Open Journal of Biological Sciences
Phytochemicals screening, cytotoxicity and antioxidant activity of the Origanum majorana growing in Casablanca, Morocco
Hanane Ennaji1*, Dounia Chahid1, Saadia Aitssi2, Abdallah Badou2, Naima Khlil1 and Samir Ibenmoussa1
2Laboratory of Cellular and Molecular Pathology, Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, B.P 5696, Casablanca, Morocco
Cite this as
Ennaji H, Chahid D, Aitssi S, Badou A, Khlil N, et al. (2020) Phytochemicals screening, cytotoxicity and antioxidant activity of the Origanum majorana growing in Casablanca, Morocco. Open J Biol Sci 5(1): 053-059. DOI: 10.17352/ojbs.000026Origanum majorana is a plant from the Lamiaceae family. It is a medicinal plant used in traditional medicine in Morocco to treat various diseases. This work aims to determine the phytochemical composition of Marjoram, as well as to evaluate its cytotoxic effect on the cells of healthy subjects. All parts of the plant (roots, leaves, stems, etc.) were subjected to selective extraction with different solvents of increasing polarity (Diethyl ether, Dichloromethane, Ethanol, Methanol) using the rotary steamer. The yields obtained are respectively 1.34%, 4.57%, 9.98%, and 10%. The phytochemical tests carried out have detected the presence of polyphenols, tannins, flavonoids, terpenoids, sterols, saponins, and reducing sugars. In contrast, the absence of the family of cardiotonic alkaloids, quinones, and heterosides. Origanum majorana L. exhibited concentration-dependent inhibitory effects on 2,2′-diphenylpicrylhydrazyl (DPPH) with IC50 equal 2.308 mg/ml, related to the presence of the same content of polyphenols and flavonoids but with the lowest concentration of tannin content. The cytotoxicity of the hydro-ethanolic extract of Marjoram was evaluated by the MTT colorimetric method. However, the results obtained that the examined extract was devoid of cytotoxic activity, on the other hand, it induced cell proliferation. O. majorana has good potential to prevent diseases caused by the overproduction of free radicals, and that it can be used as natural antioxidant agents and cell proliferation dependant on concentration.
Introduction
For centuries, traditional medicinal plants have been used by humankind to cure disease. Nowadays, many researchers became interested to treat various disorders with those medicinal plants [1].
Origanum majorana L. is a medicinal plant of the Lamiaceae family, knowing as Zaatar in traditional Moroccan medicine [2]. This plant is distributed around the Mediterranean regions, Asia, and North Africa, in particular, Morocco, Algeria, Egypt, Spain, and Portugal [3]. Origanum majorana showed various biological activities such as allergies, fever, hypertension [4,5], respiratory infections [6], antidiabetic [7], painful menstruation, Kidney Yang deficiency, stomach ache, cough [8], rheumatism, headache, insomnia [9], also in intestinal antispasmodic [10]. Moreover, Origanum majorana L. exhibits a wide effect spectrum with antioxidant, antibacterial, antifungal, nephroprotective, anti-proliferative, anti-cancer activities [11-16]. These effects are mediated by the presence of bioactive compounds such as thymol, carvacrol, tannins, hydroquinone, sitosterol, cis-sabinene hydrate, limonene, terpinene, camphene, and flavonoids like diosmetin, quercetin, luteolin, and apigenin [17,18].
Recently, the excessive use of antibiotics was conducted to the development of antibiotic resistance and without a doubt, the incidence of multidrug-resistant pathogens is considered a major disadvantage in medication strategy, which has led attention towards innovative antibiotic sources. On the other hand, the plant had a therapeutic potential against drug-resistant microbial infections [19]. The herbal made four major groups of antimicrobial compounds like phenolics and polyphenols, terpenoids and essential oils, lectins and polypeptides, and alkaloids, those bioactive plant extracts could yield an enhanced effect (Cowan,1999). A lot of mechanisms against the bacteria were used via these compounds, including inactivation of proteins, adhesins, and enzymes, among other targets.
