jeddah. Edwards and Head [41] recorded the lowest temperatures in January and February 21 °C) and the highest temperatures in August and September 27 °C in the North of the Gulf of Aqaba. Bleaching event caused by increased seawater temperatures [42].
The main feature of the seawater temperature along the coast of Saudi Arabian Red Sea generally decreases from South to North. The ranges between maximum and minimum, seawater temperature in Jeddah is lower than those found in Gazan [18,39,43-45]. The Red Sea is characterized by strong and opposing salinity-temperature-nutrient gradients [46,47].
Light: In summer, the longest duration of sunlight was 12.75 hours with integrated daily irradiation of 11.26 µE. m-2.d-1 at 5m depth, whereas in winter the day length had shortened to 11.75 hours and the integrated daily irradiation was 7.32 µE. m-2.d-1. Al- Sofyani [34], recorded the longest duration of sunlight in summer as 13.75 hours with integrated daily irradiation of 30.12 µE. m-2.d-1 at 3m depth while in winter the day length had shortened to 10.5 hours and the integrated daily irradiation was 14.99 µE. m-2.d-1. In Hawaii, and in the turbid water of the fringing reef of Coconut Island, Oahu, Davies [48] recorded an integrated daily irradiance value of 14.39 µE. m-2.d-1 at 3m depth.
In general, P.A.R. decreases with increasing depths. The daily integrated P.A.R. in August 2011 at 10 m depth was only 39% whereas it was only 61% at 5 m in July. Al- Sofyani [34] recorded similar results in January 1990 as 39% at 10 m depth and 33% in July at 1 m depth. Head [49] recorded average light levels of about 45% of surface irradiance at the depth of 10 m in other areas of the Red Sea. Oliver, et al. [50] recorded transmission of 65% and 28% of surface light to depth of 3 m and 10 m depths respectively at Davies Reefs, Australia. Edmunds [51] recorded 26.9 and 19.4 % transmission of surface light to 10 m in clear water and turbid water respectively in Discovery Bay, Jamaica.
Skeletal densities for both S. hystrix and L. corymbosa are 2.5 g.cm-3 and 2.75 g.cm-3 respectively which are compared with 2.78 g.cm-3 and 2.77 recorded for both Pocillopora damicornis and Pocillopora verrucosa, [37,52]. In addition, skeletal variation of 2.78 g.cm-3 was recorded for Stylophora pistillata and Echinopora gemmacea [34] , 2.783 g.cm-3 for Pocillopora eydouxi [32,34], 2.822 g.cm-3 for Porites porites [33] and lowest of 2.95 g.cm-3 for Fungia fungites [53], 2.78 g.cm-3 for Sariatopora hystrix [54] , 2.4 g.cm-3 for Lobophyllia corymbosa [55].
The variations in the skeletal density among the species may be due to the difference in amount of organic matrix in the skeleton [32,34]. The difference of the skeletal density of both species in the present study may reflect a difference in percentage of the organic matrix in their skeleton.
S. hystrix has the lower tissue biomass of 37.12% per mg in the case of dry tissue weight 5m depth and 45.26% per mg dry tissue weight 10m depth in summer than the L. corymbosa while in winter, it was 63.96% at 5m depth and 73.47% 10m depth and was lower than the L. corymbosa at 5 m and 10 m depths.
Had the lowest tissue biomass than L. corymbosa in the case of surface area like 14.83% and 30.13% at depths 5 m and 10 m respectively in summer. Similarly, in winter, the biomass of tissue per surface area of S. hystrix was lower than L. corymbosa 5 m and 10 m depths 49.26% and 71.31% respectively. This may due to the differences in the growth form of the two species or from the tissue location within the skeleton [37,39,48].
The tissue biomass values of both depths were 24.9 mg.d.t.g-1skel for S. hystrix and 39.6 and 45.49 mg.d.t.g-1skel for L. corymbosa at depths in summer respectively. In winter they were 13.3 and 11.7 mg.d.t.g-1skel for S. hystrix and 36.9 and 44.1 mg.d.t.g-1skel for L. corymbosa at the 5m and 10m depths. Floos [37] reported that 28.1 mg.d.t.g-1skel and 24.92 mg.d.t.g-1skel for Pocillopora damicornis and Pocillopora verrucosa from the Red Sea at 3m depth and Al-Sofyani [39] reported 10.30 mg.d.t.g-1skel and 12.86 mg.d.t.g-1skel for Stylophora pistillata and Echinopora gemmacea from the Red Sea at of 3m depth. Al-Sofyani and Nias [54] recorded 12.50 mg.d.t.g-1skel for Sariatopora hystrix from the Red Sea at 3m depth. Al-Lihaibi, et al. [55] recorded 39.6 mg.d.t.g-1skel for Lobophyllia corymbosa from the Red Sea at 2 m depth. Other studies on Montipora verrucosa and Porites lobata revealed that 44.29 and 45.02 mg.d.t.g-1skel for at 3m depth respectively [32,48]. In addition to that another study which showed 2.2 mg.d.t.g-1skel for Fungia fungites at 2m depth [53].
On the basis of unit area (mg.d.t.cm-2), the values were 7.81 and 7.7 mg.d.t cm-2 in summer at 5m and 10m depths respectively for S. hystrix. In L. corymbosa they were 9.17 and 11.02 mg.d.t cm-2 in summer at the same depths respectively. While in winter, at 5m and 10m depths they were 4.14 and 3.32 mg.d.t cm-2 for S. hystrix as well as 8.16 and 11,57 mg.d.t cm-2 for L. corymbosa close to the range of 5.56 mg.d.t cm-2 to 9.65 mg.d.t cm-2 for Montastrea annularis at 2m and 10m depths respectively [56]. Similarly the surface area range of 2.8 to 12.5 mg.d.t cm-2 was observed for six species at 2.5m depth from Barbados, West Indies [57].
