Open Journal of Bacteriology
Hericium erinaceus powder inhibits the growth of Porphyromonas gingivalis
Hirono Ichikawa, Junya Kawai and Koichiro Mori*
Cite this as
Ichikawa H, Kawai J, Mori K (2021) Hericium erinaceus powder inhibits the growth of Porphyromonas gingivalis. Open J Bac 5(1): 017-020. DOI: 10.17352/ojb.000020Disruption of the oral microbiota may contribute to the pathogenesis of oral diseases such as gingivitis. It is desired to explorer for a natural material that is safe for the body and specifically suppresses the growth of periodontopathic bacteria. Hericium erinaceus, which is consumed as food or used as a herbal medicine in East Asia, has been previously reported to inhibit bacterial growth. However, few studies addressed its effects on oral bacteria. Here we determined the effects of H. Erinaceus Powder (HEP) on the periodontal pathogenic bacterium Porphyromonas gingivalis and a nonpathogenic commensal bacterium Streptococcus gordonii. The antibacterial activity of HEP were evaluated by colony forming unit assay. As a result, HEP significantly inhibited the growth of P. gingivalis in a concentration-dependent manner, but in contrast, did not exhibited significant antibacterial activity against S. gordonii. H. erinaceus may selectively inhibit the growth of P. gingivalis and be useful for prevention of periodontal disease.
Abbreviations
ATCC: American Type Culture Collection; CFU: Colony Forming Unit; eTSB: Enriched Tryptic Soy Broth; HEP: Hericium Erinaceus Powder; P. gingivalis: Porphyromonas gingivalis; S. gordonii: Streptococcus gordonii
Introduction
Maintenance of the oral microbiota contributes to health. Homeostasis between resident oral microbes may be disrupted by external factors such as diet, smoking, and consumption of antibiotics [1]. Disruption of the oral microbiota may contribute to the pathogenesis of oral diseases such as gingivitis [2]. The periodontal bacterium Porphyromonas gingivalis causes gingivitis and periodontitis [3]. Streptococcal species, which dominate the bacterial population of the human oral cavity, comprise beneficial commensals such as Streptococcus sanguinis, Streptococcus cristatus, Streptococcus mitis, Streptoccus oralis, and Streptococcus gordonii. Commensal streptococci inhibit the growth of pathogenic species and maintain oral health [4]. For example, S. gordonii generates alkali from arginine in salivary and dietary substrates to raise the pH of an acidic cariogenic microenvironment [5]. Furthermore, S. gordonii generates H2O2, which suppresses the proliferation of pathogens such as P. gingivalis [6]. Cetylpyridinium chloride and chlorhexidine inhibit the growth of beneficial as well as pathogenic oral bacteria [7]. Moreover, certain antibiotics may lead to an increase in the populations of antibiotic-resistant bacteria [8]. Inappropriate use of antibiotics may cause adverse effects such as allergic reactions and end-organ toxic effects [9]. Therefore, safe and specific antibiotics that inhibit the growth of oral bacterial pathogens are required. Certain mushrooms possess antimicrobial activity [10-13]. For example, H. erinaceus, known as Yamabushitake in Japan, has served since ancient times as food and natural medicine in East Asia. We found that H. erinaceus possesses strong antibacterial activity against P. gingivalis, consistent with the findings of others [14-16]. However, little information is available about the antibacterial potential of H. erinaceus against oral bacteria. Here we used a colony forming assay to determine the effects of H. erinaceus on oral bacteria.
Material and methods
Materials and bacterial strains
Fruit bodies of H. erinaceus cultivated by the Hokuto Corporation (Nagano, Japan) were freeze-dried and milled to prepare H. Erinaceus Powder (HEP). The bacterial strains P. gingivalis ATCC 33277 and S. gordonii ATCC 10558 were purchased from the American Type Culture Collection (ATCC: Manassas, VA, USA) and cultured under anaerobic conditions at 37ºC in enriched Tryptic Soy Broth (eTSB; Difco Laboratories, Detroit, MI, USA) containing 1 g/L yeast extract (BD, Franklin Lakes, NJ, USA) supplemented with 0.005 g/L hemin (Wako Pure Chemical industries, Ltd., Osaka, Japan) and 0.001 g/L menadione (Wako Pure Chemical industries, Ltd., Osaka, Japan).