Some recent research revealed that certain molecules in the plant could also block cell-to-cell signaling pathways, extinguish the production of virulence factors and disrupt or inhibit the formation of biofilms, which bestow a protective advantage to pathogens during infection [20].
Nevertheless, Origanum majorana leaves wielded significant antimicrobial activity against a huge spectrum of Gram-positive and Gram-negative bacteria: Bacillus cereus, Escherichia coli, Staphylococcus coagulase-positive, Enterobacter spp, Proteus spp, Acinetobacter spp, Klebsiella spp., Pseudomonas spp S, aureus, Klebsiella pneumonia, and Pseudomonas spp. Besides, the sensitivity results obtained are almost the same as those observed for the antibiotic gentamicin considered as a positive control [10].
The purpose of the present study was to characterize the flavonoid, polyphenols, and tannins content, antioxidant properties, and cytotoxic activity of O. majorana.
Materials and methods
Plant material and extract preparation
Origanum majorana (O. majorana) was collected from Casablanca, Morocco between February and April. The plant was identified by a botanist. The collected plant was dried in the shade at room temperature for two weeks. One kg of the plant was powdered with an electric grinder, 20 g of the pulverized plant material was extracted in 200 ml of hydro-ethanol and hydro-methanol (80:20) separately. Another 20 g of plant material was extracted in 200ml of diethyl ether and dichloromethane separate. Then, th-e separated extracted were t filtered through Whatman’s No. 1 filter paper. The excessive solvent was evaporated with a rotary vacuum evaporator using the boiling time of each solvent. The yield percentage was calculated using the following equation:
Humidity determination
Two g of O. majorana was placed in previously weighed glass capsules. The whole was placed in a vacuum oven at 105 ° C for 24 hours. Three repetitions were performed according to a previously reported method of Cunniff, 1997 [21]. When drying was finished, the humidity percentage was calculated according to the following formula:
T1: the weight of the empty capsule, T2: the weight of the capsule containing the fresh sample, T3: the weight of the capsule containing the dry sample.
From the moisture content we can determine the dry matter content which is calculated by the following formula:
Dry matter content (%) = 100 - Humidity
Preliminary qualitative phytochemicals screening
The extract of the plant material was subjected to phytochemical screening to qualitatively determine some types of interested phyto-organic constituents which are responsible for biological activities, alkaloids, polyphenols, quinones, saponins, tannins, sterols, carbohydrates, glycoside cardiac, triterpenes, and terpenes which were the major cheeked groups using standard methods.
Determination of total phenolic content
Folin-Ciocalteu reagent method described by Zhishen, et al. 1999 [22] was used to detect total phenolic content (TPC) present in the hydro-ethanolic extract of O. majorana. A volume of 1.5 mL of 10% Folin-Ciocalteu reagent was added to 0.5 ml of extract and mixed for 5 min. Then, 3 mL of sodium carbonate solution 7.5% (Na2CO3) was added and further incubated at 300C for 2 h. Finally, the absorbance was calculated at 760 nm using a UV-visible spectrophotometer against a blank composed of the same previously reagents except for the extract. The quantity of total phenolic compounds was detected from the standard curve of gallic acid and expressed in mg gallic acid equivalent (GAE) per g of the dry weight of extract (mg GAE/g DW).
Determination of total flavonoid content
Aluminum Chloride (AlCl3) colorimetric method is described by Dewanto, et al. 2002 [23]. Was used to detect the Total Flavonoids Content (TFC). Briefly, one mL of 2% AlCl3 solution was added to 1 mL of the hydro-ethanolic extract of O. majorana and incubated for 30 min. The absorbance was measured at 420 nm and the flavonoid content was detected from a quercetin standard curve and expressed in mg quercetin equivalents per g of the dry weight of extract (mg QuE/g DW).
Determination of condensed tannins content
Condensed Tannins Content (CTC) was determined according to the method of Sun, et al. 1998. An aliquot of the extract with different concentrations (0.5-2-4mg/mL) was mixed with 3mL of vanillin (4%) and 1.5 mL of sulfuric acid concentred. After homogenization, the tubes are incubated in darkness at ambient temperature for 15min.