Al-Sofyani [34] reported lower values, 3.52 mg.d.t cm-2 for Stylophora pistillata and 4.91 mg.d.t cm-2 for Echinopora gemmacea at of 1m and 3m depths respectively. Floos [37] recorded lower values, 5.33 mg.d.t cm-2 for Pocillopora damicornis and 6.92 mg.d.t cm-2 for Pocillopora verrucosa, while Edmunds and Davies [33] showed much higher value of 18.59 mg.d.t cm-2 for Porites porites at 10m depth. These differences in this present study may due to the growth form or the methods used for measuring surface area [33,34] .
The density of zooxanthellae by means of dry tissue weight for S. hystrix was approximately 73.7% and 77.8% at of 5 m and 10 m depths in summer respectively. While in winter it was lower 91.6% and 95.3% at 5m and 10m depths when compared with the number of zooxanthellae on the basis of biomass unit in winter and summer respectively. On the other hand, the surface area of S. hystrix was 58.3% and 63.8% at 5m and 10m depths in summer respectively, while in winter it was lower when compared L. corymbosa 81.6% and 82.8% at 5m and 10m depths in winter and summer respectively.
Floos [37] recorded the zooxanthellae density in winter was approximately lower as 13.3% in summer and 2.63% at 3 m depth in P. verrucosa. On the other hand, P. damicornis was also lower 39.36% and 42.9% when compared with P. verrucosa on the basis of surface area in winter and summer respectively. In addition, the other published data showed that in summer the density of zooxanthellae at 5m and 10m depths for both S. hystrix and L. corymbosa were (1.2×106 ± 0.6, 0.50×106 ± 0.27) and (0.58×106 ± 0.26, 1.6×106 ± 0.67) g-1skel respectively. However in winter, it was (19×106 ± 8.9, 3.51×106 ± 1.86) for S. hystrix and (25.7×106 ± 12.9, 4.42×106 ± 1.53) g-1skel for L. corymbosa. at 5m and 10m depths respectively.
Floos [37] stated that the density of zooxanthellae at 3m depth of both Pocillopora damicornis and Pocillopora verrucosa was 2.40×106 ± 0.57and 2.7 ×106 ± 1.07 g-1skel respectively, where as other values were similar to Stylophora pistillata (2.87×106 g-1skel) but lower in Echinopora gemmacea (1.63×106 g-1skel) than Fungia fungite (1.5×106 g-[34,53].
On the basis of dry tissue weight, the density of zooxanthellae of S. hystrix was 0.19 ×105 ± 0.23 .mg-1 d.t at 5m depth and was 0.27×105 ± 0.14.mg-1d.t at 10m depth and for L. corymbosa, it was 0.05×105 ± 0.02.mg-1 d.t at 5m depth and 0.43×105 ± 0.18.mg-1d.t at 10m depth. This was lower than Stylophora pistillata,2.78 ×105 mg-1d.t and Echinopora gemmacea 1.27.mg-1 d.t studied earlier [34]. It was much lower when compared to Fungia fungites 6.91×105 mg-1 d.t [53]. The number of zooxanthellae per unit area of S. hystrix, 1.2 ×105 cm-2 1.6×105 cm-2 and L. corymbosa 0.5×105 cm-2, 0.58 ×105 were lower than of P. damicornis 4.76 ×105 .cm-2 and of P. verrucosa 7.85×105.cm-2 [37]. And also it was lower than Stylophora pistillata 9.82×105 cm-2 [34].
When compared Stylophora species it was lower that the light-adapted shallow water Stylophora pistillata 10.0×105 cm-2 [58], for light-adapted Stylophora pistillata 16×105 cm-2 [59], Stylophora pistillata 15.6×105 cm-2 [60]. And it was also much lower than Stylophora mordax 48.8 ×105 cm-2 even at 1m depth [25]. These differences may be due to using of different methods for measuring the surface area. Stimson [61] reported high variability in the algal densities within coral species. Drew [60] and Porter, et al. [58] reported that the density of zooxanthellae per area unit were same at different depths and among coral genera. Dustan [62,63] described that the number of zooxanthellae in Montastrea annular was increased with increasing depth and the zooxanthellae appeared to be photo adapted to lower light intensity by increasing the size of the photosynthetic units.
Both species showed annual density cycle of zooxanthellae, lower in summer than the winter. The S. hystrix had lower density of zooxanthellae (6.5% and 6.2%) in summer than the winter at 5m and 10m depths respectively. Similarly, L. corymbosa also possessed lower density (13.6% and 12.23%) in summer than the winter at 5m and 10m depths respectively. Many studies reported that the number of annual cycle of zooxanthellae was changing due to the environmental parameters, such as irradiance, UV radiation, nutrients and temperature. There was a negative correlation between the number of zooxanthellae with increasing irradiance and temperature and a positive correlation with increasing nutrients. The density of zooxanthellae decreased in response to increasing irradiance [59,64,65]. In the present study, the seawater temperature increased during months of summer and photosynthetically active radiation (P.A.R. µE.m-2.s-1) at depth of 5m was at a maximum in August 2011 with a value of 512 µE.m-2.s-1 and at a minimum at the end of February 2011 when the value was 389µE.m-2.s-1 while at depth of 10m was at a maximum in August 2011 with a value of 204.8 µE.m-2.s-1and at a minimum at the end of February 2011 when the value was 204.8 µE.m-2.s-1.
In addition Al-Sofyani [34] reported that the photosynthetically active radiation (P.A.R. µE m-2 s-1) at depth of 3m was at a maximum in June 1990 with a value of 1212 µE m-2 s-1 and at a minimum at the end of November when the value was 727 µE m-2 s-1. Furthermore, at depth of 10m was at a maximum in July 1990 with a value of 455 µE m-2s-1 and at a minimum at the end of January 1990 when the value was 430 µE m-2 s-1.
Dark respiration
The mean percentage of dark respiration rates may vary between two species on the basis of milligram dry tissue weight (mg.d.t.wt. h-1). In this study, the percentage of dark respiration rate of S. hystrix (77.3%) was higher than the L. corymbosa in summer (32 °C) and similarly the rate of S. hystrix was as higher as 73.3% than the L. corymbosa in winter 30 °C at 5m depth respectively. Similarly, in 10m depth also the mean percentage respiration rate was higher in S. hystrix (75.1%) in summer 31 °C and higher (67.1%) in winter 29 °C than the L. corymbosa respectively.
