Open J Biol Sci
Environmental toxicants and Infant Mortality in the USA
David Kennedy1, Stephanie Seneff2, Robert M Davidson3*, John W Oller Jr,4, Boyd E Haley5 and Roger D Masters6
2Computer Science and Artificial Intelligence Laboratory, MIT, Cambridge, MA 01890, USA
3Independent physician/medical scientist, P.O. Box 1785, Kilgore, TX 75663-1785, USA
4Hawthorne/LEQSF Endowed Professor of Communicative Disorders, University of Louisiana, Lafayette, LA 70504-3170, USA
5Department of Chemistry, University of Kentucky, Lexington, KY 40506-0174, USA
6Department of Government, Dartmouth College, Hanover, NH 03755, USA
Cite this as
Kennedy D, Seneff S, Davidson RM, Oller Jr JW, Haley BE, et al. (2016) Environmental toxicants and Infant Mortality in the USA. Open J Biol Sci 1(1): 036-061. DOI: 10.17352/ojbs.000005Despite enjoying a high standard of living, the United States ranks 46th among nations reporting infant survival rates to the World Health Organization. Among factors that increase infant mortality are environmental toxicants. Toxic metals such as mercury, aluminum, and lead interact synergistically with fluoride compounds to produce metal fluoride complexes (e.g., AlF3 and AlF4−). Such toxicants act as biophosphate mimetics disrupting biological signaling processes governing development, immune defenses, and ordinary maintenance systems. Sources for the metals include mother’s mercury amalgams, mercury and aluminum in injected medicines, and lead contaminated drinking water. All of them are made even more toxic by fluorides as evidenced recently by water contamination in Flint, Michigan. Fluorides interact with other toxins increasing their harmful impact. Among the interactants are glyphosate and phosphate containing fertilizers that end up in the food and water because of their widespread use in agriculture. The negative synergy for neonates in the U.S. is increased by the hepatitis B injection containing both mercury and aluminum, and infant formula contaminated with aluminum and the glyphosate in genetically modified soy milk reconstituted with water containing fluoride, aluminum, lead, and other toxic substances. The harmful interactions of such chemicals are associated with rising infant mortality in the U.S. We propose, therefore, a modest but urgent policy change: under TSCA §5, silicofluoride addition to public water supplies should be suspended.
Introduction
Why does the United States have a higher infant mortality rate (IMR) than many other nations, including both developed and developing nations, many of which spend a great deal less on health care per person than the U.S. does?[1]. Given the general availability of health care in the U.S., why should the U.S. rank lower with respect to the IMR indicator of general health than even some nations in the less developed category? [2] In the selective review of theory and research provided here, an interdisciplinary team of authors show that manufactured toxicants are implicated.
We examine various toxicants and certain experimentally demonstrated interactions but, for reasons that will become clear to our readers, we give special attention to silicofluorides, and to interactions involving aluminum, mercury, lead, and organophosphorus-based pesticides. The general U.S. population is increasingly exposed to all of these. For instance, toxicants containing aluminum and mercury are introduced to women of child-bearing age and neonates through medical procedures such as dental amalgam fillings and childhood vaccines [3-5]. Drinking water is deliberately loaded with silicofluorides by governmental policy [6]. Food and water supplies are contaminated with organophosphonates (and their adjuvants [7–9]) through genetically modified crops engineered to withstand the exposure [10]. The organophosphorus-based pesticides, then, along with government supplied silicofluorides and other toxicants that interact with them, end up in the supplies of food and water and are inevitably consumed by the population at large [4,11,12]. For the interactions of these toxicants there are no governmental guidelines concerning “safe” amounts of daily exposure through breathing or swallowing or absorption through the skin.
At the top of the known neurotoxins, mercury in various forms is perhaps the most studied of the toxins linked to disorders of the brain and body, especially during prenatal and early post-natal development [3,13–17]. However, with no danger of being contradicted we can assert categorically that governmental guidelines from different agencies concerning “safe” exposures are dramatically inconsistent with each other as well as with studies showing that, once inside the body very tiny doses, amounts measured in parts per billion, many times smaller than those recommended as safe in any published government estimates, have been shown to be harmful especially during early development [3,5,18–20]. In general, because of the virtual impossibility of sorting out the myriad interactions that need to be taken into account, it is our considered judgment, for reasons to be explained in this review, that meaningful estimates of “safe” dosages of the plethora of potentially interacting toxicants are apt to be misleading. This is not to say that methodologies will not be developed in the perhaps not distant future permitting highly accurate chemometric modeling, but with respect to the extremely complex and often over-riding interactive synergies [21,22], any estimates of “safe” dosages are likely to be very rough approximations.
Nevertheless, a necessary outcome of the increasing toxic burden on the U.S. population in particular, as we demonstrate in the theory reviewed here, is an inevitable and not necessarily commensurate disruption of the delicate biosignaling systems essential to human health and well-being. In this review, we focus on informative experimental and epidemiological research concerning known interactions and harmful effects especially of silicofluorides in combination with aluminum, mercury, lead, manganese, and organophosphorus-based pesticides. For reasons demonstrated and explained in this review, the most vulnerable individuals in any given segment of the population at large are babies during their prenatal and post-natal development [17,23,24]. Simply put, the greatest number of cell divisions and correct DNA replications must occur during this period beginning from just a single pair of cells. For that reason, the earlier toxic disruptions of biosignaling processes occur, all else being equal, the more likely they will cause an injurious cascade including fatalities. Such effects are predicted by strict logicomathemaical reasoning [23,25–27].
Therefore, as has long been known, fluctuations over time in the measured rate of infant mortality, the nation’s IMR, is not only one of the best epidemiological indices of national health and well-being, but must logically also be sensitive to the known generally increasing levels of toxicant exposure. The IMR as reported to the World Health Organization is measured by deaths occurring between birth and the child’s first birthday, but, of course, exposure to toxicants does not end at the child’s first year. It continues to have consequences throughout the lifetime of any given cohort that might be studied. Looking to adult populations, research reviewed here shows that a population measure of violent crime in densely populated cities of the U.S. is surprisingly sensitive to the level of intoxication through drinking water with silicofluorides and other interacting manufactured contaminants [28–31].
Interactions between toxicants in fact are the norm and are well-demonstrated in controlled experimental studies. For instance, exceedingly small doses of toxicants containing aluminum and mercury, ones that might have negligible effects if administered separately, become injurious or lethal if combined. The biophysical impact of such two-way interactions is well-established in the research literature across all species that have been studied including humans, but clinical studies of three-way, four-way, and higher levels of biochemical interactions are virtually non-existent in experimental clinical trials of medicines or consumable products. The fundamental contribution of this review, therefore, is to show how and why the cumulative toxicant burden, including interactions, over time is certain to be injurious and eventually fatal in proportion to the increase in their cumulative effects. In early development, sad to say, measured IMR must be sensitive to such a toxic burden imposed on any given cohort of infants prior to their first year. This outcome we demonstrate at the top of the review with data from the U.S. compared to other nations reliably reporting infant mortality data to the WHO.
The fundamental mystery addressed is this: Why has the U.S. fallen, over several decades, from a position near the top of all the nations reliably reporting infant mortalities to the WHO, to near the bottom? Also, why and how could increased exposure to manufactured toxicants not only stagnate or increase the nations measured IMR in spite of the development of better medical technologies and facilities over the same time period? Additionally, how is the greater toxic burden of certain urban populations, contrasted with less contaminated settings, contributing to increased levels of violent crimes in the more toxic contexts?
The U.S. spends more but gets less as IMR Increases
Before Obamacare the U.S. spent 2.4 times more per capita for health care than the average for the 30 member-states in the Organization for Economic Co-operation and Development (OECD) [1]. With respect to infant survival in the first year of life, the U.S. ranked 46th out of 224 reporting nations in 2008 and by 2015 had fallen to 59th in the same reporting system of the CIA [2,32]. The U.S. also has the highest IMR in the world on the first day after birth [33]. Why should the U.S. IMR over the last decade, between 63 and 69 per 10,000, be so much higher than in Sweden, between 24 and 28, for the same time frame? In 2010, an estimated 4,130,665 babies were born in the U.S., where the infant death rate was 18 per 10,000 live births higher than the OECD average [34–37]. The graph in Figure 1 suggests that America’s high cost health care is also yielding the highest IMR of all the industrialized nations. Why is this so?
According to recent reports, the U.S. spends more, has more doctors, and yet has a worse record for IMR than less developed countries such as Guam and Cuba [2,32]. The rising IMR in the U.S. must be attributed in part to the increasing exposure of U.S.-born infants to toxicants, including aluminum, mercury, silicofluoride, glyphosate, phosphate fertilizers, and lead. The facts show that present policies of the CDC and U.S. Public Health Service (PHS) ensure the continuation and acceleration of such exposures. The recent public outrage about lead, fluorides, and other toxicants in Flint, Michigan has served to emphasize the importance of the negative synergies to be examined in this paper [38–41]. It is noteworthy that by ordinance (legislative policy), the Flint water supply is contaminated with silicofluorides, the most harmful variety of fluoride treatment, as will be seen below in this paper [42]. Interestingly, the toxic effects of fluoride compounds in consumable products have long been side-stepped in official letters from the Food and Drug Administration explaining policy and regulations to consumers and consumer representatives.
No U.S. agency ever tested silicofluorides for public safety
The main relevant legislation at the federal level was enacted in 1976 in the Toxic Substance Control Act (Public Law 94-469), which excluded chemicals already in use, including the fluoride compounds, which were thus “grandfathered in” without testing for safety. So far, efforts to reform that legislation have made little progress, and the flouride compounds to be examined carefully in this paper remain exempted because they were in use from 1942. To get a sense of how the bureaucracies were created to monitor the use of “toxic substances,” the three exchanges labeled A, B, and C in the paragraphs following this one are useful. Each exchange involved an official in one of the branches of the U.S. Public Health Service. In general, officials in a particular branch characterize the toxic impact of fluoride compounds as “mild” while directing consumers and their advocates to some other government agency (e.g., the Environmental Protection Agency, or to a different branch of the U.S. Public Health Service). Meanwhile, officials do not actually deny that fluoride compounds are toxic, some more than others, but neither do they take responsibility to investigate the nature and levels of toxicity.
Here are three examples of letters to U.S. agencies showing prior knowledge that fluoride compounds are harmful. The first example comes from Edna M. Lovering answering a complaint by a private citizen. Lovering, speaking for the Food and Drug Administration on August 15, 1963 admitted that if “sodium fluoride” was being “used for therapeutic effect” [to prevent tooth decay it] would be a drug” and thus subject to FDA regulation. But, she said it was not known to be “essential to human nutrition”; no “minimum daily requirement” had ever been established; and, thus, FDA had no way of identifying “a safe amount”. The complainant was then directed to possibly contact “the Division of Dental Public Health Services and Resources [43].
Another complaint to the FDA, dated December 21, 2000, was answered by Melinda K. Plaisier, Associate Commissioner for Legislation, responding to Congressman Ken Calvert, then Chairman of the Subcommittee on Energy and Environment of the Committee on Science in the U.S. House of Representatives. Calvert had asked the Director of the FDA about the toxicity of fluoride compounds. Plaisier denied FDA responsibility. She sent him to “the Environmental Protection Agency” which she said “regulates fluoride in the water supply”. She admitted that “dental fluorosis”, a known deterioration, can be caused by “ingestion of fluoride” but attributed the problem to two-year olds having “swallowed a fluoride toothpaste”[44].
Finally, our third example comes from an exchange in 2015 between Roger D. Masters (personal communication) and Dr. Maria J. Doa, then, Director of the Chemical Control Division at the U.S. Environmental Protection Agency. When asked if the EPA would test the silicofluoride compounds H2SiF6 and Na2SiF
Although there have been several recent efforts to amend and improve the 1976 law, with respect to the fluoride compounds that are in focus in this paper, the Toxic Substance Control Act of 1976 still exempts them from testing just as it did four decades ago. As we will show by reviewing relevant research in this paper, by putting fluoride compounds into drinking water, U.S. public health agencies, while claiming to improve the health of tooth enamel, have long been exposing the U.S. population to toxic chemicals that can only increase infant mortality, while also worsening a host of life threatening neurological and genetic disorders and diseases. In this article, we consider both the nature of the problem and feasible steps toward solving it.
toxicants, including silicofluorides, are known to interact
According to the National Cancer Institute, a toxicant is “a poison that is made by humans or that is put into the environment by human activities” [45]. In real life contexts, low-level toxic injuries are rarely caused by just one toxicant in isolation, but rather by some combination of toxicants working synergistically. The underlying principle was demonstrated nearly four decades ago by Schubert et al. [46]. Rats injected with a dose of a mercury salt that would leave 99% of them still surviving for at least 10 days (a dose judged to be LD1, meaning lethal to only 1% of the animals), but which simultaneously also received an LD1 dose of lead, or of cadmium, all died. Of the three metals studied, mercury was the most toxic, then lead, followed by cadmium. Combining the mercury salt with only 1/24 of the LD1 of the lead salt killed 50% of the animals [46]. Synergistic effects, therefore, were shown to greatly magnify the injuries produced by the toxins studied. That aspect of their research has been shown to generalize to humanly manufactured chemicals which we refer to throughout as toxicants. Bringing the Schubert findings up-to-date, advances in molecular biology have confirmed their findings, especially concerning synergies, and have shown their applicability to human populations [47–51].
With respect to the silicofluoride compounds which are the chemicals of greatest interest in this paper, H2SiF6 and Na2SiF
Silicofluorides plus lead and manganese can lead to violence
Based on a study of data from 3,141 U.S. counties reported to the Environmental Protection Agency in 1991, Masters, Hone and Doshi (1998) had found that children’s blood lead levels are consistently increased when the exposure to environmental sources of lead occurs in a community adding either hydrofluorosilicic acid (H2SiF6) or sodium silicofluoride (Na2SiF
Based on examples of violent individuals with high lead or manganese provided by Everett “Red” Hodges, President of the Violence Research Foundation, Roger Masters initiated studies linking the EPA’s reports of lead pollution with the murder rates reported in FBI statistics for affected cities. In 1991 the murder rate in cities with lead pollution stood at 8.7 per 100,000 population and was 3.48 times greater than in those without the lead pollution. The murder rate with manganese pollution (5.0 per 100,000 population) was double the murder rate in cities not exposed to either lead or manganese (2.5 per 100,000 population). However, as Masters and Coplan discovered in their 2007 unpublished study titled “The ten most violent cities in the U.S.” (available on Research Gate in its entirety as a PDF, [30]), by using FBI data on violence and public records of water fluoridation as well as data on metal pollutants, the synergy of combining both lead and manganese pollution in the same cities was correlated with a reported murder rate at 50.1 per 100,000 population — 20 times higher than in cities not exposed to either of those neurotoxicants. Further, the 1991 data and follow up studies also revealed a significant interaction of lead and/or manganese with either hydrofluorosilicic acid (H2SiF6) or sodium silicofluoride (Na2SiF
Recently, such toxic effects have been spotlighted in news stories about lead poisoning in Flint, Michigan that is undoubtedly made worse, as we will see in this paper, by Flint’s water fluoridation policy [41,42]. Using the data from 1991, as shown in Table 1, Masters and Coplan [30], had discussed the fact that Flint, Michigan was not only ranked third among the 10 most violent cities in the U. S. by the FBI at the time they collected their data on metals, but it was also already among those using hydrofluorosilicic acid (H2SiF6) in its public water supply. More recent FBI data shows Flint, Michigan as the number one most violent city in America for 2011-2012 [31,63–65].
As discovered in this review, longitudinal data across multiple generations has also shown increased exposure and uptake of environmental toxicants in low-income groups. Therefore, the interactive effects of aluminum, mercury and lead in combination with silicofluorides can be expected to be more severe for groups of historically lower socio-economic status. It is, therefore, unsurprising that significantly higher blood lead levels have been found among Black or Hispanic children than in White children from the same communities. Controlled experiments involving rats exposed to lead, or to lead plus silicofluoride, demonstrated a two- to three-fold higher lead concentration in calcified tissues for the rats that were exposed to both (p<0.001) [46]. Unfortunately, research to be reviewed in this paper shows that low income groups are more at risk for every known combination of environmental toxicants. Also, simultaneous exposures are known to produce greater harm than a linear sum of effects for the same toxicants in isolation [21,46,66-68].
Materials and Methods
As noted above in our Introduction, to assess factors that can have harmful effects on living organisms, attention in this selective review is directed to the disruption of biological signaling processes resulting in disorder, inflammation and disease, and in extreme instances death [4,25,26,69-76]. The biosignaling (or biosemiotic) perspective offers a comprehensive understanding of how toxicant insults can change normal phenotypic traits and behaviors from the molecular level [71,74,77], all the way to the highest levels of cognitive functions of the human brain [23,24,27,78–81].
General cascading effects
A toxic impact resulting in a genetic mutation (s) can modify the regulation of cellular functions throughout the affected organism. Toxic insults are often pleiotropic [23,82]. They are known commonly to impact multiple factors including structural development, hormonal expression, neurotransmitter activity, and essentially all regulative functions connected with our feeling of well-being (or malaise), behavioral and emotional normalcy (versus stress and anxiety), and overall physical health (as contrasted with disorder and disease). In the broad context of gene-environment interaction, in this paper we focus on the molecular level of biosignaling and the communications that coordinate gene expression, cellular integrity, neurological development, organism- and population-level behavior, and life-sustaining activities [66,71,74,83].