Colony Forming Unit (CFU) assay
After two passages from thawing of frozen stocks of P. gingivalis and S. gordonii, the bacterial cells were diluted 1/20 (v/v) with eTSB medium, and 500 µL of the bacterial suspensions and 500 µL of HEP suspensions (0.25 –4 mg/mL) were poured into a 24-well plate and incubated at 37°C for 3, 6, 9, and 24 h. Sterile water was used as a control instead of the HEP suspension. After incubation with HEP, bacteria were harvested and serially diluted in degassed phosphate-buffered saline (pH 7.4). P. gingivalis was plated on Trypto-Soya agar (Nissui, Tokyo, Japan) containing 5% (v/v) defibrinated sheep blood (Nippon Bio test laboratories INC., Saitama, Japan). S. gordonii was plated on Trypto-Soya agar without sheep blood. Plates were incubated under anaerobic conditions at 37°C, and the colonies were counted.
Statistical analysis
Data were expressed as the means ± standard deviation (S.D.). All statistical analysis were performed using EZR (v1.42) [17]. Data were analyzed using one-way analysis of variance (ANOVA) with Dunnett’s test, and p < 0.05 indicates a significant difference.
Results
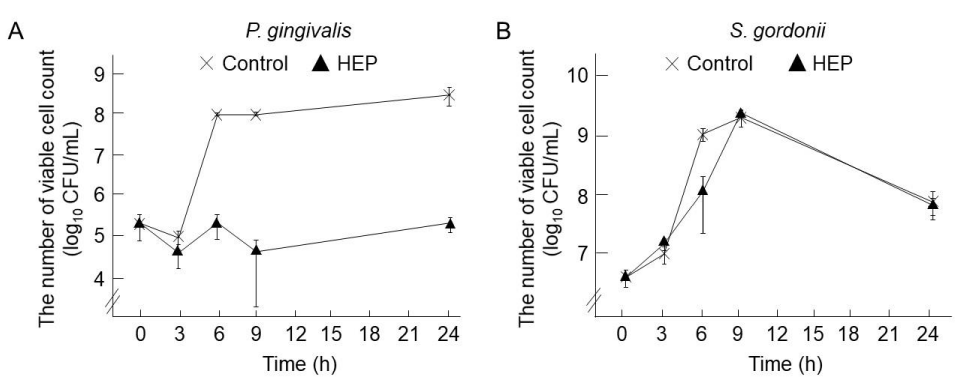
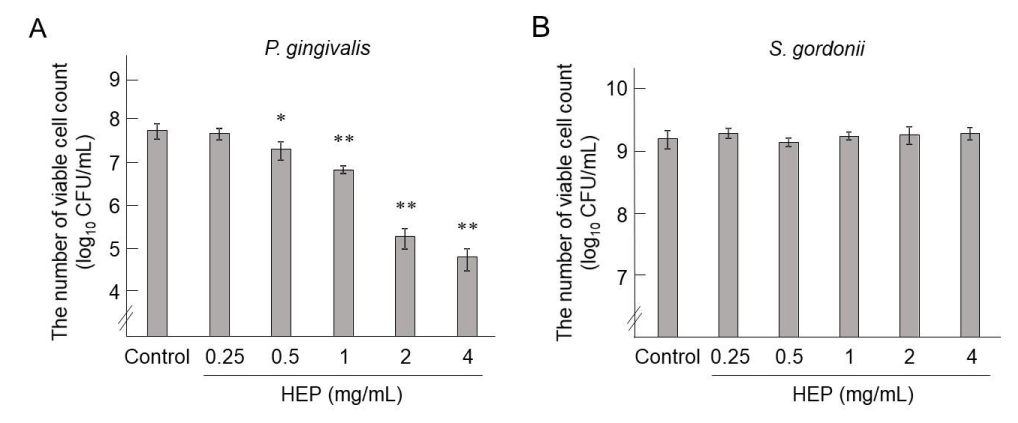
Figure 1 shows the CFU/mL of P. gingivalis and S. gordonii cultured with or without 4 mg/mL of HEP for 3, 6, 9, and 24 h. The growth of P. gingivalis was almost completely inhibited by 4 mg/mL of HEP after 6, 9, and 24 h. In contrast, in the absence of HEP, the CFUs increased approximately from 105 to 108 cells in 24 h (Figure 1-A). The peak number (9 h) of CFUs of the S. gordonii culture was not affected by HEP, although the growth rate seemed to be decreased from 3 h to 6 h in the presence of HEP (Figure 1-B). We next determined the growth rates of P. gingivalis and S. gordonii as a function of HEP concentration after 9-h incubation. The numbers of viable P. gingivalis were as follows: 5.4 ± 2.0 × 107 CFU/mL (control), 4.5 ± 1.3 × 107 CFU/mL (HEP 0.25 mg/mL), 2.0 ± 0.9 × 107 CFU/mL (HEP 0.5 mg/mL), 6.6 ± 1.3 × 106 CFU/mL (HEP 1 mg/mL), 2.0 ± 1.0 × 105 CFU/mL (HEP 2 mg/mL), and 6.5 ± 3.4 × 104 CFU/mL (HEP 4 mg/mL) (Figure 2-A). HEP, from 0.5 mg/mL to 4 mg/mL, significantly inhibited the growth of P. gingivalis. The numbers of S. gordonii were as follows: 1.5 ± 0.5 × 109 CFU/mL (control), 1.9 ± 0.3 × 109 CFU/mL (HEP 0.25 mg/mL), 1.3 ± 0.2 × 109 CFU/mL (HEP 0.5 mg/mL), 1.7 ± 0.2 × 109 CFU/mL (HEP 1 mg/mL), 1.8 ± 0.6 × 109 CFU/mL (HEP 2 mg/mL), and 1.9 ± 0.4 × 109 CFU/mL (HEP 4 mg/mL) (Figure 2-B). The growth of S. gordonii was not inhibited by HEP at concentrations ranging from 0.25 mg/mL to 4 mg/mL.