Then, the absorbance was measured at 500nm. The standard curve was prepared by using different concentrations of catechin and the absorbance was measured on the same wavelength. Total condensed content was expressed as mg catechin equivalents of the dry weight of individual extract (mg CE/g DW).
Antioxidant activity
The DPPH free radical scavenging activity to hydro-ethanolic extract of O. majorana was quantified according to the method of Wu, et al. 2019 [24], with some modifications. Briefly, 50 μL of extract with different concentrations (0.5-2-4 mg/mL) was added to 1950 μL of 60 μM DPPH ethanol solution (2.3mg of DPPH in 100mL ethanol).The mixture was shaken strenuously and incubated 30 min at room temperature. Absorbance was recorded at 517 nm. The scavenging activity was calculated using the formula:
Where A0 and AE are the absorbance of the control and extract after 30 min, respectively.
PBMCs isolation and cell culture
The whole blood was collected from healthy adults informed with written consent. The PBMCs were isolated using Ficoll-Histopaque (d=1.077g/mL) (Sigma, USA), by centrifugation at 900g for 25 min at 20°C. After washing cells in NaCl 0.9% at 400g for 15 min and resuspended in RPMI-1640 media (Gibco, UK), enriched with 10% of Fetal Bovine Serum (FBS) (Sigma, USA), 26.3g/L penicillin, and 4.2g/L Streptomycin, the viable cell counting was performed by Trypan blue method using microscopy. 100µL of complete media containing 1x105 cells were seeded in triplicate using 96 well plate culture, supplemented with 20 µL of different concentrations of O.majorana, and the final volume (200µL/well) was reached with complete media. Then, the PBMCs were incubated for 96 hours at 37°C under 5% CO2 and 95% humidity.
MTT assay
Viability and proliferation were measured by the MTT (3-(4,5 dimethyl thiazol-2-yl)-2,5 diphenyltetrazolium bromide) test [25] in each well and compared to that of the untreated group. After incubation, 20µL (5mg/mL) (Sigma, USA) was added to each well and the plates were incubated 4h at 37°C under 5% CO2 and 95% humidity.
Thereafter, the supernatant was removed from each well after centrifugation, and 100µL DMSO was added to dissolve the formazan crystals produced following the viable cells able to metabolize MTT salts. The extent of the formazan product was measured at 492nm using FLUO star Omega microplate reader.
Cell viability was calculated using the following formula:
Cell viability (%) = (mean of optical density of treated cells /mean of optical density of negative control cells) x100
And cell proliferation was calculated using the following formula:
Statistical analysis
The statistical significance between the mean values was performed by an independent t-test. using SPSS statistical software version (15.0.1) (Chicago, IL) to determine the difference between groups. All the [* p < 0.05; ** p < 0.01; *** p < 0.001] were considered significant.
Ethics
The study was assessed and approved by the Faculty of Medicine and Pharmacy, Hassan II University, Ethics Committee, following the declaration of Helsinki. Consents were obtained for the collection of PBMCs samples.
Results
Extracts yields and humidity
The yield extracted from O. majorana by four solvents namely, hydro-methanol, hydro-ethanol, diethyl ether, and dichloromethane is presented in Figure 1. The highest extract yield was obtained by hydro-methanol extraction followed by hydro-ethanol, dichloromethane, and finally by diethyl ether. The yields of both hydro-methanol and hydro-ethanol were 9.98 and 10 % respectively. Diethyl ether showed as a solvent was lower than the efficiencies of all other solvents.
Although, O. majorana exhibited the lowest humidity percentage with a value of 10.107 ± 0.25%. then, the water content allowed to determine the percentage of dry matter estimated at 89.893%.
Phytochemicals screening
The preliminary phytochemicals of O. majorana revealed the presence of important secondary metabolites such as tannins, polyphenols, flavonoids, and saponin. Carbohydrates, terpenoids, sterols, and triterpenoids were found in moderate concentration while quinone and glycosides cardiac was not found. Although, the alkaloids were present by the Mayer test but not by Dragendorff (Table 1).