In this present study, the Summer respiration rate of S. hystrix was 4.563 µlO2 mg.d.t.wt. h-1 at 5m depth whereas was 2.477 µlO2 mg.d.t.wt. h-1 at 10m depth. Similarly in L. corymbosa, the rate was about 1.035 µlO2 mg.d.t.wt. h-1 at 5m depth and 0.618 µlO2 mg.d.t.wt. h-1 at 10m depth respectively. The mean respiration rates of this study was compared (mg.d.t.wt. h-1) with other published values of this region. In summer, Pocillopora damicornis rate was lower than the present study (1.190 µlO2 mg.d.t.wt. h-1) and also respiration rate of the Pocillopora verrucosa was also lower (1.37 µl O2 mg.d.t.wt. h-1) at 3m depth of the same study site [37]. In Echinopora gemmacea also the respiration rate was lower (1.37 µl O2 mg.d.t.wt. h-1) than the present study on S. hystrix at the same study area [34]. Studies on the other regions like Hawaii also showed minimum respiration rates in Mintipora annularis (1.65 µlO2 mg.d.t.wt. h-1) and Porites lobata (1.19 µlO2 mg.d.t.wt. h-1) than the present study [48].
In winter, the mean respiration rate of S. hystrix was 3.164 µlO2 mg.d.t.wt. h-1 and 1.432 µlO2 mg.d.t.wt. h-1. The L. corymbosa respiration rate was lower than the S. hystrix 0.846 µlO2 mg.d.t.wt. h-1 and 0.471 µlO2 mg.d.t.wt. h-1 of the present study at 5m and 10m depths. It was (L. corymbosa) even lower in respiration rates when compared to the other studies on Echinopora gemmacea (1.21 µlO2 mg.d.t.wt. h-1) and Stylophora pistillata (1.77 µlO2 mg.d.t.wt. h-1) at the same study site [34]. This lower respiration rate of L. corymbosa of the present study may due to the higher tissue biomass than the S. hystrix.
Davies [56] stated that, the corals with lower surface area consumed less oxygen than the corals with higher surface area. Furthermore, Kawaguti [66] reported that the corals with large polyps contracted during daytime resulted in the lower respiration rate than the corals with small and expanded polyp of the day time. Hence the mean dark respiration rates of this study on the both species were higher in summer (32 °C and 31 °C) than the winter (30 °C and 29 °C) at 5m and 10m depths respectively. But, Nakamura Eriko, et al. [20] reported that the dark respiration rate of coral Acropora pruinosa was higher in winter at 20 °C than the summer at 30 °C.
In overall depth, the respiration percentage rate of S. hystrix was higher in summer (54.3%) than the winter (45.3%), similarly in L. corymbosa, the overall respiration rate was higher (59.7%) in summer and lower (55.7%) in winter.
In general, the worldwide studies revealed that the respiration rates of corals were decreased with increasing depths [34,56,67-69]. Furthermore, studies described that the corals in shaded area respire less oxygen than the open area corals [58,70].
Al-Sofyani [34] reported that the respiration rate percentage of Stylophora pistillata at 10m depth was 81% of the 1m depth both in summer and winter seasons. Whilst in Echinopora gemmacea, the respiration rate percentage was 93% at 10m depth and 79% 3m depth during both summer and winter. Similar to this, in Montastrea annularis off Discovery Bay, Jamaica, the dark respiration rate at 10m depth was 46% of that of 3m depth [56]. In shade-adapted Stylophora pistillata, the oxygen consumption was 54% of that of the light-adapted one [71].
The reduction in coral respiration rates with depth or shade may be related to the reduction in translocation of organic material from the algae which in turn was a function of decreased irradiance [51,56].
On the basis of surface area, the mean dark respiration rate of S. hystrix was higher 16.974 µlO2cm-2 h-1 in 5m depth and also higher in 10 m depth (8.09 µlO2cm-2 h-1) than the L. corymbosa (8.082 µlO2 cm-2 h-1, 5.7610 µlO2 cm-2 h-1) respectively at during summer season.
In winter also, the mean dark respiration rate of S. hystrix was higher (22.01 µlO2cm-2 h-1 and 9.958 µlO2cm-2 h-1) than the L. corymbosa (5.871 µlO2 cm-2 h-1and 3.167 µlO2 cm-2 h-1) at 5m and 10 m depths respectively. In the case of Pocillopora eydouxi it was 8.1 µlO2 cm-2 h-1 at Guam [32]. and in Stylophora pistillata it was 9.84 µlO2 cm-2 h-1[34] and 9.7 µlO2cm-2 h-1 [58] at Red Sea. In Barbados, West Indies the respiration was within the range of 3.5-8.76 µlO2cm-2 h-1 for six species of coral [72]. The present values were lower than that of Porites lobata (11.38 µlO2 cm-2 h-1) observed at 7 m depth [73] and of Porites porites (11.91 µlO2 cm-2 h-1) of at 10 m depth [33]. This comparison study revealed that the respiration rates of corals were decrease with increasing depths [56,67-69]. There is a difference in the mean dark respiration rate of two species on the basis of zooxanthellae count per hour. In S. hystrix it was 4.15 (5 m depth) and 2.69 (10 m depth) µlO2 10-6zoox-1. h-1 and in L. corymbosa it was 17.84 (5m depth) 5.11 (10m depth) µlO2 10-6zoox-1. h-1 during summer respectively. But in winter, the mean dark respiration rates of S. hystrix was (2.43 µlO2 10-6zoox-1. h-1 and 1.73) µlO2 10-6zoox-1. h-1 but in L. corymbosa it was ( 7.59 µlO2 10-6zoox-1. h-1 and 2.97) µlO2 10-6zoox-1. h-1 at 5m and 10m depths respectively,
Other studies reported that the Pocillopora damicornis respiration rate was 2.39 µlO2 10-6zoox-1. h-1 (25 °C) to 3.03 µlO2 10-6zoox-1. h-1 ((30 °C) during winter. In Pocillopora verrucosa it was 2.84 µlO2 10-6zoox-1. h-1 at 25 °C and it was 2.48 µlO2 10-6zoox-1. h-1 at 30 °C during winter, while in summer (35 °C) the values recorded, ranging from 7.46 µlO2 10-6zoox-1. h-1and 5.78 µlO2 10-6zoox-1. h-1 for Pocillopora damicornis and Pocillopora verrucosa respectively in the Red Sea [37]. In the case of Stylophora pistillata it was 2.51 µlO2 10-6zoox-1. h-1 at 1m depth and was 2.15 µlO2 10-6zoox-1. h-1 at 10m depth in the summer (30 °C) similarly at 3m depth it was 3.1 µlO2 10-6zoox-1. h-1 and at 10 m depth it was 2.63 µlO2 10-6zoox-1. h-1 in the Red Sea [34]. Other studies on Montastrea cavernosa showed 2.61 µlO2 10-6zoox-1. h-1 at 5m depth [32] and the studies on Porites porites showed 1.84 µlO2 10-6zoox-1. h-1 at 10m depth [33].
