Open Journal of Biological Sciences
Histological characterization of sexual reproduction in the coral Pocillopora damicornis (Coelenterata: Scleractinia) from the Red Sea
Yahya AM Floos*
Cite this as
Floos YA (2022) Histological characterization of sexual reproduction in the coral Pocillopora damicornis (Coelenterata: Scleractinia) from the Red Sea. Open J Biol Sci 7(1): 011-018. DOI: 10.17352/ojbs.000030Copyright
© 2022 Floos YA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The scleractinian coral is common along the Red Sea coast, and its reproductive mode and period of reproduction were assessed using histological preparations. the sexuality, and reproductive mod timing of reproductive of Pocillopora damicornis from adjacent to the fringing reefs of the Ubhur Creek in the Red Sea, were assessed using a serial histological section. Sexual reproduction in Pocillopora damicornis a shallow water hermatypic coral was studied from December 2011 to November 2012. Pocillopora damicornis is a simultaneous hermaphrodite with ovary and testis in the project into the body cavity on the same mesentery. Sperm and eggs were usually released simultaneously from the same polyp. The onset of the reproductive period of Pocillopora damicornis was found to be limited (April to May). In the number of eggs and testes observed in this period, the gonads were found in the polyps. The Pocillopora damicornis egg size ranged from 49.80 µm (in March) to 125.0 µm (in May). Four stages were chosen, to reflect very immature ovaries, the early stages of oocyte development, ova near maturity, and mature ova, and also four distinct stages of sperm development were identified. The state of gonads development (eg. testis and eggs) was measured by a calibrated eyepiece micrometer of a compound light microscope. Zooxanthellae were presented in the mature oocytes in Pocillopora damicornis . This study aimed to examine the reproduction mode and timing of Pocillopora damicornis .
Introduction
Coral reefs are ecosystems as productive as tropical rainforests and biodiversity [1-4]. Reef-forming coral reefs have an important role in underwater life as well as for the many human communities that depend on the ecosystem services they provide. [5,6].
Scleractinian corals show a range of sexuality, there are four basic patterns of sexual reproduction, Knowing among scleractinians such as hermaphroditic broadcast spawners (dominant group), hermaphroditic brooders, gonochoric broadcast spawners, and gonochoric brooders [7,8].
Scleractinian corals can reproduce asexually by fragmentation [9-11], buds, polyp bail-out [9], or sexually by gametogenesis. Free-spawning corals release their gametes into the water column, where fertilization and larval development occur. Brooders release only sperm, with fertilization and maturation of larvae taking place within the polyps of females [8,12,13]. Sexual reproduction in corals has been studied in many locations but research has been concentrated on a few geographical regions. By 1986, detailed information on reproduction in a wide range of coral was available primarily from the Great Barrier Reef (156 species), Northern Western Australia (28 species), the Caribbean (20 species), Red Sea (13 species), Okinawa (11species), Hawaii (10 species) and Palau (10 species) [7]. Coral identified as Pocillopora damicornis produces asexual planulae and broadcast spawners gametes in Western Australia [14], while is only a broadcast spawner for the same species in the Eastern Pacific [15]. Similarly Pocillopora verrucosa brooders at Enewetak Atoll [9,16] and broadcast spawners in the Red Sea [17-19]. Pocillopora damicornis colonies are mostly delicately branched with slender cylindrical branches and large wart-like projections that are often difficult to distinguish from true branches, as the two intergrade. It occurs in all shallow water habitats from exposed reef fronts [20]. It has a wide range of distribution from East Africa; to the Red Sea to Mexico and to Ecuador (Batangas, Luzon, Philippines) [21]. Fecundity and the ratio of male to female colonies may be regulated by a variety of internal and external processes [22-24] The reproductive mode may be determined by the coral’s morphological traits such as colony size, polyp size oocyte diameter, and gonad location [24-26].
The studied species contribute approximately 60 to 80 of the total living coral cover in the Ubhur Creek in the Red Sea and are therefore very important in this ecosystem. furthermore, the present study adds much-needed data about scleractinian corals’ reproduction in the Red Sea. The results are discussed in light of different reproductive pathways, with special emphasis on the question of phylogenetic constraints on gonad parameters and the relationship between reproductive strategy (brooding versus broadcasting) and gonad morphology. This study aimed to examine the reproduction mode and timing of Pocillopora damicornis .
Materials and methods
The study was carried out in the on-shore laboratory of the Faculty of Marine Science, King Abdulaziz University, Jeddah, which is 35 km away from the north of the city and located adjacent to the fringing reefs of the Ubhur Creek (E39 05 46.11 N 21 42 34.26 ) at 5m depth (Figure 1). The study site was located in the northern side mouth of the Creek. The entrance of the Sharm is about 36 m deep and the depth decreases gradually to 3 m at its northernmost extremity.