Determination of total polyphenols, flavonoids, and tannins content
Based on the absorbance values of the hydro-ethanolic extract solution, which reacts with the Folin-Ciocalteu reagent and compared to standard solutions of gallic acid equivalents (y=0.0064x - 0.1213, R2 =0.9288) a polyphenol content of (3.87 to 5.15 mg GAE/g DW) was found (Table 2).
The result of flavonoids content revealed their presence of a concentration between 3.02 to 5.62mg QuE/g DW this result was differentiated with a standard curve of quercetin. The flavonoid contents were calculated using the equation y=0.0352x+0.0273, R2 =0.9889.
While the number of condensed tannins varied by increasing concentration in the hydro-ethanolic extract of O. majorana and ranged from 1.9 to 4.4 mg CE/g of dry material (Table 2). Catechin was used as a standard compound and the condensed tannins content were expressed as mg/ml catechin equivalent using the standard curve equation: y = 0.003x + 0.021, (R2= 0.995).
Antioxidant activity
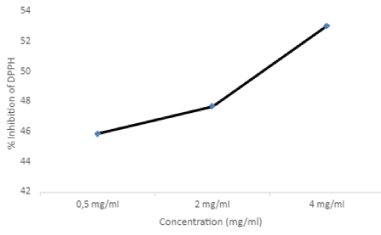
The radical Scavenging activity of hydro-ethanolic extract of O. majorana was examined using the DPPH radical at different concentrations. Scavenging activity against the DPPH radical was concentration dependant. The IC50, the concentration at which 50% of DPPH molecules are inhibited, is obtained from the point of intersection of the scavenging activity and the antioxidant activity curves (Figure 2). IC50 corresponds to 2.308 mg/mL of O. majorana. The maximum scavenging activity of the hydro-ethanolic extract was 53.068% at 4 mg/mL (Table 2).
Cytotoxicity assay
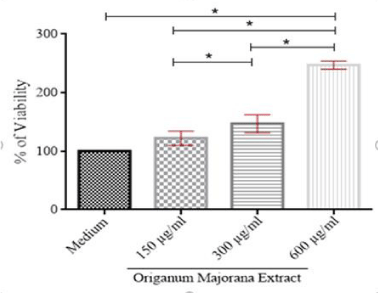
MTT assay investigated the effect of hydro-ethanol extract of O. majorana on PBMCs viability. A dose-dependent MTT reduction was observed by a color change from yellow to purple. After 5 days of application, O. majorana showed a statistically significant increase in the percentage of cell viability between the medium and the concentrations of 300 and 600 μg/mL. Whereas the comparison with the concentration of 150 µg/mL showed only a slight increase. This means O. majorana extract increases the viability of immune cells (Figure 3).
However, the cut off showed in Figure 4 at which the extract induces a proliferative effect is set at 0.392. At a concentration of 150 μg/mL, the proliferation index is below the positivity threshold, which means that at this concentration the extract of O. majorana has no proliferative effect, while at 300 and 600 µg/mL the proliferation index is statistically higher than the positivity threshold when the concentration increases, this being said that from 300 µg /mL the extract showed a proliferative effect.
Discussion
Recently, medicinal plants are known for their various biological activities to cure humans against many diseases. Their activities were related to the presence of secondary metabolites such as alkaloids, terpenes, flavonoids, lignans, plant steroids, curcumins, saponins, phenolics, flavonoids, and glucosides [26].
The extraction method adopted is successive exhaustion using four solvents of increasing polarity. The best yields are obtained with the more polar solvents, namely hydro-methanol and hydro-ethanol, while the lowest yields have been obtained with dichloromethane and diethyl ether, which are less polar solvents.
This difference of yield could be first caused by the physicochemical characteristics of the solvents used, in particular on their polarity. Secondly, according to several studies, geographical origin of species, drying time, as well as the extraction method, and conditions such as pH and temperature are among other factors that may also have a direct impact on extraction yields, which makes it difficult to compare our results with those found in other studies [27].