There is good evidence that disease and early death are often linked to breakdowns in the integrity of cell membranes and the structures that enable communications between and within cells [4,69,73,84]. Many types of breakdowns are known which can give rise to macromolecular changes often reported in the literature. We propose that fluorides generally and more specifically aluminofluorides, silicofluorides, and interacting phosphates as found in agricultural applications ending up in our food and water as well as other toxic elements (such as lead, mercury, or manganese) can trigger multilevel disruptions of biological signaling processes and thus lead to pathological changes due to mechanisms as diverse as abnormal gene expression, unsuitable nutrition, and neurotoxicity. Such disruptions can only increase biosignaling entropy (leading toward disorder, disease, and death) as discussed by Oller and others [25,26,70,71,73]. The research shows that known synergies produced by toxic combinations of seemingly small exposures can have surprisingly harmful, sometimes even catastrophic effects, especially on the delicate processes of gamete loading in meiosis, and the interpretation and subsequent multiplications that must take place in prenatal and very early post-natal development [80–90]. The greater vulnerability of prenates, neonates, and infants undergoing rapid development is largely because of the delicacy of the billions of instances of mitosis that must be carried out almost flawlessly in order for the developing organism to survive, not to mention achieve a reasonable level of well-being.
It must also be noted, of course, that the threats to human health posed by the environmental toxicants we examine in this paper are merely added to the sources of traumatic injuries and diseases on which the medical profession has more commonly focused its collective attention. We must also bear in mind the fact that the harmful effects of trauma (at a macro-level, e.g., as in a concussive blow to the head) [91–96], a meso-level (as in burns that may be small or large), or at a micro-level (as in exposure to electromagnetic fields, or nuclear radiation, or in cumulative small injuries), as well as damage owed to invasive pathogens, generally must trend toward increasing the damage from toxins of the types considered here. We are especially concerned with man-made toxins for which we use the term toxicants, and in the latter class, we single out certain toxicants with which policy-makers have the greatest influence: in this paper we are particularly focusing attention on man-made chemical derivatives of fluorine.
Pregnancy presents unique immune neurodevelopmental vulnerability to infectious environmental pathologies and to toxic injuries from even before fertilization and afterward, e.g. congenital Rubella syndrome, CMV retinitis, Zika-associated microcephaly, etc., added to any disorders that may arise during the delicate processes of meiosis and mitosis (as discussed later in this paper). During pregnancy, the immature blood-brain-barrier of the developing fetus, previously weakened by environmental neurotoxicants, may become sensitized and susceptible to penetration by neurotropic viruses contracted by the mother prior to or during pregnancy. Of particular prospective interest, in light of the recent microcephaly “outbreak” in Brazil, is to determine whether such toxicants co-localize with infectious pathogens. This can be determined in principle by histopathological (postmortem) study of fetal brain tissue [97,98].
As will become clear in our Results and Discussion section, we speculate that Flint, MI (USA) residents with elevated blood lead levels and Brazilian microcephalic fetuses may share a common pathophysiology related to exposure to silicofluorides in public drinking water, which is synergistically-neurotoxic in the presence of oral, inhaled, or parenteral aluminum adjuvants, lead, mercury amalgam fillings, phosphate fertilizers, and glyphosate containing herbicides, which are widespread contaminants of the food and water supply in both countries. Zika virus may represent an associated “opportunistic” viral overgrowth, whereas the underlying causality might conceivably lie with the silicofluorides. Of particular interest would be to learn whether the calcifications noted at necropsy of Zika-associated microcephalic fetal brain and placental tissue contain lead. The paucity of inflammatory cells in these fetal brain and placental calcifications is suggestive [98], and may show that the underlying pathophysiological disturbance in the current microcephaly epidemic is non-infectious. The manifested microcephaly may be owed in part or in whole to toxic injuries that occurred well prior to their manifestation.
Aluminum and silicofluoride toxicity
Because of its chemical property of electronegativity, derivatives of fluorine are of special interest when introduced into biological systems. Because fluorine is the most electronegative element in the entire periodic table, and given the relevance of “ions in water” to “a wide range of systems, including biological environments” as pointed out by Tielrooij, et al. [99,100], fluoride containing compounds are especially biologically active and, as we will see from the research, almost always harmful to living organisms. Fluoride anion (F-) is the smallest halide ion and it is categorized as a hard anion according to Pearson’s theory of Hard and Soft Acids and Bases (HSAB) [101]. Under the HSAB principle, a metal in a chemical reaction involves an electron pair acceptor (Lewis acid) interacting with an electron pair donor (Lewis base) to form various chemical groups, such as an ion pair, a metal complex, a coordination compound, or a donor-acceptor complex [101]. Any positively charged ion is able to accept electrons, thus defining it as a Lewis acid. According to Duffus (2002), “The classification of metals by their Lewis acidity indicates the form of bonding in their complexes. Class A metal ions, which are hard or nonpolarizable, preferentially form complexes with similar nonpolarizable ligands, particularly oxygen donors, and the bonding in these complexes is mainly ionic” [102]. This fact has implications for the formation of aluminofluoride complexes, both in vitro and in vivo [103,104].
Electronegativity is a measure of the power of an atom to draw bonding electrons to itself. Positive ions can have the effect of polarizing (electrically distorting) nearby negative ions. The polarizing power, which depends on the charge density in the positive ion, increases as the positive ion gets smaller and the number of charges gets larger. Darwent (1970) showed that fluorine forms silicon bonds (540 kJ-mol-1) as well as aluminum bonds (663.6 kJ-mol-1) [105] that are comparable only to scandium bonds in strength (589 kJ-mol-l) [103,106]. Thus, any time aluminum and fluorine are both present there is a high likelihood that they will unite. Aqueous Al3+, a hard metal cation, interacts most strongly with hard donors, such as F-. Under Pearson’s HSAB principle, hard Lewis acids prefer to bind to hard Lewis bases [101]. Klopman quantified Pearson’s HSAB principle using frontier molecular orbital theory [107], providing a theoretical basis for the empirical observation that F- donor ligands produce very stable complexes with Al3+.
Although any fluoride compound might enhance the bioavailability of aluminum, water treatment with either of two silicofluorides hydrofluorosilicic acid (H2SiF6) or sodium silicofluoride (Na2SiF
H2SiF6 + Al2O33H2O → 2 AlF3 + SiO2 + 4 H2O (1)
According to their study, the heat evolved brings the temperature of the mixture to 90-100°C, the reaction is complete within 20 minutes, and the precipitated silica apparently easily filterable.
We suggest that a similar exothermic in vivo reaction may account for reports of in vivo toxicity of nano-sized alumina [111] and silica [112]. In a recent study in mice, aluminum oxide (alumina) nanoparticles induced apoptosis via up regulation of the caspase-3 gene and also was associated with impaired spatial learning behavior, suggesting that in addition to neurotoxicity, nano-alumina may be causing mitochondrial impairment [111]. A recent in vitro study using a dopaminergic PC12 cell line indicated that SiO2-NPs decreased cell viability, triggered oxidative stress, disturbed cell cycle, and induced apoptosis mediated by the p53 signaling pathway [113].
The 2006 19F-NMR study by Finney et al, specifically undermined claims that silicofluorides are harmless, despite the limitations they noted in their study. They identified a peak at an 19F NMR chemical shift of -130.5 ppm (parts per million) attributed to SiF62- and, importantly, a second peak at an 19F NMR chemical shift of -129.5 ppm attributed to the hydrolysis intermediate SiF5(H2O)- [109]. Their study refutes the long-held claim that silicofluorides completely dissociate in water. If that claim were true, it would theoretically render SiF harmless, but the relevant research shows this hope to be false in some if not all instances, and the false dissociation claim misleadingly suggests another almost probably false hope: that SiF residues cannot re-associate themselves in some biological environments to produce such harmful species as silicon tetrafluoride (SiF4).
Prior to the Finney et al., study in 2006, both Urbansky (2002) and Morris (2004) indicated that, at pH<5, silicofluoride (SiF62-) would be found [114,115]. The possibility of biological effects of SiF62-, as opposed to free fluoride, is increased by the facts that many acidic beverages such as soft drinks have a pH<3, and because most fruit drinks have a pH<4. It was hypothesized by Ciavatta et al. (1988) that incompletely dissociated SiF residues might re-associate at intra-gastric pH around 2.0, thereby exposing the consumer to a toxic cascade of as yet unknown but almost certainly harmful consequences [108]. At low pH range, e.g. pH<4, equilibria prevail and dissociation is not complete at equilibrium. We suggest that in relatively “de-wetted”, hydrophobic environments, such as the human CNS, the equilibrium may actually favor association over dissociation of SiF intermediates. During food preparation and consumption, moreover, low pH soft drinks are thought to produce SiF species, including silicon tetrafluoride (SiF4), a known hazardous toxicant (see the U.S. National Library of Medicine, Hazardous Substances DataBase [87]).
If aluminum is present in a medium to which silicofluorides have been added, the resulting chemical events potentially expose cells to toxic aluminum fluoride, thereby compromising delicate structures of the blood-brain barrier [111,112] and increasing deposition of aluminum or silicate residues in the brain. As studies of “Autoimmune-inflammatory syndromes induced by adjuvants” (also called “ASIA”) have documented [116], the widespread use of aluminum “adjuvants” to augment the response of the human immune system during vaccination [4,116–119], combined with public water treated with silicofluorides being delivered to over 120 million Americans, can only make toxicants like silicofluoride and aluminum more injurious than they might be in isolation.
In 1998, Varner et al. showed in Brain Research that giving rats water containing fluoride at 1 ppm led to kidney damage, brain damage, and a greater uptake of aluminum into the brain, and increased the formation of beta amyloid deposits often characteristic of Alzheimer’s disease [120]. The aluminum level in the brains of the fluoride-treated group was double that of the controls. Histopathological changes similar to those traditionally associated with Alzheimer’s disease are also seen in rats chronically exposed to AlF3. Because fluoride can increase the uptake of aluminum into bones [121] and the brain [120], in organisms with healthy self-regulating mechanisms, aluminum can consequently appear to reduce the measurable presence of fluoride in bodily fluids [4,117], whereas it is actually being sequestered by certain vulnerable target tissues.
More recently, researchers have found that the fluoride compounds used in water fluoridation, including NaF and the silicofluorides, H2SiF6 and Na2SiF6, have similar toxicities. In 2014, it seems that Rice, et al., were the first researchers to compare toxic injury attributable to NaF with the toxicity of silicofluorides H2SiF6 or Na2SiF
Impact on human infants
In this section, to provide more comprehensive evidence on environmental toxicant exposure in the U.S., we discuss a number of toxicants known to interact with fluoride compounds and with each other: we discuss mercury, aluminum, lead, and the phosphates in fertilizers and industrial applications, to which infants and children in the U.S. are chronically exposed. We show how U.S. health policy actively promotes increased exposure to these toxicants even though they interact synergistically, necessarily increasing the number and intensity of the adverse events involved in developmental disorders, diseases, and fatalities from even before birth.
Harm to Infants: Dental amalgams are a major source of mercury, the only volatile metal and a well-established toxicant with no biological role [127]. Studies have shown that mercury from maternal amalgams bypasses the placental barrier to be taken up by the fetus, and that mercury from such sources also shows up in breast milk [54,128–133]. There is a significant correlation between number of amalgams in the mother’s teeth and autism severity in children on the spectrum [22,134,135]. Although several countries in Europe, including Norway, Sweden and Denmark, have banned the use of mercury in dental amalgams, mercury is still being used in the U.S. for dental fillings. The American Dental Association reports that mercury fillings have been placed in “the teeth of more than 100 million Americans” and continues to claim on its official website in 2016 that mercury containing dental “amalgam is a valuable, viable and safe choice” [136]. Given that it has long been known that dental amalgam is the main source of body mercury [16,128], it should be unsurprising that one in six babies at birth in the U.S. already have a sufficient body burden of mercury to cause neurological impairment [108,109]. In 1989, Snapp showed that the blood mercury level could be reduced by 50% in just 2 months by removing all mercury/silver amalgam tooth fillings from teeth, even though in this study all participants (n <10) initially experienced a transient increase in blood mercury following removal [137].
In 1990 the CDC mandated injecting newborn babies with hepatitis-B (Hep-B) vaccine which was then, and up to 17 years later continued to be (according to the official FDA website, [138]), preserved with thimerosal (approximately 50% ethylmercury). The (Hep-B) regulatory requirement for newborns remains in place today [139,140] and thimerosal containing flu vaccines are still recommended for use by the CDC, [141], even for infants and pregnant mothers, though research shows that extremely small quantities are harmful [142]. Although the Hep-B manufacturers reported thimerosal free options becoming available in 1999 and 2007, respectively, stockpiles of vaccines containing thimerosal were still in use long after those dates and are currently regarded as safe according to the CDC [143]. It must also be noted that all of the Hep-B vaccines have always contained an aluminum adjuvant, either aluminum hydroxide or aluminum phosphate [144], known to be a synergistic disruptor when combined with other neurotoxicants, especially mercury [21]. A further mercury burden is often placed on the unborn child when a pregnant woman is administered flu vaccine, as now routinely encouraged by CDC policy [139,145].
Water Treatment with Silicofluorides (H2SiF6 or Na2SiF
Although surveys conducted by the American Dental Association report that fluoridation of the water supply actually reduces tooth decay and merits support by dentists [151,152], in Europe and Australia, many consumers maintain reasonable doubts [147,153,154]. Empirical data show that tooth decay rates are similar in fluoridated and unfluoridated communities in the U.S. [155], but such indicators as kidney failure are higher in regions with higher rates of fluoride contamination in the water [156]. Fluoridation of drinking water in the U.S. was initiated in 1942 by the Manhattan Project as a means to dispose of highly corrosive hydrofluorosilicic acid, which was needed to separate weapons grade uranium from phosphate rock in the secret program to develop an American atomic bomb (Coplan, pers. comm.) It was because Hitler had gained access to high grade uranium mines when the Nazis occupied Czechoslovakia and that he had begun an attempt to develop nuclear weapons that the first water fluoridation programs in the U.S. were initiated. The public fluoridation policy was, therefore, adopted not to prevent tooth decay, but initially in the interest of the war effort and, subsequently, to guard national security with respect to the on-going nuclear weapons industries. As a result, corrosive silicofluorides would end up in drinking water in many communities throughout the US and, as an indirect consequence, phosphates would end up in the food supply. The official public explanation, however, has been that the silicofluorides in particular that are still being disposed of in the drinking water are by-products of the organophosphate pesticide industry [157].
Today a large proportion of man-made hydrofluorosilicic acid used in the U.S. is imported from China and actually is derived from the manufacture of phosphate fertilizers in addition to the on-going mining operations that extract nuclear materials from phosphate rock. The effluent wastewater from fertilizer manufacturing alone releases several thousand tons of fluoride each year [158] and careful study of the contaminants in sediments in the Gulf of Gabes, Tunisia, showed that fluoride and phosphorous along with certain toxic metals are all anthropogenic [159]. Hydrofluorosilicic acid is synthesized from hydrogen fluoride and silicon tetrafluoride in order to prevent fluorine gas from escaping into the atmosphere and polluting the air. It consists of a core of sand (silica) populated with six molecules of fluoride (Figure 2). This complex possesses a unique electrolytic attraction for lead and is therefore used as an electrolyte in the Betts electrolytic process for refining lead. In fact, proof of uniqueness of the man-made molecules can be found in patents in the U.S. office applied to the extraction of lead from brass [160,161]. Fluorine is the most reactive of the halogens and is also highly reactive with magnesium, aluminum, and zinc [104,162,163]. All of the known naturally occurring fluoro-organic compounds are toxic [164], and fluorine’s disruptive consequences for cellular physiology will be examined below.
Over a decade, Roger Masters, Myron Coplan, and colleagues analyzed raw data on blood lead levels collected by researchers in Massachusetts [1,29,52,57-60,62,165-168], New York state [58], and ultimately the National Health and Nutritional Evaluation Survey (NHANES) children’s lead study [167]. Because for many years NHANES has asked every physician who sees young children to collect blood samples for measuring blood lead levels, replication using their data from all U.S. counties with over 500,000 population is especially important. Using NHANES data from 280,000 children, the blood lead levels, as well as other known factors for lead exposure, were examined in relation to whether or not the public drinking water was supplemented with hydrofluorosilicic acid (HFSA). In all three samples, the correlations between exposure to silicofluoride and higher uptake of either lead or manganese consistently showed a highly significant interaction between HFSA and lead for each of the major effects (with p ≤ .0001 for many tests). In a total of over 400,000 samples from diverse communities in the U.S., children who were exposed to HFSA through their drinking water had almost twice the blood lead levels as those children who were not exposed. Furthermore, statistical analyses showed that behaviors associated with lead neurotoxicity, including higher drug abuse and increased rates of violent crime, are more frequent in communities using silicofluorides [52,29,60,165,167].
After publication of the first of these studies [52], the CDC argued that the research linking silicofluoride to higher blood lead was flawed and biased, and that the results could probably equally well be explained by increased dermal exposure to lead from lead paint in older homes, because some of the blood samples were from finger pricks. They theorized that poor children’s dirty hands were not adequately cleaned with the cotton swab before the blood sample was taken. The authors then followed up with a second paper based on venous blood drawn in New York state from a carefully selected subset of 151,225 children aged 0 to 6 from 105 communities with populations between 15,000 and 75,000 [58], confirming that HFSA does indeed impact blood lead levels. The larger NHANES sample confirmed earlier work as shown in Figure 3 [59]. In that figure, note that more than 60% of Black children not exposed to silicofluorides (abbreviated “SiF” in the bar graph) had less than five micrograms of lead in a tenth of a liter of blood, only about 43% of those exposed to SiF had lead levels that low. Reading left to right across the graph, note that the level of lead in the blood increases with SiF water treatment.