All fieldwork has been carried out underwater by using SCUBA diving techniques
Temperature: Water temperature was measured by using two maximum and minimum thermometers. These were attached to a piece of iron bar and hidden among coral colonies at a depth of 5m. Readings monthly, from December 2011 to November 2012. The mean monthly temperature was calculated as the average between the mean maximum and minimum temperature for each month.
Salinity: The salinity of seawater was measured monthly on samples of surface seawater, from December 2011 to November 2012, using a hand-held salinity refract meter (Atago Co. ltd) which could be read to the nearest 1.0 ‰.
Reproduction
Samples for the reproductive study of Pocillopora damicornis were taken every morning and every two weeks for the duration of one year starting from December 2011 to November 2012.
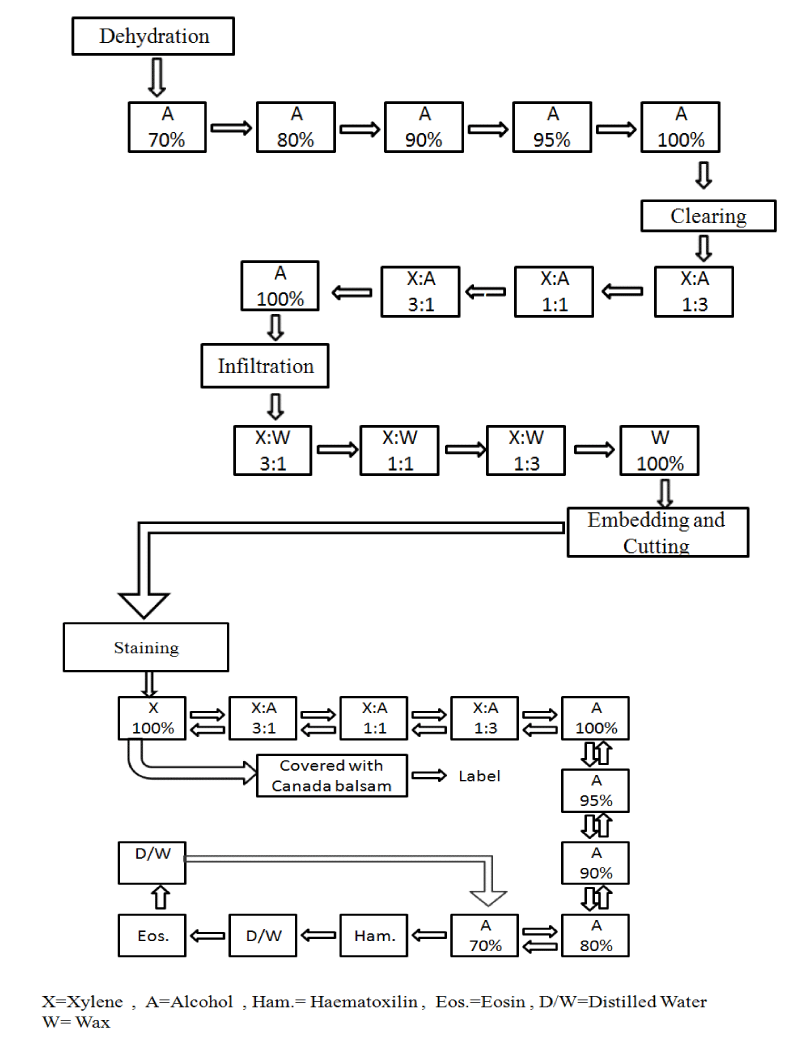
Coral specimens were cut off coral colony by chisel and hammer and fixed in a container with 7% seawater formalin for three days. Thereafter, they were transferred to a 10% solution of nitric acid to decalcify the skeleton. The decalcified polyps were washed with distilled water and preserved in a solution of 70 % Ethanol for further histological study. The preserved polyps were passed through a series of Ethanol of increasing strength from 70% Ethanol and ending with absolute alcohol, then cleared with xylene. The cleared specimens were infiltrated and embedded in pure paraffin of melting point 58-60 °C. Serial sections with a thickness of 7 µm were cut through the polyps and then stained with Haematoxylin and Eosin (Flowchart 1). Thereafter, the stained sections were cleared with xylene and mounted in Canada balsam.
In an attempt to quantify the state of gonad development, the maximum and minimum dimension of a transverse section through a single testis and ovary of six or seven specimens was measured with a calibrated eye-piece micrometer on a compound light microscope. An approximation of gonad size was then obtained from the mean of the maximum and minimum dimensions.
Statistical analysis
I am used one way- ANOVA.