During summer, the overall depth percentage of respiration rate by means of zooxanthellae in S. hystrix was 15.7% whereas in L. corymbosa it was higher 64.7%. Whereas in winter the overall depth respiration rate of S. hystrix was more in percentage 75.4% than the L. corymbosa 50.18% in the summer. The mean dark respiration rate was decreased with the increasing depths in the both species. Other studies reported that the respiration rate of the Pocillopora damicornis at 25 °C was about 7.69 % and was maximum 48.94 % at 30 °C. Similarly, in Pocillopora verrucosa the respiration rate was lower 3.7 % in 30 °C and higher in 23.53 % 35 °C respectively [37].
The work of Baker, et al. [14] suggested that members of clades D may have greater thermal tolerances than clades C in their zooxanthellae samples of Palythoa caribaeorum. Clade A and B inhabited shallow water colonies, while clade C zooxanthellae are found in deeper water colonies, or in shaded areas of the same individual coral colony[15,16] .
There was major bleaching in the Red Sea corals in the year 1998. In that period some coral species like Pocillopora verrucosa had found resistant to the bleaching event than the Pocillopora damicornis [16,18]. Moreover, Coles and Jokiel [21] reported very high response in metabolic rate due to temperature changes (19-31 °C) in Pocillopora damicornis, Montipora verrucosa, Porites compressa and Fungia scutaria in Hawaii and Enewetak. This difference in the metabolic rates may be the indication of compensatory acclimation of the species to the temperature [74]. Acclimation has the effect of increasing metabolic rate during long-term exposure to reduced temperature. If acclimation is completed, the measured metabolic rates at the temperature at which the animals are living are identical.
Photosynthesis
The mean maximum gross photosynthesis (Pgmax) on the basis of both biomass and surface area were higher in S. hystrix than the L. corymbosa. This may be due to the higher number of zooxanthellae when expressed on both biomass and surface area basis. When the values were expressed on the basis of zooxanthellae density, L. corymbosa showed higher values than S. hystrix suggesting that self-shading of zooxanthellae may occur in the latter species [75].
During summer, the value of Pgmax on the basis of surface area, was higher in S. hystrix (30.4888 µlO2 cm-2 h-1 at 5m depth) than the light-adapted Stylophora pistillata (22.9 µlO2 cm-2 h-1) at 2m depth [34,54] but at 10m depth the value of S. hystrix of the present study was 21.027 µlO2 cm-2 h-1 when compared to the Stylophora pistillata of the different regions 18.67, 20.2 and 21.34 µlO2 cm-2 h-1 [34,54,76] respectively. The Pgmax for S. hystrix during summer was similar to the Pocillopora eydouxi (36.1 µlO2 cm-2 h-1) at 5m depths [32].
The maximum gross photosynthesis values on the basis of mg.d.t.wt. h-1 for S. hystrix was 8.1965 mg.d.t.wt. h-1 at 5 m depth and 6.4339 µlO2 mg.d.t.wt. h-1 at 10 m depth and in the case of L. corymbosa it was 2.594 µlO2 mg.d.t.wt. h-1 at 5m depth and 2.067 µlO2 mg.d.t.wt. h-1 at 10m depth in summer. These values were lower than the Stylophora pistillata (8.81-5.23 µlO2 mg.d.t.wt. h-1 and higher than the Echinopora gemmacea (3.83-3.39) µlO2 mg.d.t.wt. h-1 [34]. When compared to Porites porites it was 4.76 µlO2 mg.d.t.wt. h-1 at 10m and in Porites lobata it was 4.26 µlO2 mg.d.t.wt. h-1 at 3m respectively, both these species had large tissue biomass [33,48].
The Pgmax for L. corymbosa was lower in the winter than the summer and this could be probably due to the lower water temperature in the winter. However, at 5m depth, the rate of photosynthesis of S. hystrix was higher in winter than the summer.
The values of Pgmax in the present study for L. corymbosa decreased with increasing depths in winter, but in summer increased with increasing depth. Hence in L. corymbosa the value of α increased with increasing depths, however, in S. hystrix it declined with increasing depth and hence in S. hystrix the value of α decreases with increasing depths. These results have been shown to be associated with the processes of photoadaptation in corals [35,58,69,70,77,78], although Chalker and Dunlap [79], Falkowski and Dubinsky [80], reported the increase in Pgmax with increase in depth.