In addition, the authors of these studies considered ethnic background, old housing, poverty, and other cofactors in lead exposure, and found that Black children exposed to potential sources of lead suffered a six-fold increase in blood lead levels. The increase was four-fold for Hispanic children. As a result, the differences in absorption of lead in shared environments was significantly more harmful for minority children, especially if they were poor. Since these ethnic differences are significant, controlling for up to 10 risk factors, it has been proposed that higher rates of lactose intolerance may be involved in the most seriously affected groups. It should not need emphasis that such increased burdens of lead, well established since Benjamin Franklin commented on lower intelligence among those who swallow lead, would seem to play a substantial role in increasing the educational and economic obstacles facing minority children (especially when they live in polluted environments).
Other researchers have confirmed the costly effects of silicofluorides. A recent paper [1] calculated the cost differential to society arising from using industrial grade HFSA as the principal fluoridating agent versus using the more costly pharmaceutical grade NaF. If only the additional burden of lung and bladder cancer cases are considered, these authors estimated that the U.S. could save $1 to $5 billion dollars a year by choosing sodium fluoride (tested for safety and familiar in toothpaste) instead of the less expensive but toxic silicofluorides. This does not take into account other effects of lead and arsenic besides cancer, of which the higher rates of violent crime associated with lead neurotoxicity and exacerbated by silicofluoride exposure are the most striking.
National data for rates of violent crime in each of the 3,141 counties were subjected to multivariate statistical analysis, which permits an assessment of the relative contributions of a variety of environmental factors on a negative outcome for which reasonably objective scientific data is available. Rates of crime provide a good illustration of the utility of this method, since they readily make it possible to contrast property crime (which typically entails planning and a degree of caution by at least some of the offenders) and violent crime (which is more often an impulsive loss of self-control). National data for all 3,141 counties revealed that violent crime rates were significantly increased in localities where the EPA reported lead pollution, whereas this association was not significant for property crime.
Since imprisoning each violent offender is estimated to cost American taxpayers between $30,000 and $50,000, the added number of violent offenses that seems attributable to silicofluoride use is about 100 crimes for every 100,000 people exposed to these compounds. With silicofluoride delivered to over 120 million Americans, this is another $1 billion cost of allowing addition of these compounds to continue for their supposed dental benefits.
Discussion
Why have such harmful effects been set aside (or just ignored) in governmental decisions to substitute untested silicofluorides for sodium fluoride in water fluoridation? Results have been published for well over a decade without eliciting public attention or political action.
A puzzle: why have EPA & CDC ignored toxicity of lead, manganese, & silicofluorides
When the State of Vermont established a “Get the Lead out of Vermont Task Force,” its members decided not to study locations where lead pollution was occurring, rejecting suggestions to explore why lead is being absorbed in the brain cells of many Vermont citizens. Since a major source of exposure to lead is paint in old houses (plentiful throughout Vermont), one Task Force member tried to propose screening children for high levels of lead and other toxins, using a non-invasive $60 head hair test that documents body-levels of 17 toxins and 23 necessary elements (Masters, personal communication). Unlike a pin-prick blood test, hair analysis is non-invasive, uses only a snippet of hair from the nape of the neck, and provides information about diet and combinations of toxicants and their synergies. Where levels of lead or other toxicants are high, chelation can often reduce the problem. If silicofluoride treated water increases body levels of lead or manganese, the water treatment chemicals can be changed. Besides, poor diet increases lead absorption, and supplements like calcium pills are less costly than Special Education (estimated at a minimum of $10,000 per child per year) not to mention prison costs for violent criminals.
In contrast to the media interest in “silicon breast implants” playing a causal role in breast cancer, paradoxically there has been virtually no coverage of evidence of harm associated with the drinking water of millions of Americans treated with hydrofluorosilicic acid or sodium silicofluoride since 1942. Nor has there been significant research into the biological conversion of silicon implant materials (lining and/or gel; used in FDA approved implants [169]) into the neurologically debilitating silicon tetrafluoride (also known as silane) [170]. Could this silence be entirely justified by the government’s approval without testing by law in 1976 (but secretly much earlier to conceal their use in nuclear weapons development) on the “assumption” that the silicofluorides would “dissociate” into fluoride, silicon, and either hydrogen (from H2SiF6) or sodium (from Na2SiF
To be sure, after silicofluoride use began (for military reasons) without publicity in 1942, the Public Health Service formally approved silicofluorides as safe in 1950. But this decision was based on the “assumption” of complete “dissociation” of the molecules involved, an assumption not scientifically tested until 52 years later [109]. Moreover, the findings in 2006 explicitly confirmed earlier claims of harmful effects of silicofluorides on the regulatory enzyme acetylcholinesterase [56] and documented that their use in water increases acidity (which changes many chemical reactions in the body) and leaves behind either “colloidal silica” (sheets of silicon atoms) or “oligosilicates” (strings of silicon atoms).
Several human studies have documented an association between fluoride in drinking water and the occurrence of osteosarcoma (bone cancer) in young males [171–173]. These studies are consistent with the National Toxicology Program’s (NTP) cancer bioassay, which found that fluoride-treated male rats had a dose-dependent increase in osteosarcoma [174]. There has been a dramatic increase in osteosarcoma in males aged between 9 and 19 years old. National Cancer Institute Surveillance Epidemiology and End Results Program recorded an increase of 79% of osteosarcomas in young men living in fluoridated areas of Iowa and Seattle, while in the unfluoridated areas the incidence of bone cancer decreased by 4%. In New Jersey, rates of osteosarcoma were three to seven times higher among males aged between 10‐19 than in unfluoridated regions [175]. Since over 90% of “fluoridated” public water is treated with silicofluorides, these studies usually concern the effects of these compounds, not the element “fluorine” (which is unstable unless in a compound, when the element becomes the anion “fluoride”).
Five major epidemiological studies from France, the U.K. and the U.S. show higher rates of hip fractures in fluoridated regions. The U.S. has the highest number of hip and other bone fractures and the longest history of fluoride use. In 1997, the EPA scientists went on record against the practice of adding fluoride to drinking water. Burcher quoted Dr. William Marcus, a Senior Toxicologist at EPA saying, “The EPA should act immediately to protect the public, not just on the [basis of the] cancer data, but [also taking account of higher incidence of] bone fractures, arthritis, mutagenicity and other effects” [174].
Given these findings, it is striking that neither the EPA nor the CDC has sponsored or conducted extensive studies on the harmful effects of silicofluorides on human health and behavior. Even though the National Toxicology Program nominated silicofluorides for such study in 2002 on the grounds that their “toxicology” was not known, health authorities and physicians continue to speak of “water fluoridation” without considering the well-established differences between the toxicity of hydrofluorosilicic acid or sodium silicofluoride, and the limited danger of sodium fluoride (unless a child swallows all of a large tube of fluoridated toothpaste) [176]. For the above reasons, a moratorium on silicofluoride use has been proposed as an action that should remain in force until such time as independent studies demonstrate the safety of these compounds and explain the many studies that show toxic effects in both children and adults [167].
Parallel failures with regard to other toxicants, however, show that there is little unique about the inability of Congress to act on the costly effects of the silicofluorides. Actually, however, the terms of the Toxic Substances Control Act, §5 and §6 would be sufficient for the EPA’s Administrator to list these compounds and require their sale be subject to the laws regulatory provisions, yet such action has not been taken.
Ignorance of known synergistic health effects of multiple toxicants
It is not widely known by the general public that mercury vapor exposure from dental amalgam has been demonstrated to exceed the sum of all other exposure sources for human mercury body burden [16,128]. Less well known is research showing that metallic mercury can be transferred from a mother to her fetus via the placenta and supplied to the newborn via mercury accumulation in breast milk [129,130,133]. A baby born in the U.S. with excess mercury derived from its mother’s mercury/silver amalgam tooth fillings is subject to further exposure to both mercury and aluminum from an aggressive vaccine schedule that requires more vaccinations than any other country in the world, including a Hep-B vaccine administered at birth that contains both mercury and aluminum. If the mother is unable to nurse the baby, and if economic considerations influence her to choose a powdered formula reconstituted with tap water, then it is likely that the infant will also be chronically exposed to fluoridated water during the first year of its life. The baby is now exposed to an amount of silicofluoride-treated tap water that equals its body weight every 4 to 6 days. This transports lead into the baby’s blood stream [66], that otherwise would have passed on through without harm [161,167]. Infant formula is also commonly contaminated with aluminum, contributing an additional toxic burden [177–179]. The baby is also apt to be exposed to glyphosate from food sources routinely treated with the herbicide Roundup®. Glyphosate disrupts gut bacteria [68,180,181], leading to “leaky gut syndrome” and increased absorption of other orally ingested toxicants [182]. Glyphosate also inactivates cytochrome P450 enzymes, which are needed to detoxify environmental toxicants [180,181,183,184]. The U.S. market represents 25% of all the Roundup® sold in the world, and monitoring of glyphosate in the food supply is hardly being done.
Taking account of Schubert’s experiment with rodents, it must be noted that a nearly equivalent experiment on human babies is being carried out, albeit unintentionally, all across America with the entire population of newborns as they continue to arrive. Can we expect outcomes of increasing levels of toxic exposure for human infants to differ radically from what Schubert observed in rodents? Given the aluminum compounds known to enhance gastrointestinal absorption [185] that are being combined with exposure to glyphosate, known to be a potent chelating agent [186] that forms compounds with aluminum [187], we must predict an increased absorption of aluminum by human infants who are being exposed to these chemicals [68]. Knowing the affinity of aluminum to bind with glyphosate, which in its turn is known to be incompatible with the microbiome of the gut [181], we must predict a synergistic effect compromising both digestive and neurological functions [188]. Also, we know from a great deal of developmental research that exposure to toxicants is more apt to harm neonates because of their relatively much smaller body weight putting them in a more vulnerable place on the dose-response curve [189] and the rapidity of cell-division underway in the infant making the delicate process of mitosis many times more susceptible to injury than in a more mature person [83]. It may be noted that exposure to aluminum, mercury, and lead, along with pesticides and herbicides, some or all of them interacting synergistically with silicofluoride residues in drinking water, provides a plausible explanation for the relatively higher rate of infant mortality in the U.S. as contrasted with other industrialized nations [190]. Exposure to such poisons, which are known to cause premature births and many potentially fatal disorders [83], also cannot be easily ignored with respect to the fact that the U.S. infant mortality on the first day of life is the very highest in the world [33].
Of course, problems brought about by toxicants do not end a year after birth. For infants who survive their first year, chronic health conditions have also been increasing dramatically over the last forty years [191]. In the last five years, the prevalence of parent-reported autism spectrum disorder (ASD) diagnoses among school-aged children in the U.S. has increased significantly from 1.16% to 2.00%, and these increases were observed in all age groups [192]. At the extreme limit of the least severe edge of the autism spectrum, there is an epidemic of children who struggle in school due to attention deficit hyperactivity disorder (ADHD), and as many as 40% of those near the center of the spectrum will end up requiring full-time care or institutionalization [193,194]. A recent analysis of the cost of autism in the U.K. determined that the lifetime cost for an individual with ASD and intellectual disability is approximately £1.23 million [195]. Children in general are increasingly manifesting debilitating conditions such as severe food allergies, asthma, depression, violent behavior, obesity, and adult-onset diabetes [196,197].
The “Petkau Effect” & toxicity of multiple metals: the example of the OujéBougoumou Cree
Debilitating health conditions often illustrate what has been described as the “Petkau Effect” wherein harm from chronic low levels of exposure to one or more injurious factors turns out to be greater than from a single, much larger exposure. Such an effect was first discovered in what seemed to be negligible exposures to radioactive alpha particles released in processing nuclear materials for weapons and/or power plants [198,199]. While discovered with respect to cumulative injuries to persons living near nuclear power plants, working with X-ray machines, or being exposed to any known source of low radioactivity, the Petkau effect can be found in exposure to cumulative injuries from any source. Legislators and policy-makers, however, have generally focused their attention on events where an injurious event is known to kill about 50% of the laboratory animals exposed to it. Calibration of injury estimates, therefore, have been linked to the known “lethal dose” (LD) or a percentage thereof (%LD). As a result, policy-makers have sought to protect against single-event exposures expected to cause death to 50% of lab animals given such an exposure (LD-50). The thinking has been that much smaller exposures are likely to be negligible. But the Petkau Effect shows that the LD-50 standard was inadequate to prevent fatal injuries from radiation, and the inadequacy generalizes to cumulative injuries in general. It is necessary to consider cumulative effects across longer time spans and across multiple generations. It is also important to consider synergistic interactions of multiple sources of harm.
A case in point can be found in a study conducted by Masters of cumulative toxic injuries to the children of the OujéBougoumou Cree Nation living on the shores of Lake Chibougoumou in Northern Quebec [200]. After copper mining came to that relatively remote area of Québec, multiple toxins from the tailings and slag piles leached into the soil, streams, and lake water until they could be measured in the head hair of resident children. Analysis by Doctor’s Data laboratory in Chicago showed the children’s hair to contain PCBs, lead, and copper in proportion to samples taken from different locations where the children were exposed to mine tailings. Concerns were raised by the fact that 40% of the Cree children formerly unaffected by asthma, after exposure to toxins coming from the mining operation, were being diagnosed with asthma. Upon authorization of the mining there, the Quebec Provincial Government set up a million dollar indemnity fund to protect the Cree bands living where contaminants would invariably be introduced by the mining into the soil and water. After the hair samples of asthmatic Cree children were analyzed, speaking for the government, E. Nieboer (and other representatives of Quebec’s public health authorities and universities) would question whether the contaminants found in the children’s hair could be reasonably attributed to the copper mining. The authors of the Nieboer report acknowledged that a “2001 survey by the Quebec Ministry of the Environment [had already] confirmed the presence of toxic elements in sediments near mine tailings sites” but they asked for an additional “environmental risk assessment . . . as well as a human health study” ([201], p. iii).
When Christopher Covel, a geologist, working on behalf of the Cree Nation was able to show that the proportion of a given bouquet of toxins in the children’s hair was differentially correlated with soil and water samples taken from the different locations where the children were being chronically exposed to the Chibougoumou copper mine tailings, the Canadian court finally agreed that the source of the injurious contaminants linked to asthma, cancers, and other medical problems was the copper mining. The Cree Nation claims were substantiated by the correlation between toxins in the soil and water in different locations and those found in similar quantities in the hair of children in each of the several communities. Whereas an LD-50 measure would make subtle Petkau-type cumulative effects and the synergy of the distinct toxic cocktails undetectable, the more subtle geological, chemical, and biological measures showed the mine tailings to be the proximate cause of a host of medical problems for the Cree inhabitants near Lake Chibougoumou. In this case, the geographical isolation of the mining operation and the remarkable correlation of hair samples of the Cree children with distinct levels of exposure in soils and water to specific toxins leaching from slag piles, left no doubt about the causal relation between the toxins brought to the surface by mining and the subsequent increase in disease for Chibougoumou Cree individuals consuming water, fish, and wild-life in the affected areas. It is important to learn from such isolated cases and apply the knowledge gained to the general population.
Lead, fluoride, and dental fluorosis
In the field of toxicology, another well-established case with subtle Petkau-type cumulative effects ranging across generations and differentially affecting certain minorities of the U.S. population, is found in lead poisoning studies, especially those by Herbert Needleman and his colleagues. Needleman, a professor of psychiatry and pediatrics at the University of Pittsburgh School of Medicine, has called attention to the fact that minute amounts of chronic lead exposure are demonstrably associated with the diminution of IQ scores in exposed children [202]. Lead toxicity has also been shown to be a causal factor in learning deficits and violent crime [29,60,203,204]. Needleman’s findings have resulted in legislative reductions in lead exposure through the introduction of lead-free gasoline, but, as the research reported in this paper shows, any such reduction is offset by legislation requiring water treatment with silicofluorides (or sodium fluoride), which act as lead-binding agents ensuring more rapid and complete biological uptake of any traces of lead in food, water, air, from any source whatever.
In the CDC Fluoridation Census 1992 [148], Public Health Service data indicated that over 116 million Americans were exposed to fluoridation in their water supply, with over 90% of these systems using either hydrofluorosilicic acid (H2SiF6) or sodium silicofluoride (Na2SiF
The CDC estimates, according to their latest website updates, that about 75% of community public water systems have substantial amounts of fluoride [150,205], but to the time of this writing, there is no mention on their official websites of the mounting evidence of synergistic toxic effects of fluoride compounds added to drinking water. Given that Needleman found that lead disturbs neural regulation leading to impulsive violent behavior, and given that other researchers showed it blocks dopamine [202], a crucial neurotransmitter enabling both learning and the inhibition of undesirable behavior, it is unsurprising that children and adults exposed to silicofluorides in drinking water have higher lead levels in their blood and are more likely to be diagnosed with learning deficits and disorders such as ADHD [57,167, 207]. As adults, persons exposed to fluoridated water are also more likely to be apprehended for violent crimes and to be incarcerated [57,167].
The unhealthy synergies of fluorides need to be considered when assessing reports that claim to test the effects of fluoride ingestion. It has been demonstrated conclusively that fluoride compounds, especially the silicofluorides, but also NaF, can cause neurotoxicity, deficits in learning and memory [208,209], reduction in reproductive capacity, and deleterious genetic alterations in laboratory animals [122,124–126]. In research with humans exposed to fluorides, as of March 2016, 36 of 49 studies had already found that elevated fluoride exposure is associated with reduced IQ, while 16 of the 17 animal studies found that fluoride exposure impairs learning and memory capacity [210]. The human studies, which are based on IQ examinations of over 11,000 children, provide compelling evidence that fluoride exposure during the early years of life can damage a child’s developing brain [176,206,209–214].