Generally, the plants are rich in water while the analysis of our sample revealed a low humidity rate of 10%, this low rate reflects good conservation of the powder therefore very low degradation of the bioactive molecules of the plant.
Our results are a little high with those of Shahidi, et al. 2018 [28] who stated that the moisture content of O.majorana is between 6.5 and 8%. Numerous studies have shown that the humidity level is only relative can be related to environmental factors such as climatic conditions, geographical origin as well as the conditions, methods, and applied drying time.
The preliminary assessment of the phytochemical composition of the O. majorana species revealed the presence of secondary metabolites. Indeed, the results of the phytochemical screening revealed the presence of polyphenols, flavonoids, catechin, and gallic tannins, terpenoids, sterols, and triterpenes as well as saponin and carbohydrates, while other compounds seemed to be absent in our sample, in particular, glycoside cardiac, alkaloids, quinones, and heterosides. Our result is following those of Adam, et al. [29] and similar to those of Bhardwaj, et al. [30].
However, Vasudeva reported the absence of alkaloids and glycosides in this species, which is comparable to our results [31]. Another study carried out on the methanolic extract of O. majorana in Yemen confirms the results of our work by confirming the absence of alkaloids and the presence of other chemical compounds [32]. Contrariwise, the literature has shown the existence of various alkaloid molecules while they were not detected in our plant sample. This disagreement on the chemical composition of the same species can be justified by differences in geographical location and extraction method [33].
The choice of using hydro-ethanol extract was because the ethanol was a better solvent than the others in extracting phenolic compounds from the extracts due to his polarity and good solubility for phenolic components from plant materials [34].
Under our study, we found a slight variation between total phenolic and flavonoids content in the three concentrations while a low concentration of tannins (Table 2). That was enabled the correlation between the total flavonoid, polyphenols content, and antioxidant activity. Our results are in an agreement with those [35] and disagreed with [36]. We suggest that could be concerning high levels of polar compounds in the plant materials which are soluble in solvents with a high polarity such as water, methanol, or ethanol.
The determination of the antioxidant activity of O.mjorana extracts was based on the scavenging activity using 1,1- diphenyl-2-picrylhydrazyl (DPPH) radicals. DPPH radicals are widely used for the preliminary screening of compounds capable of scavenging activated oxygen species [37]. Ascorbic acid was used as a standard.
In this study, the extract of O. majorana exhibited IC50 values for the radical scavenging activity was found to be 2.308 mg/mL, this activity could be associated with phenolic compounds and how they played an important role in stabilizing lipid peroxidation. Previous work revealed that O. majorana essential oils and ethanolic extract exhibited high antioxidant activity, also water, methanol, and chloroform extracts [38].
The cytotoxicity and cell viability of the hydro-ethanolic extract of O. majorana species to human PBMC from healthy donors was carried out using an in vitro (MTT test), which is known for specificity, sensitivity, and reliability [39]. Our data revealed that doses (150, 300, 600 µg / mL) of O. majorana extract exhibited a stimulating effect on the cell viability of human PBMCs, resulting in a significant increase in cell proliferation from the concentration 300 µg/mL. This increase in cell viability shows that the extract has no toxicity effect on human cells, on the contrary, it potentially modulates the cellular immune response. RPMI did not affect PBMCs; therefore, the effect observed will be that of the extract used. Our results are consistent with an Indian study evaluating the effect of ethanolic, methanolic, and aqueous extract of O. majorana on normal lymphocytes at a concentration range of 40 to 120 μg / mL. The ethanolic and aqueous extracts were found to be non-cytotoxic on peripheral blood lymphocytes, while the methanolic extract was slightly toxic [14].
Besides, the cytotoxic and antiproliferative effect of extracts of O. majorana on human cancer cell lines of the breast and colon was elucidated by a Moroccan study where concentrations of extracts ranging from 15.6 to 500 µg / mL induced DNA damage, growth arrest, and apoptosis, resulting in a considerable decrease in cell viability [40].