Results
Temperature
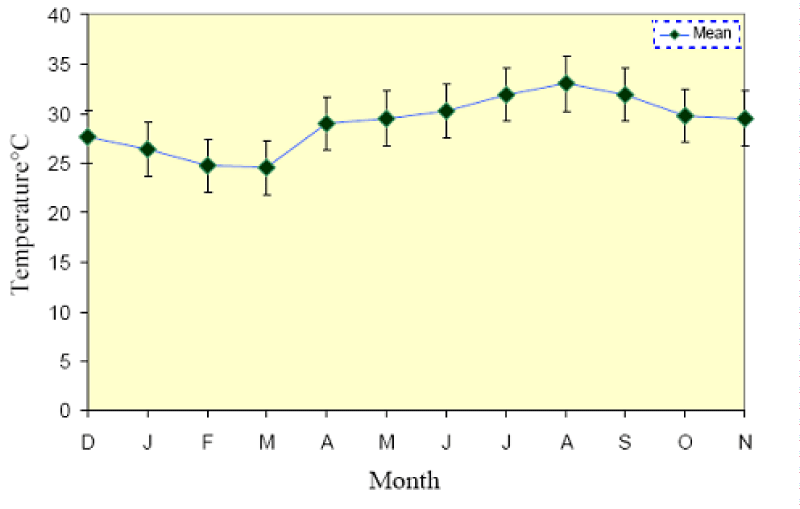
The mean monthly temperature together with the mean maximum and minimum temperatures for each month is shown in (Figure 2). The lowest minimum temperature was 24.0 ± 0.25 °C in March 2012 and the highest maximum was 33.5 ± 0.51 °C in August 2012, where the lowest monthly average was 24.5 °C in March 2012 and the highest was 33.0 °C in August 2012. The mean difference between the maximum and minimum readings was 1.7 °C. The annual range of monthly mean temperature was between 24.6 ± 1.07 and 33.0 ± 0.75 °C, a difference of 8.6 °C.
Salinity
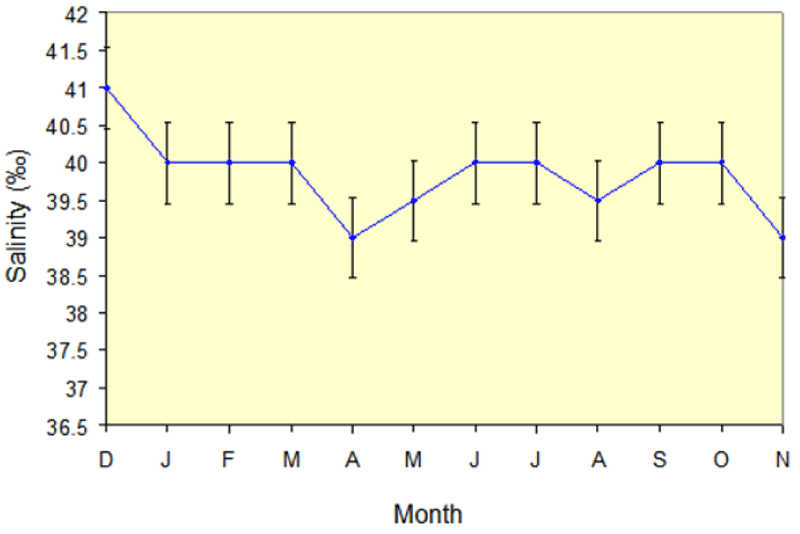
Salinity values ranged from a minimum of 39.0 ± 2.3‰ to a maximum of 41 ± 1.15‰); (Figure 3), the lowest values occurring in the months of April, May, August, and November of the year 2012, and the highest values found through the rest of the year.