Yiamouyiannis (1994) [216], followed Wedeen [217], showing the health hazards of fluoride. The findings of Yiamouyiannis [155,216,218,219] only confirmed and reinforced those of other researchers. In many of the relevant studies, researchers merely used tap water in urban communities that were adding silicofluorides (hydrofluorosilicic acid, H2SiF6, or sodium silicofluoride, Na2SiF
A study by Leite et al. 2011 using Wistar rats also showed that dental fluorosis is exacerbated by concurrent exposure to lead, providing additional confirmation of the known synergistic uptake of lead in the presence of fluorides and especially in the presence of silicofluorides [67]. More recently, de Figueiredo, et al. in 2014, exposed prenatal rats to 30mg/L of Pb in drinking water (probably fluoridated with a silicofluoride) for 28 or 60 days post-birth. They found that bone lead was five times higher than blood lead in the rats exposed for 60 days [223].
Whereas certain effects of fluoride on tooth enamel have been judged positive, thus providing the tenuous foundation for public messages in support of fluoridating drinking water, dental fluorosis, as acknowledged by the California Department of Health at least since 1986 and by the National Research Council (NRC) [211], has been universally recognized as an adverse health effect. Also, as can be seen from Figure 4, the policy of water fluoridation has increased the number of children with fluoride-damaged teeth as seen in Figure 5. In the words of the National Center for Health Statistics: “In 1986-1987, 22.6% of adolescents aged 12-15 had dental fluorosis, whereas in 1999-2004, 40.7% of adolescents aged 12-15 had dental fluorosis” [221]. The CDC does not offer current data on dental fluorosis at the time of this writing, but independent research suggests that it continues to be closely correlated to exposures through drinking water and continues to be influenced negatively by fluoridation policies [222]. Moreover, results continue to accumulate showing that any exposure is detrimental to development in humans and to physical and mental health [176,210,212–214].
A high fluoride concentration in the body causes the bone to become hardened and less elastic, a condition referred to as skeletal fluorosis, producing increased risk of bone fractures [206,223–226]. The U.S. Environmental Protection Agency (EPA) limits the fluoride that can be present in public drinking-water supplies to 4 mg/L (maximum contaminant level, or MCL) as appropriate for guarding against crippling skeletal fluorosis, with a secondary maximum contaminant level (SMCL) of 2 mg/L aiming to protect against objectionable enamel fluorosis [227]. Heller et al. (1997), found that dental fluorosis had increased nearly threefold by 1987, such that 29.9% of children in fluoridated communities had dental fluorosis on at least two teeth, and not all of it in the very mild category [228]. There is also evidence of disproportionate harm to minorities, as a 2005 study from the CDC determined that 1.92% of White (Caucasian) children have moderate/severe dental fluorosis, a number that rises to 3.43% for Black children and 4.82% for Mexicans and Hispanics [222].
Aluminum adjuvants in vaccine and autism
A lesson can be learned by examining the situation relating autism to environmental toxicants in Vietnam. Vietnam represents a microcosm where a sharp recent increase in autism suggests one or more environmental factors as causative. According to recent news reports, the autism rates in Ho Chi Minh City, Vietnam, have increased by 160 fold over the past eight years [229–231]. Over the same time period, the practice of administering the Hep-B vaccine to newborns has risen sharply [232]. A U.S.-based study published in 2010 found a three-fold increased risk of autism in association with neonatal administration of Hep-B vaccine [233]. In parallel, aggressive campaigns by manufacturers of powdered infant formula have caused a majority of Vietnamese mothers to abandon breast-feeding in favor of reconstituted powdered formula [234].
Concern for infant risk through exposure to fluoride, in the context of increased incidence of fluorosis in children in Ho Chi Minh City, led to a study which confirmed that fluoride was already present in the unreconstituted milk powder, particularly powder from imported sources [230]. A reasonable hypothesis predicts the aluminum in the milk powder [121], would act synergistically with the fluoride compounds due to reactions that researchers have found take place both in vitro and in vivo (see §4.7 below). If so, the increased rate of autism could thus be attributed in part to the interaction of the Hep-B vaccine and the increased use of reconstituted formula originally produced with silicofluoride treated water (present in the products imported from the U.S.). Immunosuppression, prematurity, and low birth weight, all potential outcomes from fluoride, mercury and aluminum exposure, are risk factors for infection with Enterobacter sakazakii contaminating reconstituted powdered milk. Infection can lead to meningitis, neurological complications, and death [234,235]. Hence the hypothesis of synergistic toxicity needs to be more generally studied in cases like this unexpected outcome in Vietnam.
Synergistic effects of toxicants on health: cystic fybrosis and preterm birth
Each of the foregoing examples shows that chemicals rarely act without synergistic interactions producing greater effects. As a result, the effects of toxic metals such as lead, mercury, and aluminum, when interacting with fluoride compounds, merit further study. Reviews of the known effects of lead, mercury and aluminum can be found: lead [202,236], mercury [128,129,237,238], and aluminum [4,53,68,117,119,239–244]. The following sections will therefore review synergies involving toxic metals, silicofluoride compounds, and/or genetic mutations now found in a number of specific health conditions in which normal biochemical and biophysical processes have been disrupted.
Since the problem posed in this article concerns the high rates of infant mortality in the U.S., skeptics might dismiss the example of cystic fibrosis, insofar as many infants born with this condition survive albeit with compromised health and quality of life. CF is, however, an example of a disorder evident at birth and hence the result of what we have called “biosemiotic entropy” during fetal development. As a result, it will be useful to summarize briefly both current evidence that mercury plays a key role in the etiology of CF and then, more broadly, the facts concerning the role of prematurity in the high rate of infant mortality on day of birth (which recent evidence shows is also linked to events harming the fetus during gestation).
Cystic Fibrosis (CF) is the most frequent hereditary lethal disease in Caucasians, where death usually follows from impaired lung function and chronic lung infection. Airway inflammation is already present in 4-week old infants with CF. While CF is a hereditary disease, environmental factors can promote an accelerated disease progression leading to early death. A combined exposure to glyphosate from herbicide application to ingested food sources along with organomercury compounds derived from mercury amalgams could only contribute negatively to CF. Several strains of Streptococci are capable of methylating mercury derived from pulverized dental amalgam to produce organic mercury compounds [245], which would be readily absorbed through an impaired gut barrier subsequent to glyphosate exposure from contaminated food sources [77,181]. Organic mercury compounds are highly effective at activating matrix metalloproteinases (MMPs) [246], which break down the extracellular matrix proteins. MMPs play a major role in CF [247]. The alveolar levels of MMPs are elevated in CF patients, and proteolysis activities increase over the lifetime of CF patients. MMPs are capable of degrading essentially all connective tissue components, and therefore lead to extensive destruction of lung tissue such as the alveolar epithelium. Lung dysfunction is the major cause of death in CF.
Premature birth is another condition that does not preclude survival, but is associated with high rates of infant mortality. Several quotations from a neonatologist’s recently published description of the facts concerning premature birth in the U.S. are therefore of exceptional importance. It’s not widely realized that, despite the “biomedical advances” in neonatology, of babies “born before 28 weeks 30,000 of the half million babies born prematurely each year in this country many will have serious physical social or cognitive problems. Consider that a one-pound, one-ounce girl born unexpectedly at 23 weeks’ gestation has a 92 percent chance of dying early or having moderate to severe neurodevelopmental impairment. Most extremely premature babies will experience complication at least once bleeding in the brain, infections, intestinal perforation, and severe lung damage before discharge. Many will need treatment long after birth, sometimes for life, at great financial cost to them and those around them” [248].
It should be evident that “bleeding in the brain, infections, intestinal perforation, and severe lung damage” can often be traced to one or more toxic insults impacting developmental processes which are especially vulnerable to disruption in the earliest stages of the pregnancy. As cellular differentiation forming the basic organization of the human body is taking place and while the central nervous system is developing, regulatory systems from the genome upward are known to be particularly vulnerable to toxic injury. Drug use, alcohol consumption, products consumed, medicines administered, radiation exposures, and so forth, are all factors to consider in explaining America’s increasingly higher rate of infant mortality. From the vantage point of biosignaling systems, it is unsurprising that the susceptibility of tubulin fibers essential to mitosis (also to meiosis prior to conception) to disruption by toxicants such as silicofluorides and the metals they interact with synergistically, makes pre-fertilization meiosis along with post-fertilization mitosis, critically vulnerable. Except for corrective systems built into biosignaling processes, we must suppose that disorders, diseases, and fatalities attributable to toxicants are most likely to be caused, dosage being equal, if the toxicants are introduced earlier rather than later. The very nature of the processes of meiosis as contrasted with mitosis makes the former more vulnerable to disruption by toxicants that impact spindle assembly and the mating and sequencing of chromatids. By the same token, although mitosis involves fewer steps and less complexity than meiosis, at every single juncture where cell division/multiplication is occurring, spindle assembly, centromere pairing, and the dynamic functions of tubulin fibers [85–90], are known to be vulnerable to disruption by the very toxicants under consideration in this paper [237,249–252].
The systems at issue, as we have argued earlier in this paper and in related works, need to be understood in the context of biosemiotic entropy (i.e., disorder, disease, or death due to disturbance of normal genetic, epigenetic, and protein-linked programs that regulate organic development, growth, and environmental adaptation). Evidence linking infant mortality to specific toxic exposures is consistent with the predictions and expectations that flow from such a theoretical perspective. Recognizing the interactions among the three major factors known to cause disorders, diseases, and mortality — toxins, pathogens, and macro- or micro-trauma — preterm birth is certain to play a role in the frequency of infant mortality. It is equally certain also to be linked to the same injurious factors known to disrupt biosemiotic processes which are likely to cause the pre-term birth, if not a still-birth or early “spontaneous” abortion. The fact is that abortions of any kind do not arise spontaneously any more than the mold in Pasteur’s most famous experiments [253,254], manufactured itself out of nothing. It is known that maternal absorption of toxins and toxicants plays a role in this degradation of the normal process of gene-environment adaptation during the earliest stages of embryonic development. Also, it is known that, although vulnerability to injuries leading to disorders, diseases, or death of the organism decreases over the first year of life, the risks remain higher throughout the first year than they will be at any later time. Hence the hypothesis from which this article started namely that America’s unusually high rate of infant mortality is due mainly to manufactured toxicants and their synergistic interactions with one another and with other naturally occurring hazards is not merely a statistical correlation: it has a functional biological explanation. Moreover, as we have demonstrated in this extensive review of research literature and theory, variations in toxicant exposures are known to be causally related to fluctuations in infant mortality rates.
The hypothesis that aluminum adjuvant in Hep-B vaccine is among the toxic metals with negative effects on normal development and survival needs to be assessed with particular care because of the lengthy ideological controversies over the process of “vaccination” as a process. Despite the success in controlling massive epidemics of lethal diseases like rheumatic fever, tuberculosis, or polio, commonly attributed to vaccinations, sanitation is known to play the larger and more important role. Particular attention must be paid to the Hep-B vaccine, the use of which in infancy is mandated by the CDC in the U.S. Since it is unusual to apply the principles of neurotoxicology to a legal requirement established by the Federal Government, it is essential that the scientific accuracy of the hypothesis under discussion be beyond question. For this purpose, it is essential to focus on the toxicity of the aluminum “adjuvant” used to increase the intensity of the immune response engendered by the injection. Moreover, given the evidence of synergistic effects due to the combination of silicofluoride treated water and aluminum adjuvants, it is necessary to consider aluminum neurotoxicity in relationship to the co-exposure to silicofluoride residues.
Aluminum and fluoride neurotoxicity and the effects of aluminum fluoride
Aluminum is the most abundant metal and the third most common element on earth, yet it is not an essential element for any biological system [255]. A recent neurotoxicological study has emphasized the fact that aluminum is still being ingested in food sources by humans [256,257]. Even low levels of aluminum in the drinking water of experimental animals induce elevation of inflammatory markers in their brains, and inflammation is a significant factor in Alzheimer’s disease, Parkinson’s disease, autism, and other neurological disorders.
Millimolar amounts of fluoride in water will etch aluminum from a glass container into the solution [258]. Aluminum binds fluoride more strongly than 60 other tested metals [259,260]. Treatment with aluminum sulfate is a means of removing suspended solids containing aluminum that may reduce the total aluminum concentration in filtered water as compared to the untreated water source. There is, however, evidence that treatment with aluminum salts can also increase the concentration of low-molecular-weight, dissolved aluminum species [259]. The average value of 101 microgram/L, associated with the various aluminum salt-treated water supplies, was used for the purpose of assessing exposure of the Canadian population to aluminum in drinking water [261]. This value represents just one of the many routes of lifelong exposure to cationic aluminum species. It does not include the parenteral route from aluminum adjuvants in vaccines, the inhaled route from inhaled aluminum nanoparticulates, the topical route from anti-perspirants and sun-blocks, or the oral route from both antacid drugs and food processing and cooking of food.
The values given for the aluminum levels in the human body vary widely, in part because it distributes unequally to the tissues. Typically, the aluminum concentration in blood is 5-10 microM/L [258]. Thus, the apparent Kact of Al3+ (1-5 microM/L) is well within the range of usual concentrations of the metal in blood and soft tissues, raising the question of what effect Al3+ might have on adenylate cyclase in vivo. Adenylate cyclase depletes energy reserves by converting ATP (adenosine triphosphate) to cAMP (cyclic adenosine monophosphate). Perhaps more importantly, however, species of aluminum fluoride (notably AlF3 and AlF4-) are thought to act as messengers of false information by their activation of G-proteins, thereby initiating an amplification cascade, which includes the second messenger, cAMP (Figure 6). Since Rall and Sutherland’s discovery published in 1958, fluoride has been known to activate adenylate cyclase [262], but whether there is a physiological equivalent in living organisms to an aluminum-fluoride complex is still in question [258].
When present, Al3+ is the main complexing agent of F- in drinking water [263]. Of all common ligands, F- binds more strongly by a factor of 10-times to A13+ than to Fe3+. Representative successive stability constant logarithms for the addition of one to five fluorides to Al3+ at 25-37o and 0.16 ionic strength are 6.4, 5.2, 3.8, 3.3, and 1.3, respectively [264]. Human red blood cells (RBCs) incubated with 10 µM AlF3 lose their normal discoid shape and develop blebs or protuberances on their surface, a strong indicator of disruption of membrane properties [255], as illustrated in Figure 6. This effect is remarkably similar to the change in RBC shape in hypotonic saline solution in the absence of membrane cholesterol sulfate, suggesting that AlF3 may somehow disrupt the anionic effects of cholesterol sulfate and/or sphingosine-1-phosphate (S1P) in RBC membranes [73,265].
Both aluminum [266,267] and fluoride [268], inhibit glycolysis, and suppressed glycolysis is implicated in Alzheimer’s disease. Fluoride also inhibits phosphatase [269] and cholinesterase [270], and it is highly likely that some of these effects are due to its reaction with aluminum to form AlF4−. Impaired phosphate metabolism is implicated in many diseases currently on the rise, such as allergies, asthma, obesity, myocardial hypertrophy and Alzheimer’s disease [271]. Fluoride interferes with calcium-calmodulin activity, preventing calmodulin activation of phosphodiesterase [272]. Impaired calmodulin function is associated with sudden death due to ventricular arrhythmias [273]. Fluorides alter the permeability of plasma membranes, likely leading to the observed phenomenon of excess loss of potassium by RBCs in the presence of fluoride, a trigger of sudden death [274].
G-protein coupled receptors are the main class of membrane-spanning proteins that mediate the communication of external signaling molecules to the interior of the cell [275] by activating G-proteins. Their means of action involves binding of G-proteins to either guanosine diphosphate (GDP: inactive state) or guanosine triphosphate (GTP: active state) [276]. Magnesium reacts with fluoride in a similar way and the product has a similar effect on falsely activating G-protein-GDP complexes [277]. Although the reaction with magnesium is not as strong as that with aluminum, magnesium is naturally found in the body whereas aluminum is not [4]. GTP binding energy activates a molecular switch that turns on the production of cAMP, initiating a signaling phosphorylation cascade, via the enzymatic detachment of the terminal phosphate group (Pi) from ATP [278]. The cAMP activated protein kinase, by phosphorylating different enzymes, results in both activation and inactivation of specific enzymes, increasing glycogen breakdown to glucose and preventing glycogen synthesis. In this way, G-protein transmits signals from many hormones, neurotransmitters, and signaling phospholipids such as lysophosphatidic acid (LPA) and S1P.
Aluminum fluoride (AlF4−), as a phosphate mimetic, is a major disruptor of G-protein signaling: it binds to G-protein-GDP complex forming a mimic or facsimile of the G-protein-GTP complex, switching the G-protein into an active state, which is however protected from the step of Pi detachment [104]. The high electronegativity of fluoride results in tight hydrogen bonding to nearby amino acids, thus preventing the removal of the phosphate mimetic and maintaining the G-protein in a sustained active state 279. This locked state has the effect of enhancing the cell’s sensitivity to all external stimuli [103].
AlF4− also potentiates LPA receptor signaling, and totally (irreversibly) prevents the degradation of LPA, resulting in its accumulation in the brain [280]. Impaired degradation of LPA is another consequence of the phosphate-like appearance of AlF4−. It binds to the Pi-recognizing pocket of lipid phosphate phosphatases (LPPs), and prevents them from dephosphorylating their substrate [280]. It takes only trace amounts of aluminum (micromolar concentrations) in the presence of fluoride for these disruptions to take place [258,281]. They no doubt also impact many phosphatases with unpredictable but almost certainly deleterious consequences, such as a buildup of S1P in the serum, a known factor in cancer [282] and heart disease [283].