The anticancer potential of O. majorana has also been shown by other studies that have shown inhibition of dose-dependent proliferation of human cancer cell lines such as fibrosarcoma, leukemia, lung cancer cells, larynx, and carcinomas. hormone-dependent prostate [10,14,41]. An Indian study has suggested that O. majorana has particularly anti-tumor biological properties on cancer cells seem attributed to the chemical composition of the plant which is rich in polyphenols, flavonoids, and tannins. P-coumaric acid has proven its antiproliferative effect against colon cancer cells by causing mitochondrial disturbances and apoptosis in these cells [42-46].
Conclusion
In conclusion, in vitro efficacy of the hydro-ethanolic extract of Origanum majorana in the proliferation of the cells. Theis herbs contain high phenolic and Flavonoid compounds that showed antioxidant and free radical scavenging activities. More studies will be required to purify and isolate the bioactive fractions of this extract also the effect on the immune system.
The authors are grateful to Dounia Chahid1,Imane Nait Irahal for their contribution and ni and acknowledge Faculty of Medecine and Pharmacy, Hassan II university-Casablanca for facilitating the accomplishment of the current study.
Funding
This work was supported by The Moroccan Ministry of Higher Education and Research (grant PPR1, type B for A.B.).
- Qasim M, Gulzar S, Shinwari ZK, Aziz I, Kahn AM (2010) Traditional ethnobotanical uses of halophytes from hub, Balochistan. Pak J Bot 42: 1543-1551. Link: https://bit.ly/35ZgQs6
- Vasudeva P, Neeru (2015) Origanum majoranaL. Phyto-pharmacological Review. IJNPR 6: 261-267. Link: https://bit.ly/367pyEV
- Ietswaart JH (1980) A Taxonomic Revision of the Genus Origanum (Labiatae). (Labiatae) Leiden Botanical Series. Link: https://bit.ly/3nYSZ1X
- Tahraoui A, El-Hilaly J, Israili ZH, Lyoussi B(2007) Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in southeastern Morocco (Errachidia province). J Ethnopharmacol 110: 105-117. Link: https://bit.ly/3mghGpS
- Benali T, Khabbach A, Ennabili A, Hammani K (2019) Ethnopharmacological Prospecting of Medicinal Plants from the Province of Guercif (NE of Morocco). 14: 1-14. Link: https://bit.ly/3nRlRJi
- Ennacerie FZ, RhaziFilali FF, Abdelilah R (2017) Ethnobotanical study of medicinal plants used in traditional medicine in the province of Sidi Kacem, Morocco. Asian J Pharm Clin Res 10: 121-130. Link: https://bit.ly/33hyDJn
- El Hafian M, Benlandini N, Elyacoubi H, Zidane L, Rochdi A (2014) Etude floristique et ethnobotanique des plantes m´edicinalesutilis´ees au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc). J Appl Biosci 81: 7198-7213. Link: https://bit.ly/3m5i1f4
- Bouayyadi L, El Hafian M, Zidane L (2015) Etude floristique et ethnobotanique de la flore médicinale dans la région du Gharb, Maroc. J Appl Biosci 93: 8770-8788. Link: https://bit.ly/2IZYKh4
- Abouri M, El Mousadik A, Msanda F, Boubaker H, Saadi B, et al. (2012) An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int J Med Plants Res 1: 99-123. Link: https://bit.ly/2J7JvlZ
- Hachi M, Hachi T, Belahbib N, Dahmani J, Zidane L (2015) Contribution to the study and floristic ethnobotany flora medicinal use at the city of khenifra (Morocco). Int J Innovat Appl Stud 11: 754-770. Link: https://bit.ly/3q1Wu9o
- Hajlaoui H, Mighri H, Aouni M, Gharsallah N, Kadri A (2016) Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb Pathog 95: 86‑94. Link: https://bit.ly/3nUXz1e
- Amor G, Caputo L, La Storia A, De Feo V, Mauriello G, et al. (2019) Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules 24: 4021. Link: https://bit.ly/2HCvBYy