The Ik values of the PvI curve were the indication of the relative efficiency of photosynthesis at low light-levels. Similarly the lower value of Ik was indicating the higher efficiency. The Ik values of S. hystrix declined from 198.5 µE. m-2. s-1 (5m depth) to 154.3 µE. m-2. s-1 (10m depth) in summer while in winter was increased from 158.273 µE. m-2. s-1 (5m depth) to 180.7 µE. m-2. s-1 (10m depth) this might due to the photoadaptation ability of the zooxanthellae. Gattuso [77] worked on Stylophora pistillata and reported that at 1m it was 318 µE. m-2. s-1 and 10 m it was 158.3 µE. m-2. s-1. Whilst Porter, et al. [58], found values of 273 µE. m-2. s-1 at light adapted and 60 µE. m-2. s-1 at dark adapted Stylophora pistillata respectively. The Ik values for L. corymbosa declined from 291.1 µE. m-2. s-1 at 5m to 250.4 µE. m-2. s-1 at 10m in summer similarly in winter it decreased from 202.72 µE. m-2. s-1 at 5m to 180.4 µE. m-2. s-1 at 10m. According to Davies [48], Montipora verrucosa was 176 and Porites lobata was 177 µE. m-2. s-1 at 3 m depth.
Both L. corymbosa and S. hystrix displayed lower values of Ik in the winter than the summer at both depths indicating the photoadaption to the lower light levels prevailing during these months. This appears to be the first report of a seasonal photoadaption response in corals of the Red Sea. The deep-living corals exhibited difference in photosynthetic characteristics, which could be explained by photoadaptation to reduced light by the zooxanthellae. Changes were observed in the shape of the P v I curves. Notably the deep corals had lower values of Ik although values for α were similar.
Growth
Linear growth rates studies in this two species can be interpreted that, it may rapidly increase at the tips of the nubbin rather than lateral [32,34,37,81-83]. The growth rate of hermatypic corals is influenced generally by the external factors like light intensity, sedimentation [5,34,37,83-85]. The temperature is also another important parameter that can affect coral growth. In tropical corals, several studies showed that a 1 °C increment in mean annual temperature may resulted into the increase in the coral calcification rate by 3.1% especially when temperatures increased above the upper threshold limit of corals [86,87].
In L. corymbosa also, the growth rate was reduced by the internal factors such as reproduction, genetics, growth form of colony, number or type of zooxanthellae and production of mucus tunics by species. The light quality and intensity and seawater temperature during summer also affected the growth rate of S. hystrix and L. corymbosa. Hence, the mean respiration rate of zooxanthellae increased dramatically with an increasing temperature. The reduction in coral respiration rates with an increasing temperature was associated with a reduction in number of zooxanthellae. It may be possibly due to the translocation of organic material from the algae due to decrease in photosynthesis, in addition to decrease the growth rate of skeleton during winter.
The respiration of the corals was consumed the most important portion of the energy input [34,51]. However, both species, especially the S. hystrix seemed to be more sensitive to exogenous and endogenous factors. This was evidenced by the mean growth rates which recorded in the summer for S. hystrix and L. corymbosa. They were about 93.8% and 78.1% at 5m, and at 10m were about 82.6% and 77.7% in winter respectively.
In compared the mean growth with other studies, showed that the mean growth rate was 1.6 mg. skel. d-1 to 2.3 mg. skel. d-1 for S. hystrix and 7.3 mg. skel. d-1 to 6.18 mg. skel. d-1 for L. corymbosa at 5m and 10m in summer respectively. It was more or less similar to the growth rates of P. damicorni (3.90 mg. skel. d-1) and also of Pocillopora verrucosa (5.40 mg. skel. d-1) at 3m. But it was lower than the Echinopora gemmacea (13.46 mg. skel. d-1) and Stylophora pistillata (56.03 mg. skel. d-1) of even at 1m from the Red Sea [34]. In winter, the mean growth rates of S. hystrix was (1.5 mg. skel. d-1 to 1.9 mg. skel. d-1) and for L. corymbosa it was 5.7 mg. skel. d-1to 4.8 mg. skel. d-1 at 5m and 10m respectively.
Hence, the growth rate of the corals in the present study were higher in summer than the winter at 5m and 10m depths. But it was still lesser than the other studies which fallen within the range of 12.5 mg. skel. d-1 to 51.6 mg. skel. d-1 for several other coral species from 2 to 10m depths [32-34,36,48,88]. Another study by Floos [37] showed that the values in winter were 6.08 mg. skel. d-1 and 7.44 mg. skel. d-1 for Pocillopora damicornis and Pocillopora verrucosa respectively.
Conclusion
The lower respiration rate of L. corymbosa of the present study may be due to the higher tissue biomass which is not active than the S. hystrix.
The mean maximum gross photosynthesis (Pgmax) on the basis of both biomass and surface area were higher in S. hystrix than the L. corymbosa. This may be due to the higher number of zooxanthellae when expressed on both biomass and surface area basis
The reduction in coral respiration rates with an increasing temperature was associated with a reduction in number of zooxanthellae . It may be possibly due to the translocation of organic material from the algae due to decrease in photosynthesis, in addition to decrease the growth rate of skeleton.
All these findings indicate that S. hystrix is predominantly dependant on autotrophic feeding while L. corymbosa relies mainly on heterotrophic feeding.
Recommendations
- Avoiding coastal settlement (construction of building) will reduce the sediment input on the corals.
- Reef fishing and boat anchoring on reefs should be banned. Separate boat jetty should be arranged (if necessary) in reef areas to avoid reef damage.
- Dumping of wastes and drainage or ballast water discharge in the reef environment should be completely avoided.
- Awareness campaign for both public and students will be essential to know the important of coral reefs will provide positive result
- Assess the environmental impact of socio-economic development and identify hotspots of environmental stress along the shores of the Red Sea.
- You must establish the Red Sea coral ecosystem and biodiversity.
- Scheer G, Pillai CSG. Report on the stony corals from the Red Sea Zoologica. 1983; 133: 1-198.
- Veron JEN. Corals of Australia and the Indo-Pacific. Angus &Robertson Publishers. Sydney. 1986; 644.
- Veron JEN. Coral of the world. (Ed. Stafford-Smith, M.). Australian Institute of Marine Science and CRR Qld pty ltd Townsville, Queensland Vollmer, S.V., Palumbi, S.R, 2002. Hybridization and the evolution of reef coral diversity. Science. 2000; 296: 2023-2025.
- Wells JW. Scleractinia: In R.C. Moore (ed.), Treatise on Invertebrate Paleontology. Geological Society of America and University of Kansas Press. 1956; 328-444.