Cancers that originate in the lymphatic tissues are the third most common type in children. Lymphocyte function associated antigen-1 (LFA-1) is a cell surface protein on leukocytes and lymphoma cells that promotes intercellular adhesion. It plays a crucial role in lymphoma invasion and metastasis [284]. in vitro experiments have shown that AlF4− can induce adhesion of T-cell lymphoma and subsequent invasion, by activating G-protein transduced signals [285]. This strongly suggests that AlF4− would promote metastasis.
RBCs have a vestigial adenylate cyclase that is activated by AlF4−, which can convert ATP to cAMP, thus depleting ATP. A recent study involving exposing rat RBCs to sodium fluoride, in concentrations ranging from 0.1 to 10 mM NaF, showed a dramatic dose- and time-dependent decline in ATP concentrations, which diminished to extremely low levels within 24 hours [286,287]. Aluminum contamination could have facilitated this observed response, or it could be due to a similar reaction with magnesium [277]. In any event, as noted above, reduced ATP had also been observed in the sperm cells of male mice exposed to orally presented NaF [124].
FTY720 is a drug that targets G-protein S1P receptors and causes immune suppression, due to sequestration of lymphocytes into secondary lymphoid tissues and thymus [288–290]. It is used as an S1P mimetic to suppress immunity in autoimmune diseases such as multiple sclerosis and in organ transplant patients. AlF4−, acting as a phosphate mimetic, combines with sphingosine to produce a pseudo-S1P [291], likely producing a similar effect as FTY720, and may therefore contribute to increased susceptibility to pneumonia, respiratory syncytial virus and other infective agents.
Effects of aluminum fluoride on acetylcholine and rho kinase dysregulation
Synergistic effects of aluminum, fluoride, and organophosphates can be predicted to disrupt acetylcholine signaling and the Rho/ERK signaling pathways, leading to several pathologies. Acetylcholinesterase plays an important role in neural development, and mice lacking acetylcholinesterase are developmentally delayed and are extremely sensitive to organophosphates, particularly diisopropylfluorophosphate. This insecticide contains a fluorine atom bound to phosphorus, which undergoes hydrolysis when subjected to moisture, producing hydrofluoric acid [292]. Other more severe symptoms, including muscle weakness, fatigue, muscle cramps, and even paralysis, can occur in response to organophosphates, due to accumulation of the excitotoxin, acetylcholine, at motor neurons [293]. In addition to the other effects of water treated with either hydrofluorosilicic acid or sodium silicofluorides (see above), these siliocofluorides apparently function as acetylcholinesterase inhibitors [56,109].
The RhoA/Rho kinase signaling pathway, modulated by G-proteins, is a major contributor to calcium sensitization, leading to hypertension and endothelial dysfunction through excess contraction of smooth muscle cells in the vasculature [294]. Sodium fluoride (NaF) has been shown to increase smooth muscle cell sensitivity to calcium by suppressing myosin phosphatase activity, and it has been proposed that AlF4- is the active molecule that effects this dysfunction [295]. Since NaF increases both the amount of RhoA-GTP and the amount of myosin phosphorylation in rat aorta [296], it can be presumed that the fluoride dissociated from H2SiF6 or Na2SiF
Augmented RhoA activation leading to muscle contraction is also observed in hyper-responsive bronchial smooth muscle cells, leading to obstructed oxygen delivery [289,290]. Following antigen stimulation, acetylcholine induces translocation of RhoA in bronchial smooth muscle cells, an effect that is mediated by choline-derived S1P. Aluminum fluoride mimics the action of S1P in stimulating Rho/ERK and contraction in S1P2 receptor-expressing cells [291], such as smooth muscle cells [297]. Fluoride’s known inhibition of acetylcholinesterase [109,298] enhances acetylcholine bioavailability, further aggravating the effect of organophosphates.
Rho kinase activation is also implicated in muscle contraction in the uterus leading to premature birth [299], an increasingly common problem adversely impacting infant survival immediately at birth and thereafter [33,300,301]. S1P2 receptors present on the myometrium induce calcium influx stimulated by a G-protein-coupled receptor whose function could be impaired by AlF4−, in a mechanism analogous to that occurring in bronchial smooth muscle cells. Preterm infants are especially susceptible to aluminum and fluoride due to their underdeveloped kidney function, and they unfortunately receive an additional burden of aluminum following birth as a contaminant in parenteral formulations beyond the aluminum burden from vaccines [241]. Despite the fact that this problem has been known for decades, aluminum contamination in commercial parenteral formulations is still unacceptably high, particularly in the U.S.
Systemic dysfunctional effects of synergistic toxicity
The confluence of the bewildering variety of subtle biological effects described above needs to be summarized from a systemic perspective. This integration is especially necessary because U.S. government policies have increased public exposure to both the aluminum in Hep-B vaccine and silicofluorides, each of which has independent toxic effects as well as that of forming the neurotoxic aluminum fluoride compounds. These public policies clearly explain why the U.S. is not only among the nations with the highest rates of preterm births “countries with the highest numbers include Brazil, India, Nigeria and the United States of America” [268,269] but also helps explain why the U.S. has the very highest first day infant mortality rate in the world [33]. More broadly, however, the effects of neurotoxicity on health and behavior are inseparable from the paradox that, although the per capita cost of the entire U.S. health care system is about 2.4 times more expensive than the average of other industrialized members of the OECD, our overall life expectancy is about one year less and our rates of obesity are more than double OECD averages.
More than six decades ago, Hans Selye described how our environmental exposures to metals act as sensitizers for thrombohemorrhagic phenomena, Sanarelli-Shwartzman phenomena, and calciphylaxis [302,303]. Autopsy finds the body burden of fluoride is typically in the calcified tissues of the body, especially skeleton, ligaments and pineal gland [304]. Fluoride compounds can act as a provocation for both systemic and local disease which is distinctly non-linear in its dose-response. For that reason, the limits of “safe” levels of exposure recommended by our regulatory agencies need to be reevaluated. The relevant research reviewed here shows that no systemic level of ingested fluoride should be “generally recognized as safe” (GRAS).
Today, the overwhelming consensus by dental researchers is that fluoride’s primary effect on dental health is topical, not systemic [211,305–307]. The CDC stated in 1999, “fluoride prevents dental caries predominately after eruption of the tooth into the mouth, and its actions primarily are topical for both adults and children” [149,308]). The National Research Council concurred in 2006, stating that “the major anti-caries benefit of fluoride is topical and not systemic” [211]. The benefits of fluoride in preventing tooth decay are dependent on topical application directly to the tooth, not to swallowing and digesting or otherwise consuming fluoride containing chemicals.
Such conditions as dental fluorosis, skeletal fluorosis, bone cancer, bone fractures, and CNS toxicity are all manifestations of systemic toxicity of fluoride inside the body rather than on the surface of teeth. Whereas the existence of dental caries is a localized topical condition, which only infrequently has systemic sequelae (i.e., causing or being associated with bodily diseases), systemic fluoride in the body’s organs is causally implicated in many debilitating and potentially fatal disease conditions. A review of the extensive literature shows that fluoride’s toxicity is enhanced synergistically in the presence of polyvalent metal cations, including aluminum, mercury, lead, arsenic, and silicon, which are pervasive in the U.S. food and water supply [308]. While the toxicity of various individual sources of fluoride might be small, they are cumulative and tend to interact with other toxicants. Reports have also shown that susceptibilities may vary from one person or group to another, and that the brains of the developing fetus, neonate, and infant are especially vulnerable, due to high nutritional demand and an incompletely developed blood brain barrier [4,25,71].
Concomitant with the risk of a toxic developmental injury from fluoride is another large source of potential synergistic toxicity that of the scheduled vaccinations in the U.S. A study of the CDC’s Vaccine Safety Datalink by Chen et al. (1997), found that the DPT vaccine, in particular, was 300% more likely to produce seizures within a 30 day window if given 8 to 14 days after the MMR vaccine [309]. These findings demonstrated, unsurprisingly, that the chances of an adverse reaction are increased when multiple pathogens and toxicants are combined and injected at the same time or in close proximity as all the toxicology literature shows, and as sound theory predicts if all else is held equal. In a recent study of the Vaccine Adverse Event Reporting System (VAERS), Goldman and Miller (2012), hypothesized that multiple pathogens plus toxic adjuvants or excipients in multiple doses of vaccines given simultaneously are apt to interact to have synergistic, more potent, toxic effects, and showed that younger infants are more vulnerable [310]. Miller and Goldman (2011) found that the likelihood of being hospitalized after a vaccination was correlated with the number of doses administered on a given vaccination visit a so-called well-baby visit and that the likelihood of being hospitalized or dying after 1-8 doses of vaccine diminishes as the infant matures throughout the first year of life [83]. Two principles are to be noted from their findings: first, synergistic toxicity is associated with multiple vaccine doses accounting for 91 percent of the variability in hospitalizations, or deaths, of infants in the VAERS data 1990-2010; and second, the more immature the immune system of an infant, the less well equipped that child is to handle multiple toxicants and pathogens simultaneously. The age variable accounted for 95% of the variance in percentage of hospitalizations (or deaths) with 1-8 vaccines administered at the same time to infants in the VAERS data. It should be noted that geographic variations in fluoride exposure occur concomitantly, to both the fetus and the mother, for the entirety of the pediatric and adult vaccination schedules.
Our hypothesis is supported by the analysis of “how fluoride works” by John Yiamouyiannis, who proposes that fluoride inhibits enzymes by disrupting the hydrogen bonds holding the protein in its normal shape [216]. Based on evidence that fluoride inhibits the enzyme acetylcholinesterase “by breaking and reforming hydrogen bonds” [311], Yiamouyiannis suggests that, because fluoride has induced a distortion of the conformation of a protein, the body’s immune system is no longer able to recognize the distorted protein as its own and attempts to destroy it. Since this produces an autoimmune allergic reaction, neurotoxicity would seem to play a role in the increased evidence of autoimmune diseases. In addition, disruption of the hydrogen-bonding of DNA provides still another mechanism by which fluoride might cause damage to the cell. Because these multiple dysfunctional effects concern fluoride toxicity to enzymes or proteins as well as a possible direct effect on the DNA molecule itself, the disruption of cellular communication systems described as “biosemiotic entropy” provides a useful conception for future research in neurotoxicology.
Water exclusion zones are not impervious to fluorides. Rather, evidence suggests that the opposite is true. Living tissue appears to sequester fluorides, aluminum, and aluminofluorides. This likely explains how live tissue, e.g. plant, bioorganic matter, can be used to “filter out” aluminum and fluoride from water [281]. It also explains how live human tissue sequesters fluorides, aluminum, and quite a few other exogenous interfacial water stressors (typically polycationic surfactants), upon oral, parenteral, topical, and inhalational administration. The term sequester derives from Latin sequestrāre: “to put in hands of a trustee” and Late Latin sequestrāre: “to surrender for safekeeping.” In the chemical sense, the word “sequester” denotes undergoing sequestration by forming a stable compound with an ion. Bio-sequestration can be “good” (benevolent) in health or it can be “bad” (malevolent) in the presence of toxicants such as mercury, aluminum, fluoride, and their compound, aluminum fluoride (AlF3).
Examples of bio-sequestrants are ubiquitous. For example, calsequestrin is found in the brain, as well as in skeletal and cardiac muscle [312,313]. Fluoride, from the environment, is thought to form complexes with aluminum, in vivo. Such a mechanism, if it occurs in vivo, is very likely to disturb normal gene expression and cellular functions by means of its effect upon G-protein signaling systems (Figure 7). Strunecká et al., explain how the synergistic action of fluoride with aluminum in the form of AlFx, mediated through its activation of G-proteins, can potentially negatively impact a remarkable number of cell types, including hepatocytes, platelets, RBCs, neurons, photoreceptor cells, neutrophils, lymphocytes, lung endothelial cells, cardiomyocytes, microglial cells, osteoblasts and osteoclasts [104,162,163].
Conclusions
The 21st century more than any previous era, is a chemical age. Toxic chemicals, mostly man-made, present an increasingly complex problem in our environment. The idea of fluoridating the water supply may have “solved” the problem of certain hazardous waste products by representing fluoride as a harmless preventative for tooth decay rather than, as more recently demonstrated theoretically and empirically, as a hazardous systemic cause of disease. The obligatory Hep-B vaccines, administered at birth, all of them containing aluminum and some with mercury as well, both of which interact synergistically with fluoride, and with each other, can only disrupt biological processes, nonlinearly. The ubiquitous Roundup® ready seed crops across the broad spectrum exposed to glyphosate guarantees that it must end up in the soy formula and in breast milk fed to infants.
Biophosphates are known to be principal effectors of cell-cell communication, mediated by G-protein receptors, and they are carriers of energy stored as ATP. As detailed in this paper, the immediate reaction of even trace amounts of aluminum with fluoride to form AlF4− has, due to its close homology with phosphate, catastrophic consequences on signaling cascades, the immune system, phospholipid homeostasis, vascular function, uterine contraction, and energy management. Sphingosine-1-phosphate is a powerful signaling molecule whose messaging is severely disrupted by competing AlF4−. This can lead to conditions like asthma and pulmonary hypertension, as well as premature birth due to false signals causing the contraction of smooth muscle cells.
Fluoride also greatly increases the bioavailability of lead, a well-known environmental toxicant harming our children. All of these factors lead to higher mortality in infants and children. The developing brains of human embryos, neonates, and infants are especially susceptible due to their vulnerability to sequestration of fluorides and polycationic surfactants. Hence, in countries where vaccination, fluoridation, and herbicides applied to the food and water supply are being aggressively increased by policy-makers, it should not be surprising that disorders, diseases, and fatalities in early infancy are correspondingly rising.
As a way of denoting this widespread and highly complex process, the concept of “biosemiotic entropy” usefully emphasizes the underlying destructive consequences of degrading or blocking the informational functions of genes, hormones, neurotransmitters, and other regulatory systems needed by living systems. This concept usefully denotes many synergies between neurotoxins and sources of vulnerability that influence other dysfunctional traits in the health and behavior of adults. The discussion here has focused on neurotoxic effects for rates of infant mortality because the developing brains of the human embryo, neonate, and infant are especially vulnerable to sequestration of fluorides and polycationic surfactants. Whereas policies ostensibly intended to protect consumers have been put in place to limit toxic exposures flowing through medicines, fluoridation, herbicides, genetically modified products, and environmental contaminants in general, the regulators, it seems, are protecting the sources of the very contaminants that are causing measurable deleterious changes such as the mortality rate for infants. When the most medicated of the industrialized (developed) nations of the world has the highest measured IMR, policy-makers, presumably, should be asking why. In addition to the synergistic impact of fluoride and aluminum compounds on IMR, policy-makers should also consider the whole neurological spectrum including such “mysterious” disorders as Gulf War Syndrome, Chronic Fatigue, autism, ADHD and Alzheimer’s disease, and the like. Because toxicant combinations do greater harm than each would in isolation, focus on such synergistic interactions suggests a new paradigm along the lines of Kuhn (1962, 2012) for work within the field of neurotoxicology [314,315].
We therefore urge all parties (persons and agencies) of the U.S. government to reconsider policies regarding the adding of silicofluoride compounds to public water, mercury in amalgams, aluminum and mercury in vaccines, along with toxicants widely being used in herbicides and pesticides. The future of the U.S. and the global community may well depend on whether or not policy-makers in positions of leadership are willing to acknowledge past errors and change course. Scientific evidence, sound theory, and intellectual integrity require changing the public policies that have evidently made the IMR in the U.S. take a dramatic turn in the wrong direction. If U.S. policy-makers do not step up to the demands of reason and compassion, who will?
We thank Ann Lauritzen for contributions to the research and for reading drafts of this paper and providing feedback. This research was funded in part by Quanta Computers, Taipei, Taiwan, under the auspices of the Qmulus Project.
- Masters RD (2009) Cost and effectiveness in American health care: paying for a new Mercedes and getting a clunker. Int J Health Sci 2: 221-226.
- Central Intelligence Agency (2015) The world factbook: infant mortality rate.
- Pletz J, Sánchez-Bayo F, Tennekes HA (2016) Dose-response analysis indicating time-dependent neurotoxicity caused by organic and inorganic mercury—Implications for toxic effects in the developing brain. Toxicology 347-349: 1-5.
- Shaw CA, Seneff S, Kette SD, Tomljenovic L, Oller JW, et al.(2014) Aluminum-induced entropy in biological systems: implications for neurological disease. J Toxicol 491316.
- Dórea JG (2011) Integrating experimental (in vitro and in vivo) neurotoxicity studies of low-dose thimerosal relevant to vaccines. Neurochem Res 36: 927-938.
- CDC (2016) Community water fluoridation, division of oral health.
- Defarge N, Takács E, Lozano V, et al. (2016) Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels. Int J Environ Res Public Health 13: 264.
- Mesnage R, Bernay B, Séralini G-E (2013) Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 313: 122-128.
- Benachour N, Séralini G-E (2009) Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem Res Toxicol 22: 97-105.
- Studnik H, Liebsch S, Forlani G, Wieczorek D, Kafarski P, et al. (2015) Amino polyphosphonates – chemical features and practical uses, environmental durability and biodegradation. New Biotechnol 32: 1-6.
- Solomon KR (2016) Glyphosate in the general population and in applicators: a critical review of studies on exposures. Crit Rev Toxicol 46: 21-27.