- Kozáowska M, Laudy AE, StaroĞciak BJ, Napiórkowski A (2010) Antimicrobial and antiprotozoal effect of sweet marjoram (Origanum majorana L.). Acta scientiarum Polonorum. Hortorum cultus = Ogrodnictwo 9: 133-141. Link: https://bit.ly/360scw0
- Refaie AA, Ramadan A, Mossa AT (2014) Oxidative damage and nephrotoxicity induced by prallethrin in rat and the protective effect of Origanum majorana essential oil. Asian Pac J Trop Med 7: S506‑S513. Link: https://bit.ly/3l4TANu
- Rao S, Timsina B, Nadumane VK (2014) Evaluation of the anticancer potentials of Origanum marjorana on fibrosarcoma (HT-1080) cell line. Asian Pac J Trop Dis 4: S389‑S394. Link: https://bit.ly/33hzOsh
- Abdel-Massih RM, Fares R, Bazzi S, El-Chami N, Baydoun E (1998) The apoptotic and anti-proliferative activity of Origanum majorana extracts on human leukemic cell line. Leuk Res 34: 1052‑1056. Link: https://bit.ly/3l7Ed74
- Jelali N, Dhifi W, Chahed T, Marzouk B (2011) Salinity effects on growth, essential oil yield and composition and phenolic compounds content of marjoram (Origanum majoranaL.) leaves. J Food Biochem 35: 1443–1450. Link: https://bit.ly/3m4u9Nw
- Guerra-Boone L, Alvarez-Román R, Alvarez-Román R, Salazar-Aranda R, Torres-Cirio A, et al. (2015) Antimicrobial and antioxidant activities and chemical characterization of essential oils of Thymus vulgaris, Rosmarinus officinalis, and Origanum majorana from northeastern Mexico. Pak J Pharm Sci 28: 363-369. Link: https://bit.ly/3m2B8Xa
- Singh R, Sripada L, Singh R (2014) Side effects of antibiotics during bacterial infection : mitochondria, the main target in host cell. Mitochondrion 16: 50‐54. Link: https://bit.ly/378OpqP
- Akram M, Riaz M, Munir N, Rasul A, Daniyal M, et al. (2020) Progress and prospects in the management of bacterial infections and developments in Phytotherapeutic modalities. Clinical and Experimental Pharmacology and Physiology 47: 1107-1119. Link: https://bit.ly/3pXOkiz
- Cunniff P (1997) official methods of analysis of AOAC International : Food Composition, Additives, Natural Contaminations »AOAC International. Rockville MA, USA.
- Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64: 555-559. Link: https://bit.ly/360ovXc
- Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50: 3010-3014. Link: https://bit.ly/375rGw2
- Wu Z, Tan B, Liu Y, Dunn J, Martorell Guerola P, et al. (2019) Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules 24: 2825-2841. Link: https://bit.ly/2KAjCf3
- Verma A, Prasad KN, Singh AK, Nyati KK, Gupta RK, et al. (2010) Evaluation of the MTT lymphocyte proliferation assay for the diagnosis of neurocysticercosis. J Microbiol Methods 81: 175-178. Link: https://bit.ly/2JaoEhU
- Hahn NI (1998) Is Phytoestrogens Nature’s Cure for What Ails Us?. A Look at the Research. Journal of the American Dietetic Association 98: 974- 976.
- Mechergui K, Jaouadi W, Coelho JP, Khouja ML (2016) Effect of harvest year on production, chemical composition and antioxidant activities of essential oil of oregano (Origanum vulgare subspglandulosum (Desf.) Ietswaart) growing in North Africa. Ind Crops Prod 90: 32‑37. Link: https://bit.ly/2KEUMuE
- Shahidi F, Hossain A (2018) Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J Food Bioact 3: 8‑75. Link: https://bit.ly/362Yy9u
- Shama A, Ahmed TG (2014) Phytochemical Screening and Biological effect of Indigenous Medicinal plant Origanum majorana extracts. Link: https://bit.ly/3mglzes
- Bhardwaj K, Dubey W (2019) Quantitative analysis of primary and secondary metabolites of ethanol seed extract of Origanum majorana (Marjoram). 5. Link: https://bit.ly/39aiXeJ
- Vasudeva N (2015) Origanummajorana L. -Phyto-pharmacological review. 7.