- Miller NW. Growth of a temperate coral effects of temperature, light, depth and heterotrophy. Mar Ecol Prog Ser. 1995; 122: 217 - 225.
- Birkeland C. Life and death of coral reefs, University of Guam. Chapman and Hall 115 Fifth Avenue, New York. 1997; 527.
- Farag MA, Meyer A, Ali SE. Bleaching effect in Sarcophyton spp. soft corals-is there a correlation to their diterpene content? Environ Sci Pollut Res Int. 2021 May;28(20):25594-25602. doi: 10.1007/s11356-021-12483-y. Epub 2021 Jan 18. PMID: 33459982.
- Wilkinson CR. Status of coral reef of the world. Global coral reef Monitoring. Network, Australia. 2000.
- Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs JA, Hoogenboom MO, Kennedy EV, Kuo CY, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. Global warming and recurrent mass bleaching of corals. Nature. 2017 Mar 15;543(7645):373-377. doi: 10.1038/nature21707. PMID: 28300113.
- Hoegh-Guldberg O, Jacob D, Taylor M, Bindi M, Brown S, Camilloni I, Diedhiou A, Djalante R, Ebi K, Engelbrecht F, Guiot K, Hijioka Y, Mehrotra S, Payne A, Seneviratne S, Thomas A, Warren R, Zhou G, Halim S, Achlatis M, Alexander L, Allen M, Berry P, Boyer C, Brilli L, Buckeridge M, Cheung W, Craig M, Ellis N, Evans J, Fisher H, Fraedrich K, Fuss S ,Ganase A, Gattuso J, Greve P, Guillen T, Hanasaki N, Hasegawa T, Hayes K, Hirsch A, Jones C, Jung T, Kanninen M, Krinner G, Lawrence D, Lenton T, Ley D, Liveman D, Mahowald N, McInnes K, Meissner K, Millar R , Mintenbeck K, Mitchell D, Mix A, Notz D, Nurse L, Okem A, Olsson L, Oppenheimer M, Paz S, Peterson J, Petzold J, Preuschmann S, Rahman M, Rogelj J, Scheuffele H, Schleussner CF, Scott D, Seferian R, Sillmann J, Singh C, Slade R, Stephenson K, Stephenson T, Sylla M, Tebboth M,Tschakert P, Vautard R, Wartenburger R, Wehner M, Weyer N, Whyte F, Yohe G, Zhang X, Zougmore R. Chapter 3: impacts of 1.5oC globwarming on natural and human systems. In: Global Warming of 1.5 oC an IPCCSpecial Report on the Impacts of Global Warming of 1.5 oC Above Pre-industrialLevels And Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change . Intergovernmental Panel on Climate Change. 2018.
- Al-Horani FA. Study of the interaction among photosynthesis, respiration, and calcification in the scleractinian coral, Galaxea fascicularis. Ph.D. Thesis. Bremen. Germany. 2002; 99.
- Coffroth MA, Santos SR. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 2005; 156: 19-34.
- Karako-Lampert S, Katcoff DJ, Achituv Y, Dubinsky Z, Stambler N. Do clades of symbiotic dinoflagellates in scleractinian corals of the Gulf of Eilat(Red Sea) differ from those of other coral reefs? J Exp Mar Biol Ecol. 2004; 311(2): 301–314. http://dx.doi.org/10.1016/j.jembe.2004.05.015.
- Baker AC, Starger CJ, McClanahan TR, Glynn PW. Coral reefs: corals' adaptive response to climate change. Nature. 2004 Aug 12;430(7001): 741. doi: 10.1038/430741a. PMID: 15306799.
- Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Natl Acad Sci U S A. 1995 Mar 28; 92(7): 2850-3. doi: 10.1073/pnas.92.7.2850. PMID: 7708736; PMCID: PMC42316.
- Toller WW, Rowan R, Knowlton N. Repopulation of Zooxanthellae in the Caribbean corals Montastraea annularis and M. faveolata following experimental and disease-associated bleaching. Biol Bull. 2001 Dec;201(3):360-73. doi: 10.2307/1543614. PMID: 11751248.
- Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018 Jan 5;359(6371):80-83. doi: 10.1126/science.aan8048. PMID: 29302011.
- Al-Sofyani AA. Bleaching of Some Red Sea Corals at Ubhour Bay, Jeddah. Int. Symp. on the Extent of Coral Reef Bleaching. Riyadh SA. 2000; 1-18.
- Ralph PJ, Larkum AWD, Kühl M. Temporal patterns in effective quantum yield of individual zooxanthellae expelled during bleaching. J Exp Mar Biol. 2005; 316: 17–28.
- Nakamura E, Yasutsugu Y, Tanaka J. Photosynthetic activity of a temperature coral Acropora Pruinosa (Scleractinia, Anthozoa) with symbiotic algae in Japan. Phyc Rese. 2004; 52: 38 - 44.
- Coles SL, Jokiel PL. Effects of temperature on photosynthesis and respiration in hermatypic corals. Mar Biol. 1977; 43: 209-216.
- Xu S, Yu K, Zhang Z, Chen B, Qin Z, Huang X, Jiang W, Wang Y, Wang Y. Intergeneric differences in trophic status of scleractinian corals from Weizhou Island, northern South China Sea: implication for their different environmental stress tolerance. J Geophys Res Biogeosci. 2020; 125: e2019JG005451.
- Qin Z, Yu K, Wang Y, Xu L. Spatial and intergeneric variation in physiological indicators of corals in the South China Sea: insights into their current state and their adaptability to environmental stress. J Geophys. 2019.
- Allemand D, Furia P, Benazet-Tambutte S. Mechanisms of carbon acquisition for endosymbiont photosynthesis in Anthozoa. Can J Bot. 1998; 76: 925–941.
- Gattuso JP, Allemand D, Frankignoulle M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am Zool. 1999; 39(1): 160–183.
- Goreau TF, Goreau NI. The physiology of skeleton formation in corals. II. Calcium deposition by hermatypic corals under various conditions in the reef. Biol Bull. 1959; 117: 239-250.