- Bus JS (2015) Analysis of Moms Across America report suggesting bioaccumulation of glyphosate in U.S. mother’s breast milk: Implausibility based on inconsistency with available body of glyphosate animal toxicokinetic, human biomonitoring, and physico-chemical data. Regul Toxicol Pharmacol 73: 758-764.
- Nickle RA (1999) Mercury: Top of the hit parade for eight years. Drug Chem Toxicol 22: 129-142.
- Risher JF, Amler SN (2005) Mercury exposure: evaluation and intervention. NeuroToxicology 26: 691-699.
- Yassa HA (2014) Autism: A form of lead and mercury toxicity. Environ Toxicol Pharmacol 38: 1016-1024.
- Leong CCW, Syed NI, Lorscheider FL (2001) Retrograde degeneration of neurite membrane structural integrity of nerve growth cones following in vitro exposure to mercury: Neuroreport 12: 733-737.
- Dórea JG (2015) The neurological effects of prenatal and postnatal exposure to mercury need to include ethylmercury. Chemosphere 139: 667-668.
- Minami T, Oda K, Gima N, Yamazaki H (2007) Effects of lipopolysaccharide and chelator on mercury content in the cerebrum of thimerosal-administered mice. Environ Toxicol Pharmacol 24: 316-320.
- Kang D-W, Park JG, Ilhan ZE, et al. (2013) Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children 8: e68322.
- Nevison CD (2014) A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environ Health 13: 73.
- Haley BE (2005) Mercury toxicity: Genetic susceptibility and synergistic effects. Med Veritas 2: 535-542.
- Kern JK, Geier DA, Bjørklund G, et al. (2014) Evidence supporting a link between dental amalgams and chronic illness, fatigue, depression, anxiety, and suicide. Neuro Endocrinol Lett 35: 537-552.
- Pellionisz AJ (2012) The decade of fractogene: From discovery to utility - proofs of concept open genome-based clinical applications. Int J Syst Cybern Inform 17-28.
- Shaw CA (2016) Neural Dynamics of Neurological Disease. John Wiley.
- Oller JW (2010) The antithesis of entropy: Biosemiotic communication from genetics to human language with special emphasis on the immune systems. Entropy 12: 631-705.
- Oller JW (2014) Biosemiotic entropy: Concluding the series. Entropy 16: 4060-4087.
- Pellionisz AJ (2008) The principle of recursive genome function. The Cerebellum 7: 348-359.
- Masters RD, Hone B, Doshi A (1998) Environmental pollution, neurotoxicity, and criminal violence. In: Rose J, ed. Environmental Toxicology: Current Developments. London, UK: Gordon and Breach 13-48.
- Masters RD (2003) Neurotoxicology and violence. In: Bloom RW, Dess NK, eds. Evolutionary Psychology and Violence: A Primer for Policymakers and Public Policy Advocates. New York, NY: Praeger/Greenwood Publishers Inc 23-56.
- Masters RD, Coplan MJ (2007) The Ten Most Violent Cities in the US (2007 Report). Dartmouth College
- Acosta R (2013) Crime in Flint: A look back at stories from a violent three-year period in the city. MLive.com.
- Macdorman MF, Mathews TJ (2008) Recent trends in infant mortality in the United States. NCHS Data Brief 1-8.
- Manning A (2013) U.S. Top of List for First-Day Deaths in Rich Nations. National Geographic News.
- OECD (2009) OECD in Figures 2009. OECD Observer 2009/Supplement1. Paris: 1-99.
- OECD (2012) OECD Health Data 2012: U.S. health care system from an international perspective.
- OECD (2014) Infant mortality — death per 1,000 live births.
- OECD (2013) Health at a Glance 2013: OECD Indicators.
- New Market Magazine (2015) Rally to Eliminate Fluoride in Flint, Michigan Municipal Water 2/26/15.
- (2016) Flint’s water crisis - fluoridated water is corrosive water. Nat Blogs.
- Koral S (2016) Fluoridation may have worsened lead crisis in Flint, MI. IAOMT.
- (2016) Fluoridation: worsening the lead crisis in Flint, and beyond. Fluoride Action Network.
- (2016) Fluoride Legislative User Information Database (FLUID).
- Lovering EM. In reply to your inquiry of July 9 [1963] about sodium fluoride. Consumer Affairs Inquiries Section, Department of Health Education and Welfare, Food and Drug Administration, Washington, DC, USA. August 1963.
- Plaisier MK (2000) FDA letter to Congress, Dec 21, 2000 - fda-2000a.pdf.
- National Cancer Institute (2016) Toxicant. In: NCI Dictionary of Cancer Terms.
- Schubert J, Riley EJ, Tyler SA (1978) Combined effects in toxicology--a rapid systematic testing procedure: cadmium, mercury, and lead. J Toxicol Environ Health. 4:763-776.
- Innis MD (2013) Autoimmune tissue scurvy misdiagnosed as child abuse. Clin Med Res. 2:154.
- Frieden E, ed. (1984) Biochemistry of the Essential Ultratrace Elements. Boston, MA: Springer US.
- Yeter D, Deth R, Kuo H-C (2013) Mercury Promotes Catecholamines Which Potentiate Mercurial Autoimmunity and Vasodilation: Implications for Inositol 1,4,5-Triphosphate 3-Kinase C Susceptibility in Kawasaki Syndrome. Korean Circ J. 43:581.
- Mutter J, Naumann J, Walach H, Daschner F (2005) Amalgam: Eine Risikobewertung unter Berücksichtigung der neuen Literatur bis 2005. Gesundheitswesen. 67:204-216.
- Mortazavi SMJ, Mortazavi G, Paknahad M (2016) Prenatal low-level mercury exposure and infant neurodevelopment at 12 months in rural northern China. Environ Sci Pollut Res. 23:12480-12481.
- Masters RD, Coplan MJ (1999) Water treatment with silicofluorides and lead toxicity. Int J Environ Stud. 56:435-449.
- Dórea J (2015) Exposure to mercury and aluminum in early life: developmental vulnerability as a modifying factor in neurologic and immunologic effects. Int J Environ Res Public Health.12:1295-1313.
- Dórea JG (2014) Chemical mixtures, maternal exposure and infant neurodevelopment: Did we miss positive (breastfeeding) and negative (mercury) confounders. Neurotoxicol Teratol. 45:93.
- Farina M, Avila DS, da Rocha JBT, Aschner M (2013) Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem Int. 62:575-594.
- Westendorf J. The Kinetics of Acetylcholinesterase Inhibition and the Influence of Fluoride and Fluoride Complexes on the Permeability of Erythrocyte Membranes. English edition. (Coplan MJ, ed.). Hanover, NH (German edition: Hamburg, Germany): Dartmouth College (German edition University of Hamburg); 2000.
- Coplan M, Masters RD (2001) Silicofluorides and fluoridation. Fluoride. 34:161-164.
- Masters RD, Coplan MJ, Hone BT, Dykes JE (2000) Association of silicofluoride treated water with elevated blood lead. Neurotoxicology. 21:1091-1100.
- Masters RD (2001) Biology and politics: Linking nature and nurture. Polsby NW, ed. Rev Polit Sci. 4:45-369.
- Masters RD (2002) MacLean’s evolutionary neuroethology: Environmental pollution, brain chemistry, and violent crime. In: Cory GA, Gardner, Jr. R, eds. The Evolutionary Neuroethology of Paul Maclean: Convergences and Frontiers. New York, NY: Praeger Publishers Inc; 2002:275-296.
- Masters RD (2003) The social implications of evolutionary psychology: Linking brain biochemistry, toxins, and violent crime. In: Bloom RW, Dess N, eds. Evolutionary Psychology and Violence: A Primer for Policymakers and Public Policy Advocates (Psychological Dimensions to War and Peace). Westport CT: Praeger; 2003:23-56.
- Masters RD (2006) Science, bureaucracy, and public policy: Can scientific inquiry prevail over entrenched institutional self-interest. N Engl J Polit Sci 1: 58-140.
- Adams D (2016) Flint still tops in nation for violent crime but officials see signs of hope | MLive.com..
- Gliha LJ (2016) Growing up in America’s most dangerous city, Flint.
- FBI (2016) Crime statistics. FBI.
- Sawan RMM, Leite GAS, Saraiva MCP, Barbosa F Jr, Tanus-Santos JE, et al. (2010) Fluoride increases lead concentrations in whole blood and in calcified tissues from lead-exposed rats. Toxicology 271: 21-26.
- Leite GAS, Sawan RMM, Teófilo JM, Porto IM, Sousa FB, et al. (2011) Exposure to lead exacerbates dental fluorosis. Arch Oral Biol 56: 695-702.
- Seneff S, Swanson N, Li C (2015) Aluminum and glyphosate can synergistically induce pineal gland pathology: connection to gut dysbiosis and neurological disease. Agric Sci 6: 42-70.
- Rozman KK, Doul J (1998) General principles of toxicology. In: Rose J, ed. Environmental Toxicology, Current Developments. First edition. Amsterdam: Gordon/Breach 1-11.
- Oller JW (2013) Pragmatic information. In: Biological Information. World Scientific 64-86.
- Gryder B, Nelson C, Shepard S (2013) Biosemiotic entropy of the genome: mutations and epigenetic imbalances resulting in cancer. Entropy 15: 234-261.
- Oller JW (2014) ed. Biosemiotic Entropy. Special Issue of Entropy 12: 14.
- Davidson RM, Seneff S (2012) The initial common pathway of inflammation, disease, and sudden death. Entropy 14: 1399-1442.
- Dietert R, Dietert J (2012) The completed self: an immunological view of the human-microbiome superorganism and risk of chronic diseases. Entropy 14: 2036-2065.
- Dietert RR (2014) The microbiome in early life: self-completion and microbiota protection as health priorities: The microbiome in early life. Birth Defects Res B Dev Reprod Toxicol 101: 333-340.
- Dietert RR, Silbergeld EK (2015) Biomarkers for the 21st century: Listening to the microbiome. Toxicol Sci 144: 208-216.
- Paul B, Barnes S, Demark-Wahnefried W, et al. (2015) Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics 7.
- Di Ieva A, Esteban FJ, Grizzi F, Klonowski W, Martín-Landrove M (2015) Fractals in the neurosciences, part II: clinical applications and future perspectives. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 21: 30-43.
- Di Ieva A, Grizzi F, Jelinek H, Pellionisz AJ, Losa GA (2014) Fractals in the neurosciences, part I: General principles and basic neurosciences. Neuroscientist 20: 403-417.
- Boland MJ, Nazor KL, Loring JF (2014) Epigenetic regulation of pluripotency and differentiation. Circ Res 115: 311-324.
- Millan MJ (2013) An epigenetic framework for neurodevelopmental disorders: From pathogenesis to potential therapy. Neuropharmacology 68: 2-82.
- Shapshak P, Chiappelli F, Commins D, Singer E, Levine AJ, et al. (2008) Molecular epigenetics, chromatin, and NeuroAIDS/HIV: translational implications. Bioinformation 3: 53.
- Miller NZ, Goldman GS (2011) Infant mortality rates regressed against number of vaccine doses routinely given: is there a biochemical or synergistic toxicity. Hum Exp Toxicol 30: 1420-1428.
- Shaw CA. Dynamics of Neurological Diseases. Wiley-Blackwell; in press.
- Gorbsky GJ (2015) The spindle checkpoint and chromosome segregation in meiosis. FEBS J 282: 2471-2487.
- Ibrahim B (2015) Spindle assembly checkpoint is sufficient for complete Cdc20 sequestering in mitotic control. Comput Struct Biotechnol J 13: 320-328.
- Kurdzo EL, Dawson DS (2015) Centromere pairing - tethering partner chromosomes in meiosis I. FEBS J 282: 2458-2470.
- Marchetti F, Venkatachalam S (2010) The multiple roles of Bub1 in chromosome segregation during mitosis and meiosis. Cell Cycle 9: 58-63.
- Rankin S (2015) Complex elaboration: making sense of meiotic cohesin dynamics. FEBS J 282: 2426-2443.
- Zheng G, Yu H (2015) Regulation of sister chromatid cohesion during the mitotic cell cycle. Sci China Life Sci 58: 1089-1098.
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, et al. (2005) Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57: 128-133.
- Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, et al. (2006) Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery 59: 1086-1092-1093.
- Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP (2010) Chronic traumatic encephalopathy, suicides and parasuicides in professional american athletes: the role of the forensic pathologist. Am J Forensic Med Pathol 31: 130-132.
- Barrio JR, Small GW, Wong KP, Huang SC, Liu J, et al. (2015) In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad Sci 112: E2039-E2047.
- Small GW, Kepe V, Siddarth P, Ercoli LM, Merrill DA, et al. (2013) PET scanning of brain tau in retired national football league players: Preliminary findings. Am J Geriatr Psychiatry 21: 138-144.
- Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T (2013) Role of subconcussion in repetitive mild traumatic brain injury: A review. J Neurosurg 119: 1235-1245.
- Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, et al. (2016) Zika virus associated with microcephaly. N Engl J Med. 374:951-958.
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, et al. (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 18:587-590.
- Tielrooij KJ, Garcia-Araez N, Bonn M, Bakker HJ (2010) Cooperativity in ion hydration. Science. 328:1006-1009.
- Tielrooij KJ, van der Post ST, Hunger J, Bonn M, Bakker HJ (2011) Anisotropic water reorientation around ions. J Phys Chem B.115:12638-12647.
- Pearson RG (1968) Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J Chem Educ. 45:581.
- Duffus JH (2002) “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl Chem. 74:793-807.
- Bigay J, Deterre P, Pfister C, Chabre M (1987) Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J 6: 2907-2913.
- Strunecká A, Strunecký O, Patocka J (2002) Fluoride plus aluminum: useful tools in laboratory investigations, but messengers of false information. Physiol Res Acad Sci Bohemoslov 51: 557-564.
- Darwent B deB.(1970) Bond Dissociation Energies in Simple Molecules. University of California Libraries.
- Duffus JH (2002) “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl Chem 74: 793-807.
- Klopman G (1968) Chemical reactivity and the concept of charge- and frontier-controlled reactions. J Am Chem Soc 90: 223-234.
- Ciavatta L, Iuliano M, Porto R (1988) Fluorosilicate equilibria in acid solution. Polyhedron 7: 1773-1779.
- Finney WF, Wilson E, Callender A, Morris MD, Beck LW (2006) Reexamination of hexafluorosilicate hydrolysis by 19F NMR and pH measurement. Environ Sci Technol 40: 2572-2577.
- Aigueperse J, Mollard P, Devilliers D, et al. (2000) Fluorine compounds, inorganic. In: Wiley-VCH Verlag GmbH & Co. KGaA, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co KGaA 2000.
- Zhang QL, Li MQ, Ji JW, et al. (2011) In vivo toxicity of nano-alumina on mice neurobehavioral profiles and the potential mechanisms. Int J Immunopathol Pharmacol 24: 23S-29S.
- Wu J, Wang C, Sun J, Xue Y (2011) Neurotoxicity of silica nanoparticles: Brain localization and dopaminergic neurons damage pathways. ACS Nano 5: 4476-4489.
- Runnebaum I, Köhler T, Stickeler E, Kieback H, Kreienberg R (1996) p53 mutation is associated with high S-phase fraction in primary fallopian tube adenocarcinoma. Br J Cancer 74: 1157-1160.
- Urbansky ET (2002) Fate of fluorosilicate drinking water additives. Chem Rev 102: 2837-2854.
- Morris MD. The Chemistry of Fluorosilicate Hydrolysis in Municipal Water Supplies. A Review of the Literature and a Summary of University of Michigan Studies. Report to the National Academy of Science. Ann Arbor, MI: The National Academies Press; 2004. [Search domain www.actionpa.org] actionpa.org/fluoride/nrc/NRC-2006.pdf.
- Agmon-Levin N, Hughes GRV, Shoenfeld Y (2012) The spectrum of ASIA: “Autoimmune (Auto-inflammatory) Syndrome induced by Adjuvants.” Lupus 21: 118-120.
- Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56: 304-316.
- Khan Z, Combadière C, Authier FJ, Itier V, Lux F, et al. (2013) Slow CCL2-dependent translocation of biopersistent particles from muscle to brain. BMC Med 11: 99.
- Luján L, Pérez M, Salazar E, Álvarez N, Gimeno M, et al. (2013) Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA syndrome) in commercial sheep. Immunol Res 56: 317-324.
- Varner JA, Jensen KF, Horvath W, Isaacson RL (1998) Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Res 784: 284-298.
- Ahn HW, Fulton B, Moxon D, Jeffery EH (1995) Interactive effects of fluoride and aluminum uptake and accumulation in bones of rabbits administered both agents in their drinking water. J Toxicol Environ Health 44: 337-350.
- Rice JR, Boyd WA, Chandra D, Smith MV, Besten PKD, et al. (2014) Comparison of the toxicity of fluoridation compounds in the nematode Caenorhabditis elegans: Toxicity of fluoride compounds. Environ Toxicol Chem 33: 82-88.
- Sun L, Gao Y, Liu H, Zhang W, Ding Y, et al. (2013) An assessment of the relationship between excess fluoride intake from drinking water and essential hypertension in adults residing in fluoride endemic areas. Sci Total Environ 443: 864-869.
- Niu R, Wang J, Sun Z, Xue X, Yan X, et al. (2015) Transcriptional regulatory dynamics of the hypothalamic-pituitary-testicular axis in male mice exposed to fluoride. Environ Toxicol Pharmacol 40: 557-562.
- Niu R, Xue X, Zhao Y, Sun Z, Yan X, et al. (2015) Effects of fluoride on microtubule ultrastructure and expression of Tubα1a and Tubβ2a in mouse hippocampus. Chemosphere 139: 422-427.