Development of gonads and reproduction period
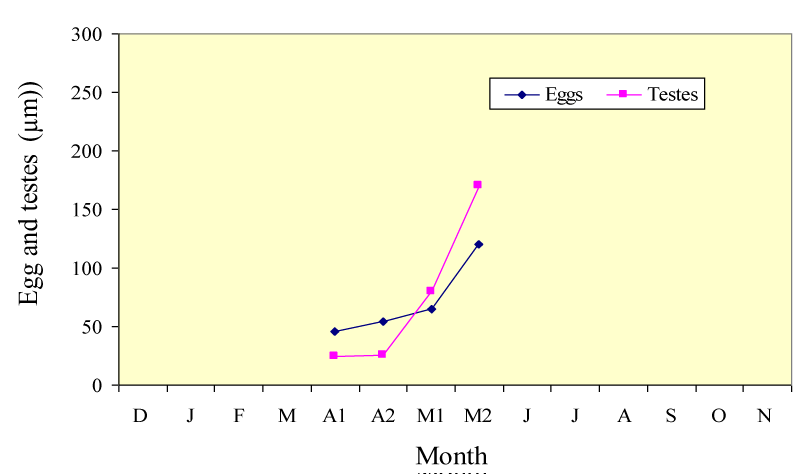
The histological sample revealed that the species P. damicornis is a simultaneous hermaphrodite where female and male gonads develop at the same time (Table 1) and (Figure 3 ). P. damicornis has a very short reproductive season (Table 1). In P. damicornis the size of the small egg was 49.80 μm ± 7.50 (20) in diameter found in April, then reached its maximum of 125.00μm ± 23.00 (20) in May. Development of the testes began in April for species and this increased progressively in size until May (Table 1 and Figure 3). In p. damcorinis from the Red Sea is a simultaneous hermaphrodite, with ova and sperm commonly seen in the same polyp. The sperm was spherical in shape in more advanced testes (Figure 5D). Small eggs have their own nucleus in the center, but as they become mature, the nucleus moves towards the surrounding cell wall of the egg (Figure 4 A, D). Zooxanthellae are present in the mature eggs of the species (Figure 4D). The species were broadcast spawners, as planulae were not observed in the coelenteron of the polyp.
Oogenesis
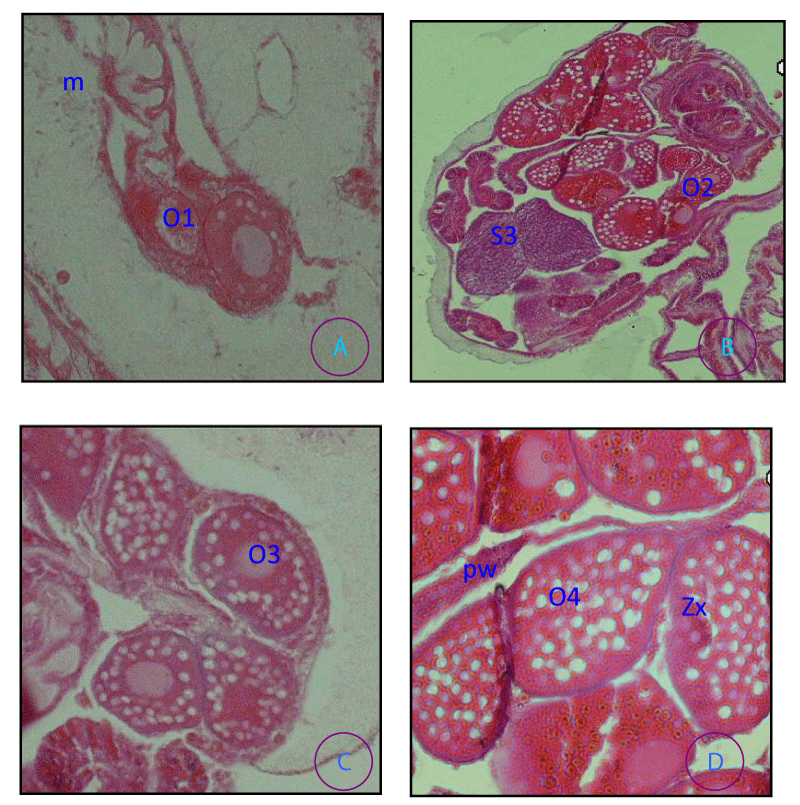
Four stages were chosen, to reflect: very immature ovaries, the early stages of oocyte development, ova near maturity, and mature ova.
Stage I (Figure 5A ). Multiple oocyte ovary; oocytes < 30 µm (smallest oocyte were 10 µm in diameter and had a germinal vesicle with a prominent nucleolus. Only a few yolk granules were present, located at one side of the oocyte
Stage II (Figure 5B ). Single oocyte ovary; <50 µm although usually >30 µm diameter and Yolk granules were arrayed in a circle around the germinal vesicle
Stage III (Figure 5C ). Oocytes from 50 to 70 µm diameter; nucleolus usually well developed and intensely staining, occasionally dispersed throughout the nucleus; granular cytoplasm invested with numerous small vacuoles. A vitelline membrane or cortical layer was observed at the surface of the oocyte
Stage IV (Figure 5D). Oocytes were about 100 µm in diameter usually surrounded by a darker membrane and contracted slightly from the ovary wall; cytoplasmic vacuoles enlarged; nucleus and nucleolus less obvious than in the previous stage.