Discussion
These results suggest HEP inhibits the growth of P. gingivalis, but not that of S. gordonii. Streptococci include many beneficial commensal species, which are associated with the homeostasis of the human oral cavity [4]. Furthermore, S. gordonii mitigates the effects of P. gingivalis on the host’s inflammatory response and the epithelial-mesenchymal transition through regulation of signalling in host epithelial cells [18]. Periodontitis participates in an increased systematic pro-inflammatory state and associated with several systemic diseases, such as diabetes, atherosclerosis, myocardial infarction, stroke and cognitive impairment [19,20]. Therefore, it is thought that maintenance of oral microbiota is very important for health and longevity. Indeed, significant correlation between severe periodontitis and all-cause and cardiovascular mortality was reported in previous study [21,22]. Therefore, the selective antibacterial activity of HEP will likely contribute to preventing periodontal disease and lowering the risk for systemic diseases.
In this study, there is no data to explain the cause of a selective antibacterial effect of HEP on P. gingivalis. On the other hand, it is known that many antibacterial drugs show different strengths in Gram-positive and Gram-negative bacteria [23]. Although further research is needed, we speculate that one possible cause may be the difference between the anti-bacterial effect of HEP on gram-negative bacterium (P. gingivalis) and that on gram-positive bacterium (S. gordonii). Aqueous and methanol extracts, and the lipid-soluble erinacines of H. erinaceus exhibit antibacterial activity [14-16]. Thus, H. erinaceus may comprise multiple active compounds. Further investigations are required to identify the active compounds produced by H. erinaceus and to determine the mechanisms underlying their antibacterial activities. As for the safety of H. erinaceus, it has been eaten in East Asia since ancient times, and no adverse effects have been reported in a human clinical trial [24].
In conclusion, our data indicate that H. erinaceus may prevent periodontal disease without causing serious adverse effects.