- Niu R, Zhang Y, Liu S, Liu F, Sun Z, et al. (2015) Proteome alterations in cortex of mice exposed to fluoride and lead. Biol Trace Elem Res 164: 99-105.
- Mutter J (2011) Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. J Occup Med Toxicol Lond Engl 6: 2.
- Lorscheider FL, Vimy MJ, Summers AO, Zwiers H (1995) The dental amalgam mercury controversy--inorganic mercury and the CNS; genetic linkage of mercury and antibiotic resistances in intestinal bacteria. Toxicology 97: 19-22.
- Vimy MJ, Hooper DE, King WW, Lorscheider FL (1997) Mercury from maternal “silver” tooth fillings in sheep and human breast milk. A source of neonatal exposure. Biol Trace Elem Res 56: 143-152.
- Vimy MJ, Takahashi Y, Lorscheider FL (1990) Maternal-fetal distribution of mercury (203Hg) released from dental amalgam fillings. Am J Physiol 258: R939-945.
- Dórea JG (2015) Methylmercury in colostrum and milk of Japanese mothers. Chemosphere 137: 221.
- Franco JL, Braga Hde C, Nunes AK, Ribas CM, Stringari J, et al. (2007) Lactational exposure to inorganic mercury: Evidence of neurotoxic effects. Neurotoxicol Teratol 29: 360-367.
- Yang J, Jiang Z, Wang Y, Qureshi IA, Wu XD (1997) Maternal-fetal transfer of metallic mercury via the placenta and milk. Ann Clin Lab Sci 27: 135-141.
- Geier DA, Kern JK, Geier MR (2009) A prospective study of prenatal mercury exposure from maternal dental amalgams and autism severity. Acta Neurobiol Exp (Warsz) 69: 189-197.
- Kern JK, Geier DA, Sykes LK, Geier MR (2015) Relevance of neuroinflammation and encephalitis in autism. Front Cell Neurosci 9. 519.
- American Dental Association (2009) Statement on Dental Amalgam: Statement adopted by the ADA Council on Scientific Affairs, August 2009.
- Snapp KR, Boyer DB, Peterson LC, Svare CW (1989) The contribution of dental amalgam to mercury in blood. J Dent Res 68: 780-785.
- FDA (2015) Thimerosal in vaccines. Thimerosal in vaccines.
- CDC (2016) Birth-18 years immunization schedule. Immunizations Schedules.
- CDC (2016) Vaccines: VPD-VAC/HepB/main page.
- CDC (2015) Thimerosal in flu vaccine-- seasonal influenza (flu).
- James SJ, Slikker W, Melnyk S, New E, Pogribna M, et al. (2005) Thimerosal neurotoxicity is associated with glutathione depletion: protection with glutathione precursors. NeuroToxicology 26: 1-8.
- CDC (2015) Thimerosal in vaccines.
- CDC, ed. Appendix B (2015) Vaccine excipient & media summary, excipients included in U.S. vaccines, by vaccine. In: Centers for Disease Control and Prevention Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th Edition, Apendix B in Grabenstein JD. ImmunoFacts: Vaccines and Immunologic Drugs – 2013 (38th Revision). St Louis, MO: Wolters Kluwer Health; 2015.
- CDC (2013) Seasonal influenza (flu).
- Griffin SO, Regnier E, Griffin PM, Huntley V (2007) Effectiveness of fluoride in preventing caries in adults. J Dent Res 86: 410-415.
- Griffin M, Shickle D, Moran N (2008) European citizens’ opinions on water fluoridation. Community Dent Oral Epidemiol 36: 95-102.
- CDC (1992) Fluoridation Census 1992. Centers for Disease Control and Prevention; 1992.
- CDC (1999) Achievements in public health, 1900-1999: fluoridation of drinking water to prevent dental caries. MMWR 48: 933-940.
- Centers for Disease Control and Prevention (CDC) (2015) CDC - community water fluoridation - oral health.
- Melbye MLR, Armfield JM (2013) The dentist’s role in promoting community water fluoridation: a call to action for dentists and educators. J Am Dent Assoc 1939 144: 65-75.
- Mork N, Griffin S (2015) Perceived safety and benefit of community water fluoridation: 2009 HealthStyles survey: Fluoridation perceived safety and benefit. J Public Health Dent 75: 327-336.
- Armfield JM, Akers HF (2010) Risk perception and water fluoridation support and opposition in Australia. J Public Health Dent 70: 58-66.
- Armfield JM, Akers HF (2010) Community water fluoridation support and opposition in Australia. Community Dent Health 28: 40-46.
- Yiamouyiannis J (1990) Water fluoridation & tooth decay: Results from the 1986-1987 national survey of US schoolchildren. Fluoride 23: 55-67.
- Wasana HMS, Aluthpatabendi D, Kularatne WMTD, Wijekoon P, Weerasooriya R, et al. (2016) Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environ Geochem Health 38: 157-168.
- Reeves TG, CDC (2000) The Manufacture of the Fluoride Chemicals. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention: 1-2.
- Gouider M, Feki M, Sayadi S (2009) Separative recovery with lime of phosphate and fluoride from an acidic effluent containing H3PO4, HF and/or H2SiF6. J Hazard Mater 170: 962-968.
- Ayadi N, Aloulou F, Bouzid J (2015) Assessment of contaminated sediment by phosphate fertilizer industrial waste using pollution indices and statistical techniques in the Gulf of Gabes (Tunisia). Arab J Geosci 8: 1755-1767.
- Cole DE, Baldwin LS, Stirk LJ (1984) Increased inorganic sulfate in mother and fetus at parturition: evidence for a fetal-to-maternal gradient. Am J Obstet Gynecol 148: 596-599.
- Maas RP, Patch SC, Christian AM, Coplan MJ (2007) Effects of fluoridation and disinfection agent combinations on lead leaching from leaded-brass parts. Neurotoxicology 28: 1023-1031.
- Strunecká A, Patočka J, Connett P (2004) Fluorine in medicine. J Appl Biomed 2: 141-150.
- Strunecka A, Patocka J, Blaylock R, Chinoy N (2007) Fluoride interactions: from molecules to disease. Curr Signal Transduct Ther 2: 190-213.
- Patocka J, Cabal J (1999) Toxicology of fluoroacetic acid and similar organofluorine aliphatic compounds. ASA Newslett 99: 16-18.
- Masters RD, Coplan MJ (1999) A dynamic, multifactorial model of alcohol, drug abuse, and crime: linking neuroscience and behavior to toxicology. Soc Sci Inf 38: 591-624.
- Masters RD (2005) A moritorium on silicofluoride usage will save $$millions. Fluoride 38: 1-5.
- Coplan MJ, Patch SC, Masters RD, Bachman MS (2007) Confirmation of and explanations for elevated blood lead and other disorders in children exposed to water disinfection and fluoridation chemicals. Neurotoxicology 28: 1032-1042.
- Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C (2008) Post-vaccination encephalomyelitis: Literature review and illustrative case. J Clin Neurosci 15: 1315-1322.
- FDA (2013) Breast Implants.
- American Chemical Society (2016) Common Chemistry - Substance Details - 7783-61-1 : Silane, tetrafluoro.
- Cohn PD (1992) A Brief Report on the Association of Drinking Water Fluoridation and the Incidence of Osteosarcoma among Young Males. New Jersey Department of Health 1-17.
- Hoover RN, Devessa SS, Cantor KP, Lubin JH, Fraumeni JF (1992) Fluoridation of Drinking Water and Subsequent Cancer Incidence and Mortality. Appendix E in Review of Fluoride Benefits and Risks: Report of the Ad Hoc Subcommittee on Fluoride Committee of the Committee to Coordinate Environmental Health and Related Programs. Washington, D.C: Public Health Service, U.S. Department of Health and Human Service; 1992.
- Bassin EB, Wypij D, Davis RB, Mittleman MA (2006) Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Cancer Causes Control CCC 17: 421-428.
- Burcher S (2005) No to Fluoridation.
- Lee JR (1993) Fluoridation and bone cancer. Fluoride 26: 79-82.
- Connett M (2016) The minimum lethal dose of fluoride. Floride Action Network.
- Weintraub R, Hams G, Meerkin M, Rosenberg AR (1986) High aluminium content of infant milk formulas. Arch Dis Child 61: 914-916.
- Hawkins NM, Coffey S, Lawson MS, Delves HT (1994) Potential aluminium toxicity in infants fed special infant formula. J Pediatr Gastroenterol Nutr 19: 377-381.
- Burrell S-AM, Exley C (2010) There is (still) too much aluminium in infant formulas. BMC Pediatr 10: 63.
- Samsel A, Seneff S (2013) Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: pathways to modern diseases. Entropy 15: 1416-1463.
- Samsel A, Seneff S (2013) Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip Toxicol 6: 159-184.
- Vindigni SM, Zisman TL, Suskind DL, Damman CJ (2016) The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Ther Adv Gastroenterol 9: 606-625.
- Lamb DC, Kelly DE, Hanley SZ, Mehmood Z, Kelly SL (1998) Glyphosate is an inhibitor of plant cytochrome P450: functional expression of Thlaspi arvensae cytochrome P45071B1/reductase fusion protein in Escherichia coli. Biochem Biophys Res Commun 244: 110-114.
- Hietanen E, Linnainmaa K, Vainio H (1983) Effects of phenoxyherbicides and glyphosate on the hepatic and intestinal biotransformation activities in the rat. Acta Pharmacol Toxicol (Copenh) 53: 103-112.
- Nieboer E, Gibson BL, Oxman AD, Kramer JR (1995) Health effects of aluminum: a critical review with emphasis on aluminum in drinking water. Environ Rev 3: 29-81.
- Glass RL (1984) Metal complex formation by glyphosate. J Agric Food Chem 32: 1249-1253.
- Purgel M, Takács Z, Jonsson CM, Nagy L, Andersson I, et al. (2009) Glyphosate complexation to aluminium (III). An equilibrium and structural study in solution using potentiometry, multinuclear NMR, ATR-FTIR, ESI-MS and DFT calculations. J Inorg Biochem 103: 1426-1438.
- Morley WA, Seneff S (2014) Diminished brain resilience syndrome: A modern day neurological pathology of increased susceptibility to mild brain trauma, concussion, and downstream neurodegeneration. Surg Neurol Int 5: 97.
- Hunt DL, Rai SN, Li CS (2008) Summary of dose-response modeling for developmental toxicity studies. Dose Response 6: 352–368.
- Central Intelligence Agency (2013) The world factbook: infant mortality rate.
- Perrin JM, Bloom SR, Gortmaker SL (2007) The increase of childhood chronic conditions in the United States. JAMA 297: 2755-2759.
- Blumberg SJ, Bramlett MD, Kogan MD, Shieve LA, Jones JR, et al. (2013) Changes in Prevalence of Parent-Reported Autism Spectrum Disorder in School-Aged U.S. Children: 2007 to 2011–2012. Hyattsville, MD: National Center for Health Statistics; 2013.
- Parrish TB (2001) Who’s paying the rising cost of special education. J Spec Educ Leadersh 14: 4-12.
- Oller JW, Oller SD (2010) Autism: The Diagnosis, Treatment, & Etiology of the Undeniable Epidemic. 1st ed. Jones & Bartlett Publishers.
- Knapp M, Romeo R, Beecham J (2009) Economic cost of autism in the UK. Autism Int J Res Pract 13: 317-336.
- Zajicek-Farber ML, Lotrecchiano GR, Long TM, Farber JM (2015) Parental perceptions of family centered care in medical homes of children with neurodevelopmental disabilities. Matern Child Health J 19: 1744-1755.
- Garbutt JM, Leege E, Sterkel R, Gentry S, Wallendorf M, Strunk RC (2012) What are parents worried about? Health problems and health concerns for children. Clin Pediatr (Phila) 51: 840-847.
- Graeub R (1992) The Petkau Effect: Nuclear Radiation, People and Trees. 1 edition. New York: Thunder’s Mouth Pr; 1992.
- Graeub R (1994) The Petkau Effect: The Devasting Effect of Nuclear Radiation on Human Health and the Environment. 2 edition. New York: Four Walls Eight Windows.
- Covel C (2001) Sediment, Surface Water and Fish Quality Investigation, Oujé-Bougoumou Cree Nation Territory.
- Dewailly E, Nieboier E (2003) Exposure and Preliminary Health Assessments of the Oujé-Bougoumou Cree Population in Mine Tailings Residues, Report of Survey.
- Needleman H (2004) Lead poisoning. Annu Rev Med 55: 209-222.
- Nevin R (2000) How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ Res 83: 1-22.
- Wright JP, Dietrich KN, Ris MD, et al. (2008) Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med 5: e101.
- CDC (2015) Community Water Fluoridation.
- Li Y, Liang C, Slemenda CW, Ji R, Sun S, et al. (2001) Effect of long-term exposure to fluoride in drinking water on risks of bone fractures. J Bone Miner Res Off J Am Soc Bone Miner Res 16: 932-939.
- Blaylock R (2004) Excitotoxicity: a possible central mechanism in fluoride neurotoxicity. Fluoride 37: 301-314.
- Mullenix PJ (2014) A new perspective on metals and other contaminants in fluoridation chemicals*. Int J Occup Environ Health 20: 157-166.
- Chioca LR, Raupp IM, Da Cunha C, Losso EM, Andreatini R (2008) Subchronic fluoride intake induces impairment in habituation and active avoidance tasks in rats. Eur J Pharmacol 579: 196-201.
- Connett M, Blank T (2015) Fluoride & IQ: the 49 studies. Fluoride Action Network.
- (2006) Committee on Fluoride in Drinking Water, National Research Council, Fluoride in Drinking Water. A Scientific Review of EPA’s Standards. Washington, D.C: The National Academies Press; 2006.
- Rocha-Amador D, Navarro ME, Carrizales L, Morales R, Calderón J (2007) Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saúde Pública 23: S579-587.
- Tang QQ, Du J, Ma HH, Jiang SJ, Zhou XJ (2088) Fluoride and children’s intelligence: a meta-analysis. Biol Trace Elem Res 126: 115-120.
- Choi AL, Sun G, Zhang Y, Grandjean P (2012) Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Perspect 120: 1362-1368.
- Yiamouyiannis J (1993) Fluoride the Aging Factor: How to Recognize and Avoid the Devastating Effects of Fluoride. Delaware OH: Health Action Press.
- Yiamouyiannis J (1994) Fluoride the Aging Factor: How to Recognize and Avoid the Devastating Effects of Fluoride. 2nd ed. Delaware, Ohio: Health Action Press.
- Wedeen RP (1984) Poison in the Pot : The Legacy of Lead. Diane Pub Co; 1984.
- Yiamouyiannis J (1977) Fluoridation and cancer. Lancet Lond Engl 2: 296.
- Yiamouyiannis J (1978) Cancer mortality and fluoridation. Lancet Lond Engl 1: 150.
- Crabbe JC, Wahlsten D, Dudek BC (1999) Genetics of mouse behavior: interactions with laboratory environment. Science 284: 1670-1672.
- Beltrán-Aguilar ED, Barker L, Dye BA(2010) Prevalence and Severity of Dental Fluorosis in the United States, 1999–2004. Natl Cent Health Stat Data Brief 53: 1-8.
- Beltrán-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, et al. (2002) Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis--United States, 1988-1994 and 1999-2002. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 54: 1-43.
- Augusto Tocchini de Figueiredo F, Gerlach RF, Andreia Mesquita Silva da Veiga M, Nakadi FV, Ramos J, et al. (2014) Reduced bone and body mass in young male rats exposed to lead. BioMed Res Int 2014: 1-5.
- Avramovski P, Avramovska M, Sikole A (2016) Bone strength and arterial stiffness impact on cardiovascular mortality in a general population. J Osteoporos 2016 :e7030272.
- Buenzli PR, Sims NA (2015) Quantifying the osteocyte network in the human skeleton. Bone 75: 144-150.
- Simon MJK, Beil FT, Rüther W, Busse B, Koehne T, et al. (2014) High fluoride and low calcium levels in drinking water is associated with low bone mass, reduced bone quality and fragility fractures in sheep. Osteoporos Int 25: 1891-1903.
- (2012) Maximum Contaminant Levels for Inorganic Contaminants. 40 CFR 141.62: 2012.
- Heller KE, Eklund SA, Burt BA (1997) Dental caries and dental fluorosis at varying water fluoride concentrations. J Public Health Dent 57: 136-143.
- Embassy US (2013) U.S. Embassy Hanoi Sponsors First-Ever National Symposium and Workshop on Autism.
- Ngo CU, Tamoon E, Hoang TH, Suarit R (2011) Fluoride content of milk-based powdered formula in HoChiMinh City, Vietnam.
- (2013) Autism on the rise in Vietnam: conference. Thanh Nien Daily.
- Nguyen VTT, Law MG, Dore GJ (2008) An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int off J Int Assoc Study Liver 28: 525-531.
- Gallagher CM, Goodman MS (2010) Hepatitis B vaccination of male neonates and autism diagnosis, NHIS 1997-2002. J Toxicol Environ Health A 73: 1665-1677.
- Nazarowec-White M, Farber JM (1997) Enterobacter sakazakii: a review. Int J Food Microbiol 34: 103-113.
- Yan QQ, Condell O, Power K, Butler F, Tall BD, et al. (2012) Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium: A review of Cronobacter species. J Appl Microbiol 113: 1-15.
- Dart RC, Hurlburt KM, Boyer-Hassen LV (2003) Lead. In: Dart RC, ed. Medical Toxicology. Third edition. Philadelphia: LWW; 2003.
- Palkiewicz P, Zwiers H, Lorscheider FL (1994) ADP-ribosylation of brain neuronal proteins is altered by in vitro and in vivo exposure to inorganic mercury. J Neurochem 62: 2049-2052.