- Pearse VB, Muscatine L. Role of symbiotic algae (zooxanthellae) in coral calcification. Biol Bull. 1997; 141: 350–363.
- Allemand D, Ferrier-Pagès C, Furla P, Houlbrèque F, Puverel S, Reynaud S, Tambutté E, Tambutté S, Zoccola D. Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. CR Palevol. 2004; 3: 453–467.
- Al-Sofyani A, Davies PS. 1992, Seasonal variation in production and respiration
- Atkinson MJ, Carlson B, Crow GL. Coral growth in high-nutrient, low-PH seawater a case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs. 1995; 14: 215-223.
- Madah F. Numerical simulation of tidal circulation in the Obhur Lagoon, on the eastern coast of the Red Sea, Saudi Arabia. Arabian Journal of Geosciences. 2022; 15(8): 119.
- Davies PS. The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydoxi. Coral Reefs 1984; 2: 181 - 186.
- Edmunds PJ. An energy budget for Porites Porites (Scleractinia). Ph.D. thesis. Glasgow University, Glasgow. 1986; 194.
- Al-Sofyani AA. Studies on Physiology and ecology of Stylophora pistillata and Echinopora gemmacea from the Red Sea. Ph. D. Thesis. Glasgow. U.K, 1991; 167.
- Chalker BE. Simulating light-saturation curves for photosynthesis and calcification by reef-building corals. Mar Biol. 1981; 63: 135-141.
- Davies PS. Short-term growth measurements of coral using an accurate buoyant weighing technique. Mar Biol. 1989; 101: 389–395.
- Floos YA. Studies on Physiology and ecology of Pocillopora damicornis and Pocillopora verrucosa in Sharm Ubhur, Jeddah, Saudi Arabia. M. Sc. Thesis, King Abdulaziz University. Jeddah. 2007; 102.
- Fadlallah YH. Reproduction in the coral Pocillopora verrucosa on the reefs adjacent to the industrial city of Yanbu (Red Sea, Saudi Arabia). In: Gabrie´ C et al. (eds.) proc 5th int coral Reef Conger. Antenne Museum- EPHE, Moorea, French Polynesia. 1985; 4:313 - 318.
- Al-Sofyani AA. Studies on growth and reproduction of Red Sea scleractinia. M. Sc. Thesis, King Abdulaziz University. Jeddah. 1987; 102.
- Behairy AKA, Osman MM, Mesha AH. Evaporation from the waste water in front of Jeddah. Jed Jour Mar Res. 1981; 1: 3 - 9.
- Edwards AJ, Head SM. Red Sea: Key Environments. A Wheaton and Co. Ltd., Exeter. 1987; 45-68.
- Raymundo L, Burdick D, Hoot W, Miller R, Brown V, Reynolds T, Gault J, Idechong J, Fifer J, Williams A. Successive bleaching events cause mass coral mortality in Guam, Microne-sia. Coral Reefs. 2019; 38: 677–700.
- Vercelli F. Recherche di oceanografia fisica esequite della R. Nave "Ammiraglio Magnaghi" (1923-24). Por. IV La Temperatura et la Salinita. Anna, Idro. 1927; 11: 1-66.
- Al-Subhi AM, AL-Aqsum MM. Temporal and Spatial Variations of Remotely Sensed Sea Surface Temperature in the Northern Red Sea, JKAU: Mar Sci. 2008; 19:61-74.
- Al-Subhi AM, Taqi AM. Remotely Sensed Sea Surface Temperature Variation in the Southern Red Sea in Relatiom to Som Meteorplogical Variables. JKAU: Mar Sci. 2010; 21(2):33-46.
- Sawall Y, Al-Sofyani A. Biology of Red Sea Corals: Metabolism, reproduction, acclimatization, and adaptation. In N. M. A. Rasul & I. C. F. Stewart (Eds.), The Red Sea: The formation, morphology, ocean- ography and environment of a Young Ocean Basin. 2015; 487–509.
- Berumen ML, Voolstra CR, Daffonchio D, Agusti S, Aranda M, Irigoien X, Jones BH, Morán XAG, Duarte CM. The Red Sea: Environmental gradients shape a natural laboratory in a nascent ocean. In C. R. Voolstra & M. L. Berumen (Eds.), Coral Reefs of the Red Sea. Springer International Publishing. 2019; 1-10. https://doi.org/10.1007/978- 3- 030- 05802-9_1
- Davies PS. Effect of daylight variation on the energy budgets of shallow water coral. Mar Biol. 1991; 108: 137-144.
- Head SM. Corasl and coral reefs of the red sea. In: Red Sea-Key Environments; Edwards, A. J., and Head, S.M. (eds), International Union for Conservation of Nature and Natural Resources, Pergamon Press. 1987; 128-149.
- Oliver JK, Chalker BE, Dunlop WC. Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier Reef, Australia. 1. Longterm growth responses of Acropora Formosa (Dana 1946). J Exp Mar Biol Ecol. 1983; 73: 11-35.
- Edmunds PJ. An energy budget for Porites Porites (Scleractinia). Ph.D thesis. Glasgow University, Glasgow 1986; 194.
- Floos YA. Seasonal Variations in the Biochemical Components of Two Species of Hard Corals Pocillopora damicornis and Pocillopora verrucosa in the Read Sea. International Journal of Scientific & Engineering Research. 2022; 13.
- Jan SAA. Effects of Zinc on Fungi corals from the Red Sea. Ma. Sc. Thesis, King Abdulaziz University. Jeddah. 2001; 92.
- Al-Sofyani AA, Niaz GR. A comparative study of the hard coral Seriatopora hystrix and the soft coral Xenia umbellate along the Jeddah coast Saudi Arabia. Revista de Biology Marina yeOceanografia. 2007; 42(3): 207-219.
- Al-Lihaibi SS, Al-Sofyani AA, Niaz GR. Chemical composition of corals in Saudi Red Sea Coast. Oceanological Acta. 1998; 21:495-501.
- Davies PS. Respiration in some Atlantic reef corals in relation to vertical distribution and growth from. Biol Bull Mar Biol Lab. (Woods Hole). 1980; 158: 187 – 194.