We thank Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
- Sharma N, Bhatia S, Sodhi SA, Batra N (2018) Oral microbiome and health. AIMS Microbiol 4: 42-66. Link: https://bit.ly/3ricSE6
- Curtis MA, Diaz PI, Van-Dyke TE (2020) The role of the microbiota in periodontal disease. Periodontol 2000 83: 14-25. Link: https://bit.ly/3B91Y86
- Silva N, Abusleme L, Bravo D, Dutzan N, Garcia JS, et al. (2015) Host response mechanisms in periodontal diseases. J Appl Oral Sci 23: 329-355. Link: https://bit.ly/3eITvz1
- Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, et al. (2018) Biology of oral streptococci. MIcrobiol Spectr 6: GPP3-0042-2018. Link: https://bit.ly/3z2xKlg
- He J, Hwang G, Liu Y, Gao L, Liverman LK, et al. (2016) L-Arginine modifies the exopolysaccharide matrix and thwarts streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol 198: 2651-2661. Link: https://bit.ly/3kqDVve
- Herrero ER, Slomka V, Bernaerts K, Boon N, Sanabria EH, et al. (2016) Antimicrobial effects of commensal oral species are regulated by environmental factors. J Dent 47: 23-33. Link: https://bit.ly/3hPbS7n
- Verspecht T, Herrero ER, Khodaparast L, Khodaparast L, Boon N, et al. (2019) Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci Rep 9: 8326. Link: https://go.nature.com/3z5CmY0
- Ready D, Lancaster H, Qureshi F, Bedi R, Mullany P, et al. (2004) Effect of amoxicillin use on oral microbiota in young children. Antimicrob Agents Chemother 48: 2883-2887. Link: https://bit.ly/3emf87J
- Tamma PD, Avdic E, Li DX, Dzintans K, Cosgrove SE (2017) Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 177: 1308-1315. Link: https://bit.ly/3rgBnkJ
- Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, et al. (2012) A review on antimicrobial activity of mushroom (basidiomycetes) extracts and isolated compounds. Planta Med 78: 1707-1718. Link: https://bit.ly/3knMMOv
- Gabreyohannes G, Nyerere A, Bii C, Sbhatu DB (2019) Determination of antimicrobial activity of extracts of indigenous wild mushrooms against pathogenic organisms. Evid Based Complement Alternat Med 2019: 1-7. Link: https://bit.ly/3xLOtZY
- Kosanić M, Ranković B, Dašić M (2012) Mushrooms as possible antioxidant and antimicrobial agents. Iran J Pharm Res 11: 1095-1102. Link: https://bit.ly/3BbftUz
- Solmaz G, Ozen F, Ekinci Y, Bird PS, Korachi M (2013) Inhibitory and disruptive effects of shiitake mushroom (Lentinula edodes) essential oil extract on oral biofilms. Jundishapur J Microbiol 6: e9058. Link: https://bit.ly/36DnbJc
- Kwak AM, Min KJ, Lee SY, Kang HW (2015) Water extract from spent mushroom substrate of Hericium erinaceus suppresses bacterial wilt disease of Tomato. Mycobiology 43: 311-318. Link: https://bit.ly/3wJI8wK
- Shen T, Morlock G, Zorn H (2015) Production of cyathane type secondary metabolites by submerged cultures of Hericium erinaceus and evaluation of their antibacterial activity by direct bioautography. Fungal Biol Biotechnol 2: 8. Link: https://bit.ly/2Ti3mnO
- Wong KH, Sabaratnam V, Abdullah N, Kuppusamy RU, Naidu M (2009) Effects of cultivation techniques and processing on antimicrobial and antioxidant activities of Hericium erinaceus (Bull.:Fr.) pers. Extracts. Food Technol Biotechnol 47: 47-55. Link: https://bit.ly/3xJSpKx
- Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458. Link: https://bit.ly/3ifzlNY
- Ohshima J, Wang Q, Fitzsimonds ZR, Miller DP, Sztukowska MN, Jung YJ, et al. (2019) Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc Natl Acad Sci USA 116: 8544-8553. Link: https://bit.ly/3if2AjL
- Sanz M, Castillo AM, Jepsen S, Juanatey JRG, D’Aiuto F, et al. (2020) Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol 47: 268-288. Link: https://bit.ly/36Jms9v
- Ide M, Harris M, Stevens A, Sussams R, Hopkins V, et al. (2016) Periodontitis and cognitive decline in alzheimer’s disease. PLoS One 11: e0151081. Link: https://bit.ly/3kDq0lZ
- Linden GJ, Lyons A, Scannapieco FA (2013) Periodontal systemic associations: Review of the evidence. J Clin Periodontol 40: S8–19. Link: https://bit.ly/3erXgIV
- Sharma P, Dietrich T, Ferro CJ, Cockwell, P, Chapple IL (2016) Association between periodontitis and mortality in stages 3–5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol 43: 104–113. Link: https://bit.ly/3kqtoAy
- Breijyeh Z, Jubeh B, Karaman R (2020) Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 25: 1340. Link: https://bit.ly/36COhjG
- Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T (2009) Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res 23: 367-372. Link: https://bit.ly/3rcsdFW

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