- Tripathy B, Satyanarayana S, Abedulla Khan K, Raja K (2017) An Updated Review on Traditional Uses, Taxonomy, Phytochemistry, Pharmacology and Toxicology of Origanum majorana. Int J Pharma Res Health Sci 5: 1717-1723. Link: https://bit.ly/2KttPKb
- Bina F, Rahimi R (2017) Sweet Marjoram: A Review of Ethnopharmacology, Phytochemistry, and Biological Activities. J Evid Based Complement Altern Med 22: 175‑185. Link: https://bit.ly/2JahrhT
- Siddhuraju P, Becker K (2003) Antioidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of Drumstick tree (Moringa oleiferea Lam.) leaves. J Agric Food Chem 51: 2144-2155. Link: https://bit.ly/3fxIIXn
- Oreopoulou A, Goussias G, Tsimogiannis D, Oreopoulou V (2020) Hydro-alcoholic extraction kinetics of phenolics from oregano: Optimization of the extraction parameters. Food and Bioproducts Processing 123: 378-389. Link: https://bit.ly/3q0NrWk
- Lahreche T, Bradaie ML, Mossadak TH (2013) In Vitro Assessment of Antioxidant Activity, Total Phenolic and Flavonoid Contents of Sweet Marjoram (Origanum majorana L.) Extract. Industrial Crops and Products 43: 827-831.
- Tominaga H, Kobayashi Y, Goto T, Kasemura K, Nomura M (2005) DPPH radical-scavenging effect of several phenylpropanoid compounds and their glycoside derivatives. Yakugaku Zasshi 125: 371-375. Link: https://bit.ly/378QmUb
- Semiz G, Semiz A, Mercan-Dogan N () Essential oil compo-sition, total phenolic content, antioxidant and antibiofilm activities of four Origanum species from south eastern Turkey. Int J Food Prop 21: 194-204. Link:
- Orlando JB, Silva BO, Pires-Cunha CL, Hiruma-Lima CA, de IOM, et al. (2019) Genotoxic effects induced by beta-myrcene following metabolism by liver HepG2/C3A human cells. J Toxicol Environ Health A 82: 176‑185. Link: https://bit.ly/39h4tcS
- Makrane H, El Messaoudi M, Melhaoui A, El Mzibri M, Benbacer L, et al. (2018) Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv Pharmacol Sci 1-9. Link: https://bit.ly/3fDxalt
- Hussain AI, Anwar F, Rasheed S, Nigam PS, Janneh O, et al. (2011) Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Rev Bras Farmacogn 21: 943‑952. Link: https://bit.ly/2Jaqhfw
- Jaganathan SK (2013) Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J Gastroenterol 19: 7726-7734. Link: https://bit.ly/39fpGUO
- Chaves RSB, Martins RL, Rodrigues ABL, de Menezes Rabelo E, Farias ALF, et al. (2019) Larvicidal evaluation of the Origanum majorana L. Essential Oil against the larvae of the Aedes aegypti mosquito. Bio Rxiv. Link: https://bit.ly/2UZx8ee
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63. Link: https://bit.ly/3nUnXZ4
- Sun B, Ricardo-Da-Silva JM, Spranger MI (1998) Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem 46: 4267-4242. Link: https://bit.ly/3kZ3L6d
- Nyati KK, Prasad KN, Rizwan A, Verma A, Paliwal VK, et al. (2010) Lymphocyte transformation test detects a response to Campylobacter jejuni antigens in patients with Guillain–Barré syndrome. Med Microbiol Immunol 199: 109-116. Link: https://bit.ly/3kZ3RuB
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