- Lewis JB, Post EE. Respiration and energetics in West Indian Gorgonacea (Anthozoa, Octocorallia). Comp Biochem Physiol. 1982; 71: 457-459.
- Porter JW, Muscatine L, Dubinsky Z, Falkowski PG. Primary production and photoadaptation in light-and shade-adapted colonies of the symbiotic coral, Stylophora pistillata. Proc Roy Soc Lond. Ser. 1984; 222: 161-180.
- Falkowski PG, Dubinsky Z. Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature. 1981; 289: 172-174.
- Drew EA. The biology and physiology of alga-invertebrate symbioses. 11 the density of symbiotic algal cell in a number of hermatypic hard corals and Alcyonarians from various depth. J Exp Mar Biol Ecol. 1972; 9: 71-75.
- Stimson JS. The annual cycle of density of zooxanthellae in the tissues of field and laboratory-held Pocillopora damicornis. J Exp Mar Biol Ecol. 1997; 214: 35-48.
- Dustan P. Distribution of zooxanthellae and photosynthetic chloroplast pigments of the reef-building coral Montastrea annularis (Ellis & Solander) in relation to depth on a West Indian coral reef. Bull Mar Sci. 1979; 29: 79 - 95.
- Dustan P. Depth-dependet photoadaption by zooxanthellae of Montastrea annularis. Mar Biol. 1982; 68: 253 - 264.
- Thinh LV. Photo-adaptation in two species of Acropora growth under controlled conditions. photosynthetica. 1991; 25: 365 - 371.
- Stimson JS, Kinzic RA. Temporal pattern and release rate of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J Exp Mar Biol Ecol. 1991; 153: 63-74.
- Kawaguti S. On the physiology of reef coral I. On the oxygen exchange of Reef Corals. Palao Trop Biol Stn Stud. 1973; 1:187–198.
- Davies PS. Carbon budgets and vertical zonation of Atlantic reef corals. Proc. 3rd Int. Coral Reef Symp, Miami. 1977; 1: 391 – 395.
- McCloskey LR, Muscatine L. Production and respiration in the Red Sea coral Stylopohpra pistillata as a function of depth. Proc Roy Soc Lond Ser B. 1984; 222: 215 – 230.
- Gattuso, JP, Pichon M. Jaubert J. Physiology and taxonomy of scleractinian corals: A case study in the genus Stylophora Coral Reefs., 1991; 9:173-182
- Wethy DS, Porter JW. Sun and shade differences in productivity of reef corals. Nature. 1976; 262: 281-282.
- Muscatine L, Falkowski PG, Porter JW, Dubinsky Z. Fate of photosynthetic fixed carbon in light and shade- adapted colonies of the symbiotic coral Stylophora pistillata. Proc Roy Soc Lond Ser B. 1984; 222: 181–202.
- Lewis, JB. and Post, EE. Respiration and energetics in West Indian Gorgonacea (Anthozoa, Octocorallia). Comp. Biochem. Physiol. 1982; 71 (A) : 457-459.
- Johannes RE, Tepley L. Examination of feeding of the reef coral Porites lobata in situ using time lapse photography. Proc.2nd Int. Coral Reef Symp. 1974; 1: 127-131.
- Hochachka PW, Somero GN. Biochemical adaptations Princeton. University press. 1984; 537.
- Crossland CJ, Barnes DJ. Gas exchange studies with the Staghorn Corals Acropora acumnata and its zooxanthellae. Mar Boil.1977; 40: 185-194.
- Dubinski Z, Falkowski PG, Porter JW, Muscatine L. Absorption and utilization of radiant energy light-and shade-adapted colonies of the hermatypic coral Stylophora pistillata. Proc Roy Soc. Lond. 1984; 222(B): 203-214.
- Gattuso JP. Features of depth effects on Stylophora pistillata, a hermatypic coral in the Gulf of Aqaba (Jordan, Red Sea). Proc. 5th Int. Coral Reef Symp, Tahiti. 1985; 6: 95-100.
- Wyman KD, Dubinsky Z, Porter JW, Falkowski PG. Light absorption and utilization among hermartypic corals: A Study Jamaica, West Indies. Mar Boil. 1987; 96: 283-292.
- Chalker BE, Dunlap WC. Bathymetric adaptations of reef buildingcorals at ies Reef, Great Barrier Reef, Australia. II. Light saturation curves for photosynthesis and respiration. J Exp Mar Boil Ecol. 1983; 73: 37-56.
- Dubinsky Z. Increase in ecosystem of the world. Coral Reef., 1999; 25:193-194.
- Rinkevich B, Loya Y. Does light enhance calcification in hermatypic coral? Mar Biol. 1984; 20: 1-6.
- Wellington GM, Glynn PW. Environmental influences on skeletal banding in Eastern Pacific (Panama) corals.Coral Reef. 1983; 1: 215-222.
- Floos YA. Seasonal Variation in Photosynthesis, Dark Respiration and Growth Rate of Two Reef Building Corals in the Red Sea Coast. Journal Tehama. 2020; 12: 1 -70.
- Wellington GM, Glynn PW. Environmental influences on skeletal banding in Eastern Pacific (Panama) corals.Coral Reef. 1983; 1: 215-222.
- Atkinson, MJ, Carlson B, and Crow GL. Coral growth in high-nutrient, low-PH seawater a case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs, 1995; 14: 215 - 223.
- McNeil BI, Matear RJ, Barnes DJ. Coral reef calcification and climate change: the effect of ocean warming.Geophys Res Letter., 2004; 31: 1–4.
- Orejas C, Ferrier-Pagès C, Reynaud S, Tsounis G, Allemand D, Gili JM. Experimental comparison of skeletal growth rates in the cold-water coral Madrepora oculata Linnaeus, 1758 and three tropical scleractinian corals. J Exp Mar Bio Ecol. 2011; 405: 1-5.
- Davies PS. A rapid method for assessing growth rates of corals in relation to water pollution. Mar Pollut Bull. 1990; 21: 346 - 348.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