- Davidson PW, Myers GJ, Weiss B (2004) Mercury exposure and child development outcomes. Pediatrics 113: 1023-1029.
- Tomljenovic L (2011) Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link. J Alzheimers Dis JAD 23: 567-598.
- Shaw CA, Petrik MS (2009) Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J Inorg Biochem 103: 1555-1562.
- Bohrer D, Oliveira SMR, Garcia SC, Nascimento PC, Carvalho LM (2010) Aluminum loading in preterm neonates revisited. J Pediatr Gastroenterol Nutr 51: 237-241.
- Shaw CA, Li D, Tomljenovic L (2014) Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy 6: 1055-1071.
- Hewitt CD, Savory J, Wills MR (1990) Aspects of aluminum toxicity. Clin Lab Med 10: 403-422.
- Seneff S, Davidson R, Liu J (2012) Empirical data confirm autism symptoms related to aluminum and acetaminophen exposure. Entropy14: 2227-2253.
- Heintze U, Edwardsson S, Dérand T, Birkhed D (1983) Methylation of mercury from dental amalgam and mercuric chloride by oral streptococci in vitro. Scand J Dent Res 91: 150-152.
- Stricklin GP, Jeffrey JJ, Roswit WT, Eisen AZ (1983) Human skin fibroblast procollagenase: mechanisms of activation by organomercurials and trypsin. Biochemistry (Mosc 22: 61-68.
- Gaggar A, Hector A, Bratcher PE, Mall MA, Griese M, et al. (2011) The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur Respir J 38: 721-727.
- Dworetz AR (2013) End of Life, at Birth. The New York Times. August 4, 2013: A17.
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, et al. (2008) Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320: 1332-1336.
- Obeso D, Pezza RJ, Dawson D (2014) Couples, pairs, and clusters: mechanisms and implications of centromere associations in meiosis. Chromosoma 123: 43-55.
- Duro E, Marston AL (2015) From equator to pole: splitting chromosomes in mitosis and meiosis. Genes Dev 29: 109-122.
- Gladstone MN, Obeso D, Chuong H, Dawson DS (2009) The synaptonemal complex protein zip1 promotes bi-orientation of centromeres at meiosis i. Hawley RS, ed. PLoS Genet 5: e1000771.
- Pasteur L (1864) On spontaneous generation. Rev Cours Sci 1863-64: 257-264.
- Pasteur L (1980) On the extension of the germ theory to the etiology of certain common diseases.
- Suwalsky M, Norris B, Villena F, Cuevas F, Sotomayor P, et al. (2004) Aluminum fluoride affects the structure and functions of cell membranes. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 42: 925-933.
- Bondy SC (2010) The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology 31: 575-581.
- Bondy SC (2014) Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration. Toxicology 315: 1-7.
- Sternweis PC, Gilman AG (1982) Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A 79: 4888-4891.
- Martin RB (1988) Ternary hydroxide complexes in neutral solutions of Al3+ and F-. Biochem Biophys Res Commun 155: 1194-1200.
- Martin RB (1986) Ternary complexes of Al3+ and F− with a third ligand. Coord Chem Rev 149: 23-32.
- Health Canada Government of Canada (2004) Canadian Environmental Protection Act, 1999: Priority Substances List Assessment Report for Aluminum Chloride, Aluminum Nitrate and Aluminum Sulfate. 2004:122.
- Rall TW, Sutherland EW (1958) Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem 232: 1065-1076.
- Brudevold F, Moreno E, Bakhos Y (1972) Fluoride complexes in drinking water. Arch Oral Biol 17: 1155-1163.
- Agarwal RP, Moreno EC (1971) Stability constants of aluminium fluoride complexes. Talanta 18: 873-880.
- Bleau G, Lalumiùre G, Chapdelaine A, Roberts K (1975) Red cell surface structure. Stabilization by cholesterol sulfate as evidenced by scanning electron microscopy. Biochim Biophys Acta 375: 220-223.
- Lai JC, Blass JP (1984) Inhibition of brain glycolysis by aluminum. J Neurochem 42: 438-446.
- Xu ZX, Fox L, Melethil S, Winberg L, Badr M (1990) Mechanism of aluminum-induced inhibition of hepatic glycolysis: inactivation of phosphofructokinase. J Pharmacol Exp Ther 254: 301-305.
- Belli WA, Buckley DH, Marquis RE (1995) Weak acid effects and fluoride inhibition of glycolysis by Streptococcus mutans GS-5. Can J Microbiol 41: 785-791.
- Lau KH, Farley JR, Freeman TK, Baylink DJ (1989) A proposed mechanism of the mitogenic action of fluoride on bone cells: inhibition of the activity of an osteoblastic acid phosphatase. Metabolism 38: 858-868.
- Cimasoni G (1966) Inhibition of cholinesterases by fluoride in vitro. Biochem J 99: 133-137.
- Bottini N, Bottini E, Gloria-Bottini F, Mustelin T (2002) Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch Immunol Ther Exp (Warsz) 50: 95-104.
- Yorio T, Sinclair R, Henry S (1981) Fluoride inhibition of the hydro-osmotic response of the toad urinary bladder to antidiuretic hormone. J Pharmacol Exp Ther 219: 459-463.
- Pogwizd SM, Bers DM (2002) Na/Ca exchange in heart failure: contractile dysfunction and arrhythmogenesis. Ann N Y Acad Sci 976: 454-465.
- McIvor ME, Cummings CE, Mower MM, Wenk RE, Lustgarten JA, et al. (1987) Sudden cardiac death from acute fluoride intoxication: the role of potassium. Ann Emerg Med 16: 777-781.
- Ji TH, Grossmann M, Ji I (1998) G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem 273: 17299-17302.
- Grigorenko BL, Shadrina MS, Topol IA, Collins JR, Nemukhin AV (2008) Mechanism of the chemical step for the guanosine triphosphate (GTP) hydrolysis catalyzed by elongation factor Tu. Biochim Biophys Acta 1784: 1908-1917.
- Graham DL, Eccleston JF, Chung CW, Lowe PN (1999) Magnesium fluoride-dependent binding of small G proteins to their GTPase-activating proteins. Biochemistry (Mosc) 38: 14981-14987.
- Gether U, Kobilka BK (1998) G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem 273: 17979-17982.
- Li L (2003) The biochemistry and physiology of metallic fluoride: action, mechanism, and implications. Crit Rev Oral Biol Med Off Publ Am Assoc Oral Biol 14: 100-114.
- Aaltonen N, Lehtonen M, Varonen K, Goterris GA, Laitinen JT (2012) Lipid phosphate phosphatase inhibitors locally amplify lysophosphatidic acid LPA1 receptor signalling in rat brain cryosections without affecting global LPA degradation. BMC Pharmacol 12: 7.
- Lubkowska A, Zylu B, Chlubek D (2002) Interactions between fluorine and aluminum. Fluoride 35: 73-77.
- Pyne NJ, Tonelli F, Lim KG, Long JS, Edwards J, et al. (2012) Sphingosine 1-phosphate signalling in cancer. Biochem Soc Trans 40: 94-100.
- Means CK, Brown JH (2009) Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res 82: 193-200.
- Roossien FF, de Rijk D, Bikker A, Roos E (1989) Involvement of LFA-1 in lymphoma invasion and metastasis demonstrated with LFA-1-deficient mutants. J Cell Biol 108: 1979-1985.
- Driessens MH, van Hulten PE, van Rijthoven EA, Soede RD, Roos E (1997) Activation of G-proteins with AIF-4 induces LFA-1-mediated adhesion of T-cell hybridoma cells to ICAM-1 by signal pathways that differ from phorbol ester- and manganese-induced adhesion. Exp Cell Res 231: 242-250.
- Agalakova NI, Gusev GP (2012) Fluoride induces oxidative stress and ATP depletion in the rat erythrocytes in vitro. Environ Toxicol Pharmacol 34: 334-337.
- Agalakova NI, Gusev GP (2013) Transient activation of protein kinase C contributes to fluoride-induced apoptosis of rat erythrocytes. Toxicol In Vitro 27: 2335-2341.
- Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, et al. (2000) FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol Baltim Md 164: 5761-5770.
- Chiba Y, Sakai H, Misawa M (2001) Augmented acetylcholine-induced translocation of RhoA in bronchial smooth muscle from antigen-induced airway hyper-responsive rats. Br J Pharmacol 133: 886-890.
- Chiba K, Matsuyuki H, Maeda Y, Sugahara K (2006) Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol 3: 11-19.
- Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y (2003) Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol 23: 1534-1545.
- Xie W, Stribley JA, Chatonnet A, Wilder PJ, Rizzino A, et al. (2000) Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholinesterase. J Pharmacol Exp Ther 293: 896-902.
- Worek F, Koller M, Thiermann H, Szinicz L (2005) Diagnostic aspects of organophosphate poisoning. Toxicology 214: 182-189.
- Somlyo AP, Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325-1358.
- Yang E, Cho JY, Sohn UD, Kim IK (2010) Calcium sensitization induced by sodium fluoride in permeabilized rat mesenteric arteries. Korean J Physiol Pharmacol Off J Korean Physiol Soc Korean Soc Pharmacol 14: 51-57.
- Yang E, Jeon SB, Baek I, Song M-J, Yoon Y-R, Kim IK (2010) Fluoride induces vascular contraction through activation of RhoA/Rho kinase pathway in isolated rat aortas. Environ Toxicol Pharmacol 29: 290-296.
- Zhou H, Murthy KS (2004) Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol 286: C1130-1138.
- Krupka RM (1966) Fluoride inhibition of acetylcholinesterase. Mol Pharmacol 2: 558-569.
- Leiber D, Banno Y, Tanfin Z (2007) Exogenous sphingosine 1-phosphate and sphingosine kinase activated by endothelin-1 induced myometrial contraction through differential mechanisms. Am J Physiol Cell Physiol 292: C240-250.
- McCormick MC, Behrman RE (2007) The quiet epidemic of premature birth: commentary on a recent Institute of Medicine report. Ambul Pediatr Off J Ambul Pediatr Assoc 7: 8-9.
- World Health Organization (2012) Born Too Soon: The Global Action Report on Preterm Birth. WHO.
- Selye H (1966) Thrombohemorrhagic Phenomena. Charles C. Thomas; 1966.
- Hans Selye (1967) In Vivo: The Case for Supramolecular Biology. Liveright; 1967.
- Luke J (2001) Fluoride deposition in the aged human pineal gland. Caries Res 35: 125-128.
- Cheng KK, Chalmers I, Sheldon TA (2007) Adding fluoride to water supplies. BMJ 335: 699-702.
- Pizzo G, Piscopo MR, Pizzo I, Giuliana G (2007) Community water fluoridation and caries prevention: a critical review. Clin Oral Investig 11: 189-193.
- Charone S, Bertolini MM, Gonçalves RM, Loivos AC, Grizzo L, et al. (2012) Lack of a significant relationship between toenail fluoride concentrations and caries prevalence. Fluoride 45: 133-137.
- Wang SX, Wang ZH, Cheng XT, Li J, Sang ZP, et al. (2007) Arsenic and fluoride exposure in drinking water: children’s IQ and growth in Shanyin county, Shanxi province, China. Environ Health Perspect 115: 643-647.
- Chen RT, Glasser JW, Rhodes PH, Davis RL, Barlow WE, et al. (1997) Vaccine Safety Datalink project: a new tool for improving vaccine safety monitoring in the United States. The Vaccine Safety Datalink Team. Pediatrics 99: 765-773.
- Goldman GS, Miller NZ (2012) Relative trends in hospitalizations and mortality among infants by the number of vaccine doses and age, based on the Vaccine Adverse Event Reporting System (VAERS), 1990-2010. Hum Exp Toxicol 31: 1012-1021.
- Froede HC, Wilson IB (1985) The slow rate of inhibition of acetylcholinesterase by fluoride. Mol Pharmacol 27: 630-633.
- Royer L, Ríos E (2009) Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J Physiol 587: 3101-3111.
- Takei K, Stukenbrok H, Metcalf A, et al. (1992) Ca2+ stores in Purkinje neurons: endoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. J Neurosci Off J Soc Neurosci 12: 489-505.
- Kuhn TS, Hacking I (2012) The Structure of Scientific Revolutions: 50th Anniversary Edition. Fourth edition. Chicago ; London: University of Chicago Press; 2012.
- Kuhn T (1962) The Structure of Scientific Revolutions. 2nd ed. Chicago: University of Chicago Press; 1962.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
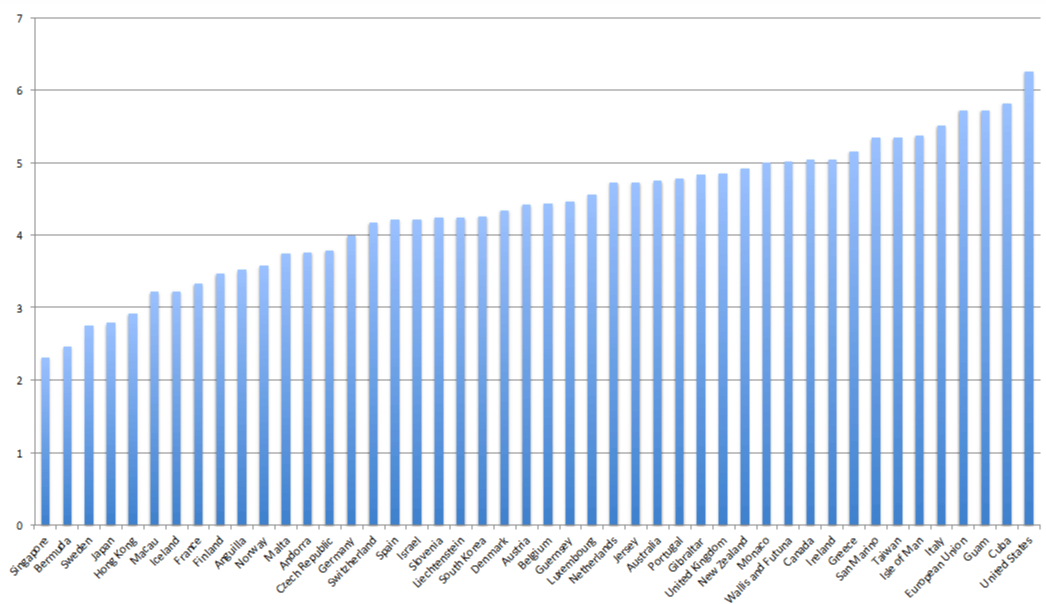
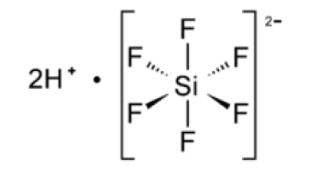
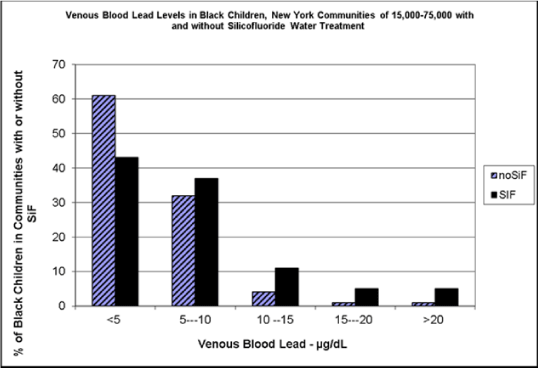
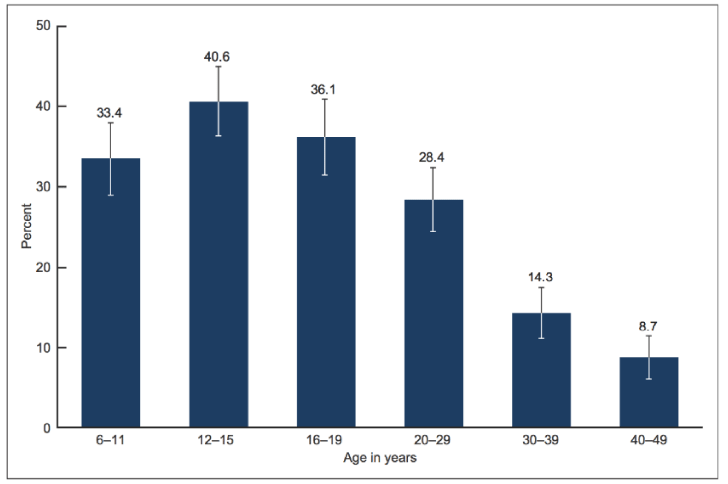
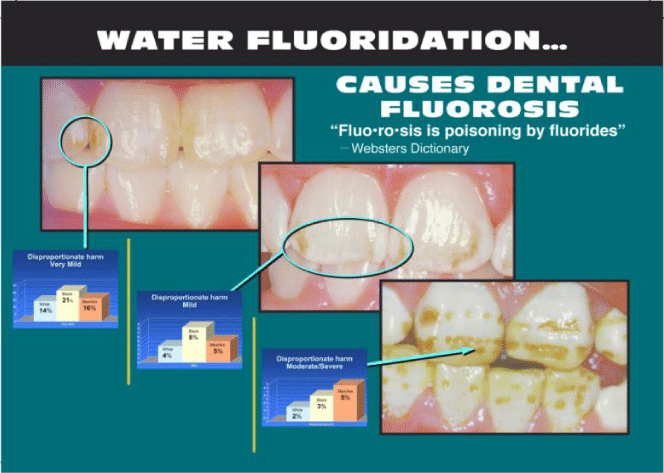

 Save to Mendeley
Save to Mendeley