Spermatogenesis
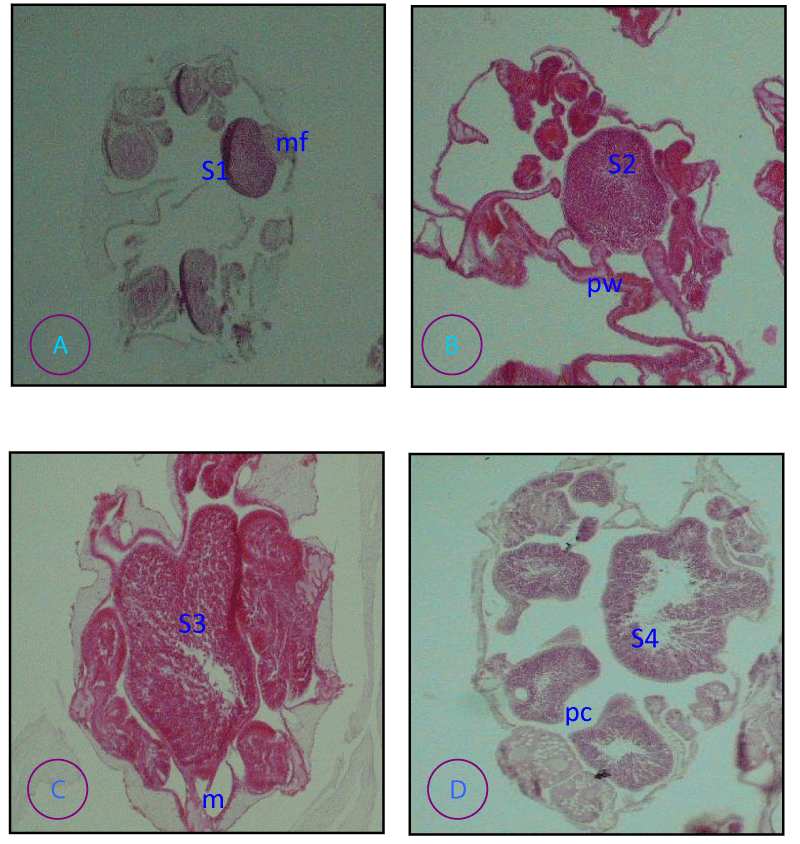
Four distinct stages of sperm development were identified.
Stage I (Figure 6A ). Spermaria usually < 30 µm diameter primary spermatocytes surrounded by a thickened spermatogonial wall
Stage II (Figure 6B ). Testes of varying size (up to 200 µm) secondary spermatocytes with a characteristic hollow circle appearance.
Stage III (Figure 6C). large (usually >100 µm diameter) Testes; densely staining spermatids; Testis not vacuolated.
Stage IV (Figure 6D ). Mature sperm with tails: the middle of the testes is often vacuolated or the whole testes loosely packed with sperm. Stage 1V sperm is sometimes observed free in the coelenterons, although this may be due to histological technique in the field or aquaria.
P. damicornis the arrangement of ovaries and testes on mesenteries was found on different pairs of mesenteries and they were carried on a stalk which is tall and thin in the case of the male and short and thick in the female illustrated in Figures 5,6 respectively.”
Small eggs have their own nucleus in the center, but as they become mature, the nucleus moves toward the surrounding cell wall of the egg illustrated in (Figure 5 A, D). Zooxanthellae are present in the mature eggs of the species illustrated in (Figure 5D). The sperm was spherical in shape in more advanced tests illustrated in (Figure 6D).
The species was broadcast spawners, as planulae were not observed in the coelenteron of the polyp. Oocytes and testes developed in mesenteries and were enveloped in mesoglea and gastrodermis. Mature oocytes and testes protruded into the gastrovascular cavity and were connected to a mesentery by a stalk.
Discussion
Studies on the reproduction of Pocillopora damicornis indicated that species were hermaphrodite broadcaster spawners with external larval developments. Embryos of species were not observed in the histological sections (Table 1) and (Figure 5). The reproductive strategy of P. verrucosa from the Red Sea was found to be a hermaphrodite broadcaster spawner [9], while Stimson [16] reported brooding in P. verrucosa from Enewetak. On the other hand, most studies on P. damicornis from different geographic locations proved that P. damicornis is a hermaphrodite brooder [14,28-30] Similar results were in agreement with the present study by Glynn, et al, [15] who found a hermaphrodite broadcaster spawner in the eastern Pacific However, in Western Australia P. damicornis includes both a brooder and broadcaster spawner [14].
Differences in the reproductive patterns of P. damicornis from different geographic areas were related to differences in many parameters such as colony morphology, polyp size, environmental conditions, and habitats [16,31]. None of these factors have been shown to give any explanation for these differences [32]. In the Red Sea P., verrucosa displays no different reproductive pattern among the different sites studied (e.g. North of Agaba Gulf, Yanbu, and Jeddah in the present study), whilst P. damicornis has a similar reproductive mode as in P. verrucosa in the Red Sea, but there are no comparative studies on P. damicornis from the Red Sea so that P. verrucosa is not the only known broadcasting Pocilloporidae as mentioned by Fadlallah [32].
p. damcorinis from the Red Sea is a simultaneous hermaphrodite, with ova and sperm commonly seen in the same polyp. Ovaries were situated on stalks projecting into the coelenterons, as reported for p. damcorinis from the Great Barrier Reef [33]. However, testes appeared as an outgrowth of mesenteries, although this was not always apparent in the transverse section of polyps as not all of a large testis was connected to the mesentery. Sex pairs of gonads were present within polyps and sperm substantially outnumbered ova in terms of the volume occupied within a polyp, and the number of the polyp with gonads of one sex. Oocytes were classified into 4 stages based on morphology and size (see Figure 5) Stoddart and Black [34].
The onset of the reproductive period of P. damicornis was found to be of very short duration i.e. from April to May 2012 (Figure 4), with a negligible number of eggs and testes observed at the end of March and early June (3-5 eggs). These species were also simultaneous hermaphrodites where oogenesis and spermatogenesis occurred at the same time during April. Fadlallah [32] and Shlesinger and Loya [17] noticed a very short reproductive period for P. verrucosa at Yanbu north of Jeddah from April to May and at Eilat, the northernmost tip of the Gulf of Aqaba in the Red Sea from July to August. This short duration of gametogenesis occurred with the increase of seawater temperature (29 °C) in April after the minimum average of seawater temperature from 24.5 °C to 24.75 °C during Feb. and Mar.2012 with a narrower range of seawater temperature from 28.5 °C min. to 30 °C, by the end of the reproductive period in May, where the mean temperature reached 30.25 °C (Figure 2) [35].
There is annual variation in salinity during the period of this study from 39‰ to 41‰ (Figure 3), while AL- Sofyani [36] showed a similar range of 39‰ to 40.5‰ in Sharm Ubhur over a broad period of 2 years. The increase from 39‰ to 40‰ in the summer months is probably due to surface evaporation and to the action of the northwesterly winds driving the more saline water from the northern Red Sea to more southerly latitudes [36-38].
Gonad development of P. damicornis is stimulated by increasing water temperature. These species prefer a slightly warmer season for their gonads development and this will explain why this species during the bleaching events in 1998 exhibited a sign of temperature stress P. damicornis and was bleached, whereas P. verrucosa was more resistant to extreme temperature. Fadlallah [32] reported that gametogenesis of P. verrucosa may be triggered by rising sea water temperature in March and April. Many suggested factors influence the reproduction among various marine invertebrates, especially corals: these are temperature [39-41], lunar tidal cycle, and day-night cycle [25].
In the present study, P. damicornis showed a similar reproductive mode as in Pocillopora verrucosa. While The Stylophora pistillata and Seriatopora hystrixhave have been reported as brooding coral species of the Red Sea [18,19,27,36,42].
In the Red Sea, the South-North gradients of seawater temperature seem to be by far from the most powerful factors influencing the reproductive cycle of scleractinian corals [36,43]. The egg sizes of P. damicornis ranged from 49.50 μm in April. to 125μm in May (Table 1). Sier and Olive [44] found zooxanthellae infestation at a later stage in this case, while Harrison and Wallace, 1990 mentioned that, an infestation can occur weeks before the spawning, as observed in the genus Porites and Montipora sp. or as little as 24 h before spawning as in Montipora digitata.
Conclusion
Pocillopora damicornis are hermaphrodite broadcasters with external larval development. Differences in the reproductive patterns of Pocillopora damicornis from different geographic areas were related to differences in many parameters such as colony morphology, polypsize, environmental conditions, and habitats. The onset of the reproductive period of oscillopsia damicornis was found to be limited (April to May). In the number of eggs and testes observed in this period, the gonads were found in the polyps. Zooxanthellae are present in the mature oocytes in Pocillopora damicornis
Recommendation
- This study revealed that is not spa pocillopora damicornis winning throughout the year but limited to particular months. Their settlement rate is also highly challenging. Hence, this is our responsibility to protect the corals by providing suitable substrates for settling.
- Develop a white paper to justify the rationale for an internationally recognized systematic terminology for coral histopathology in consultation with experts in scientific nomenclature.
- Record the normal range of histological characteristics for the priority species of healthy corals in the Red Sea. This would include developing standardized methods to collect corals on a spatial and temporal basis and conduct histology using light microscopy on selected specimens.
- Reef fishing and boat anchoring on reefs should be banned. A separate boat jetty should be arranged (if necessary) in reef areas to avoid reef damage.
- Recreational diving and other related activities should not be allowed in the core reef area.
- Awareness campaign for both public and students will be essential to know the importance of coral reefs will provide a positive result.
The author would like to thank the Help in the collection of samples, especially Mr. Kamal Aldahoudi, Mr. Rabea Khayat, Mr. Suhail Arshad, and Mr. Khaled he is duly acknowledged. My deepest thanks go to the staff of the marine station of Ubhur for their assistance. in collecting the samples and assistance in laboratory histology. I would also like to thank the administrative staff of the Faculty of Marine Sciences for their co-operation and cordial response during this study.
- Barber PH, Cheng SH, Erdmann MV, Tenggardjaja K, Ambariyanto. Evolution and conservation of marine biodiversity in the Coral Triangle: Insights from stomatopod Crustacea. In: Held C, Koenemann S, Schubar CD (eds.). Phylogeography and Population Genetics in Crustacea. CRC Press, Boca Raton. 2011.
- Carpenter KE, Barber PH, Crandall ED, Ablan-Lagman MCA, Ambariyanto Mahardika GN, Manjaji-Matsumoto BM, Juinio-Menez MA, Santos MD, Starger CJ, Toha AHA . Comparative phylogeography of the coral triangle and implications for marine management. J Mar Biol. 2011; 396982: 1-14.
- Rinkevich B. Novel tradable instruments in the conservation of coral reefs, based on the coral gardening concept for reef restoration. J Environ Manage. 2015 Oct 1;162:199-205. doi: 10.1016/j.jenvman.2015.07.028. Epub 2015 Aug 1. PMID: 26241935.
- Wooldridge SA. Instability and breakdown of the coral-algae symbiosis upon exceedence of the interglacial pCO2 threshold (>260 ppmv): The‘“missing”’ Earth-System feedback mechanism. Coral Reefs. 2017; 36: 1025-1037.
- Hoegh-Guldberg O, Pendleton L, Kaup A. People and the changing nature of coral reefs. Reg Stud Mar Sci. 2019; 30: 1-20.
- Woodhead AJ, Hicks CC, Norström AV, Williams GJ, Graham NAJ. Coral reef ecosystem services in the Anthropocene. Funct Ecol. 2019; 33: 1023-1034.
- Harrison PL, Wallace CC. Reproduction, dispersal and Hubbard DK (1986) Sedimentation as a control of reef development. Coral Reef. 1990; 5: 117-125.
- Harrison PL. Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: An ecosystem in transition. Springer Netherlands, Dordrecht 2011; 59–85.
- Kruger A, Schleyer MH. Sexual reproduction in the coral Pocillopora verrucosa (cnidaria scleractinia) in Kwazulu-Natal, South Africa. Mar Biol. 1998; 132: 703 - 710.
- Borneman EH. Aquarium corals; selection, husbandry and natural history. T. F. H. Publication. New Jersey, USA. ISBN: 1-890087-47-5. 2001.
- Spalding MD, Raviolus C, Green EP. World Atlas of coral reef prepared at the UNEP world conservation Monitoring centre. University of California press, Berkley, U. S. A. ISBN: 0-520-23255-0. 2001.
- Oliver J, Babcock R. Aspects of the Fertilization Ecology of Broadcast Spawning Corals: Sperm Dilution Effects and in situ Measurements of Fertilization. Biol Bull. 1992 Dec;183(3):409-417. doi: 10.2307/1542017. PMID: 29300507.
- Baird AH, Guest JR, Willis BL. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst. 2009; 40: 551–571.
- Ward S. Evidence for broadcast spawning as well as brooding in the scleractinian coral Pocillopora damicornis . Mar Biol. 1992; 112: 641 - 646.
- Glynn PW, Gassman NJ, Eakin CM, Cortes J, Smith DB, Guzman HM. Reef coral reproduction in the eastern pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). 1. Pocilloporidae. Mar Biol. 1991; 109: 355 - 568.
- Stimson JS. Mode and timing of reproduction in some common hermatypic corals of Hawaii and Enewetak. Mar Biol. 1978; 48: 173 - 184.
- Shlesinger Y, Loya Y. Coral community reproductive patterns: red sea versus the great barrier reef. Science. 1985 Jun 14;228(4705):1333-5. doi: 10.1126/science.228.4705.1333. PMID: 17799121.
- Floos YA. Studies on Physiology and ecology of Pocillopora damicornis and pocillopora verrucosa in Sharm Ubhur, Jeddah, Saudi Arabia. M. Sc. Thesis, King Abdulaziz University. Jeddah. 2007; 102.
- Floos YA. Histological; Mode and Timing Reproduction Studies of Pocillopora verrucosa in the Red Sea. Abhath Journal of Basic & Applied Sciences. A scientific refereed journal issued by Faculty of Education- Hodeidah University-Yemen. 2022; 1:15-28.
- Vollmer SV, Palumbi SR. Hybridization and the evolution of reef coral diversity. Science. 2002 Jun 14;296(5575):2023-5. doi: 10.1126/science.1069524. PMID: 12065836.
- Terrence M, Gosliner, David W, Behrens Gary C Williams. (1996). Coral reef animals of the indo-pacific. Monterey, California.
- Rinkevich B, Loya Y. Variabilityin the pattern of sexual teproduction of the coral Stylophora pistillata. at Eilat. Red Sea along- term study. Biol mar biol. Woods Hole 173: 335-344.
- Ward S. Two patterns of energy allocation for growth reproduction and lipids storge in scleractinian coral Pocillopora damicornis (linnaeus) J Exp Mar Bol Ecol. 1995; 187:193-206.
- Shlesinger Y, Goulet TL, Loya Y. Reproductive patterns of scleractinian corals in the North ern Red Sea. Mar Biol. 1998; 132: 691-701.
- Rinkevich B, Loya Y. The reproduction of the Red Sea coral Stylophora pistillata. 1. Gonads and planulae. Mar. Ecol. Prog. Ser. 1979; 1:133-144.
- Szmant AM. Reproductive ecology of Caribbean reef corals. Coral. Coral Reef. 1986; 5:43-54.
- Floos YA. Physiological and Ecological Studies of Two Reef Building Corals Species Seriatopora hystrix and Lobophyllia corymbosa in the Red Sea. Ph. D. Thesis., King Abdulaziz University. Jeddah. 2012;165.
- Kolinski S, Cox EF. An update on modes and timing of gamete and planula release in Hawaiian scleractinian corals with implications for conservation and management. Pac. Sci. 2003; 57:17-27.
- Zakai D, Levey O, Cadwick-Furman NE. Experimental fragmentation reduces sexual reproductive output by the reef-building coral Pocillopora damicornis Coral Reef. 2000; 19:185
- Richmond RH. Energetics, competency, and long-distance dispersal of planulae larvae of the coral Pocillopora damicornis . Mar. Biol. 1987; 93:527-533.
- Tomascik T, Sander F. Effects of eutrophication on reef-building corals I11. Reproduction of the reef building coral Porites porites Mar Boil. 1987; 94:77-94.
- Fadlallah YH. Reproduction in the coral Pocillopora verrucosa on the reefs adjacent to the industrial city of Yanbu (Red Sea, Saudi Arabia). In: Gabrie´ C et al. (eds.) proc 5th int coral Reef Conger. Vol. 4. Antenne Museum- EPHE, Moorea, French Polynesia. 1985; 313-318.
- Harriot VJ. Reproductive seasonality, settlement and post settlement mortality of Pocillopora damicornis at Lizard Island, Great Barrier Reef. Coral Reef. 1983; 2:9-18.
- Stoddart JA, Black R. Cycle of gametogenesis and planulation in the coral Pocillopora damicornis . Mar. Ecol. Prog. Ser. 1985; 23:53-164.
- Al-Sofyani AA, Floos YA. Effect of temperature on two reefbuilding corals Pocillopora damicornis and P. verrucosa in the Red Sea. Oceanologia. 2013; 55:4; 917-935.
- Al-Sofyani AA. Studies on Physiology and ecology of Stylophora pistillata and Echinopora gemmacea from the Red Sea. Ph. D. Thesis. Glasgow. U.K, 1991; 167.
- Dubach HW. A summary of temperature-salinity characteristics of the Persian Gulf. National Geographic data center. Publication G-4 in NODC general series. 1964; 223.
- Edwards AJ, Head SM. Red Sea: Key Environments. A. Wheaton & Co. Ltd., Exeter. 1987.
- Fadlallah YH. Sexual reproduction, development and larval biology in scleractinian corals. A review. Coral Reefs. 1983; 2:129-150.
- Richmond RH, Jokiel PL. Lunar periodicity in larva release in the Reef Coral Pocillopora damicornis at Enewetak and Hawaii. Bull. Mar. Sci. 1984; 34:280-287.
- Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL. Mass spawning in tropical reef corals. Science. 1984 Mar 16;223(4641):1186-9. doi: 10.1126/science.223.4641.1186. PMID: 17742935.
- Floos YA, Al-Sofyani AA, Zari TA. Sexual reproduction of two reef building corals Seriatopora hystrix and Lobophyllia corymbosa in the Jeddah coast of Red Sea Bioscience Biotechnology reaches Asia. 2012; 9: 1; 63-72.
- Al-Sofyani AA. Studies on growth and reproduction of Red Sea scleractinia. M. Sc. Thesis, King Abdulaziz University. Jeddah. 1987; 102.
- Sier CJS, Olive PJW. Reproduction and reproductive variability in the coral Pocillopora verrucosa from the Republic of Maldives. Mar Biol. 1994; 118:713-722.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
