Open Journal of Bacteriology
Study of Bacillus megaterium potential application for high metal content residues biotreatment
Andrea M Rivas-Castillo1,2 Yessica Mejía-Escobedo1, and Norma G Rojas-Avelizapa1*
2Universidad Tecnológica de Zona Metropolitana del Valle de México, Boulevard Miguel Hidalgo y Costilla 5, Los Héroes de Tizayuca, Tizayuca, Hgo., 43816, México
Cite this as
Rivas-Castillo AM, Mejía-Escobedo Y, Rojas-Avelizapa NG (2018) Study of Bacillus megaterium potential application for high metal content residues biotreatment. Open J Bac 2(1): 004-008. DOI: 10.17352/ojb.000007Previous work has demonstrated that Bacillus megaterium, besides showing an elevated resistance to heavy metals, possesses the ability to remove metals like Ni, Hg, and V from liquid media. Notably, Ni represents a highly valuable metal commonly found in industrial wastes, whose removal from environment is crucial, as it is considered a dangerous toxic metal. Thus, the aim of the present study was to determine Bacillus megaterium MNSH1-9K-1 resistance to Al, Ni, V, and Mo, metals that are commonly found in high metal content residues, and also evaluate its Ni removal capability from liquid medium. To this end, the microorganism was exposed to different metal concentrations, and its resistance was assayed by serial dilutions and viable count. Afterwards, MNSH1-9K-1 Ni resistance and removal capability was evaluated by ICP-OES. The results showed that this microorganism presents a Minimum Inhibitory Concentrations of 240, 2000, 35000 and >270000 ppm for Ni, Al, V, and Mo, respectively. Even more, MNSH1-9K-1 is able to remove up to 26 ppm of Ni. The results presented here may contribute to the knowledge regarding the application of this microorganism for the biotreatment of high metal content liquid and solid residues, mainly the ones containing Ni.
Introduction
The treatment of high metal content liquid and solid residues has been gaining importance worldwide, as they represent a major threat to ecosystems and human health, due to its accumulative capacity [1], emerging then the imminent need of developing efficient methods to minimize or even eliminate metals prior to its discharge to the environment. Although there are treatments currently available to reduce the metal content from solid and liquid wastes, they consider the utilization of chemical compounds that generate more residues [2]. Hence, it is important to develop more eco-friendly methods to treat and/or recycle such materials. Thus, biotechnological approaches are an innovative and promising technology applicable for the removal of heavy metals from solid and liquid residues, that has been proved as an effective economically feasible technology [3,4], due to their low costs and higher efficiency at low metal concentrations, where physicochemical removal methods fail [5,6].
It has been previously reported that Bacillus megaterium possesses high levels of resistance to hostile conditions [7,8], including the exposition to metals like Hg and Ni [9,10], and some B. megaterium strains also present the demonstrated ability to remove metals from solid and liquid residues [10-12], establishing their potential to treat phosphogypsum waste [13], and spent catalysts [14,15]. Specifically, B. megaterium strain MNSH1-9K-1 presents the ability to resist up to 200 ppm of each Ni and V contained in a liquid mixture, being able to remove up to 15.38 ± 1.30 ppm of Ni contained in the former liquid medium [12].
Metals like Ni, Al, Mo, and V are commonly present in spent catalysts [14], being Ni and Al often found as water pollutants too, by reason of being used in numerous industrial processes. Furthermore, Ni is considered a highly valuable metal for modern industry, mainly due to its expanding need as a crucial component of stainless steel [16]. Although Ni has biological activity in bacteria at nanomolar concentrations [17-19], it is also considered as a highly toxic metal [20], by the aim of causing the elevated production of reactive oxygen species and DNA damage [21]. In the case of Al, its embryotoxic effects in animal models and its neurotoxic effects in humans have been demonstrated, suggesting that this metal exposition may cause specific encephalopathy with a dementia syndrome [22].
Thus, the current study analyzed the resistance of Bacillus megaterium strain MNSH1-9K-1 to Al, Ni, V, and Mo, metals that are commonly found in high metal content residues, and also to evaluate MNSH1-9K-1 Ni removal potential from liquid media at different concentrations. The results presented in this manuscript may contribute to the knowledge of B. megaterium resistance to diverse toxic metals, and evenmore, to evaluate is potential for the biotreatment of high Ni content residues.
Materials and Methods
Bacterial strain and growth conditions
Bacillus megaterium strain MNSH1-9K-1 (GenBank accession number KM654562.1) was used for this study, which isolation from a high metal content site has been previously described [15]. LB liquid medium was used for microbial growth at 200 rpm and 37°C. Inoculums were prepared overnight, and microbial density was adjusted to an Optical Density at a wavelength of 600 nanometers (O.D.600 nm) = 0.1 to begin experimentation.
Metal resistance
Cultures were grown for 48 hours in 125 ml Erlenmeyer flasks containing 10 ml of LB liquid medium and different concentrations of Al, Mo, V, or Ni, provided as AlCl3, (NH4)6Mo7O24·4H2O, NaVO3, and Ni(NO3)2·6H2O, respectively, as specified for each experiment. Also, samples without metals were included, as controls. Viability was assessed by taking a 100 µl aliquot from each culture, and serial 10-fold dilutions were prepared in phosphate-buffered saline (PBS) buffer [23], followed by plating on LB solid medium. Finally, LB plates were incubated for 24-48 h at 37 °C to perform colony counting.
Ni removal from liquid medium
Experimental sets were prepared in 125 ml Erlenmeyer flasks containing LB liquid medium supplemented with different Ni concentrations, to a 10 ml final volume. Also, samples without metals were included, as controls. After 24 hours of growth at 37°C and 200 rpm, samples were centrifuged at 5000 rpm for 10 min in order to separate cells from the supernatant, biomass was discarded and the liquid phase of each sample was filtered using a cellulose acetate syringe filter (Alltech, Deerfield, IL, USA) to remove any cell debris remaining in the samples. Subsequently, the samples were acid digested. For this purpose, 1 ml samples were placed in cylindrical silicon carbide vials, and 6 ml of concentrated HNO3 and 2 ml of concentrated HCl were added, and samples were digested in a microwave reaction system (Multiwave PRO, Anton Paar), using an HF100 rotor. Digestion conditions were: 600 W for 6 vessels, 40 bar, 210-240°C, with pRate of 0.3 bar sec-1, ramp 15 min, hold 15 min, and cooling 15 min. Afterwards, 20 ml of deionized water was added to the cylindrical vial, and the supernatant was collected and filled up to 100 ml with deionized water. Metal analysis was performed at 231.604 nm by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES; Varian Model 710-ES). To determine Ni removal by B. megaterium, metal concentration remaining in liquid medium was calculated based on a calibration curve covering 0.1-10 mg kg-1, using a commercial standard (High-Purity, cat. # ICP-200-7-6) [12].
Statistical analyses
Basic statistical parameters and analyses of variance (ANOVA) were performed using the commercial statistical software OriginPro 9.0. Differences with P values of ≤ 0.05 were considered statistically significant.
Results and Discussion
It has been previously reported that members of the genus Bacillus present high heavy metal resistance properties [24-26]. Particularly, it was reported that Bacillus megaterium strain MNSH1-9K-1 possesses the ability to remove 149.5 mg/kg of Ni, and 127.5 mg/kg of V from a spent catalyst at a 16% (w/v) of pulp density [15]. Besides, MNSH1-9K-1 may tolerate up to 200 ppm of each Ni and V contained together in liquid medium [12], and removed up to 15.38 mg/kg of Ni from the latter system.
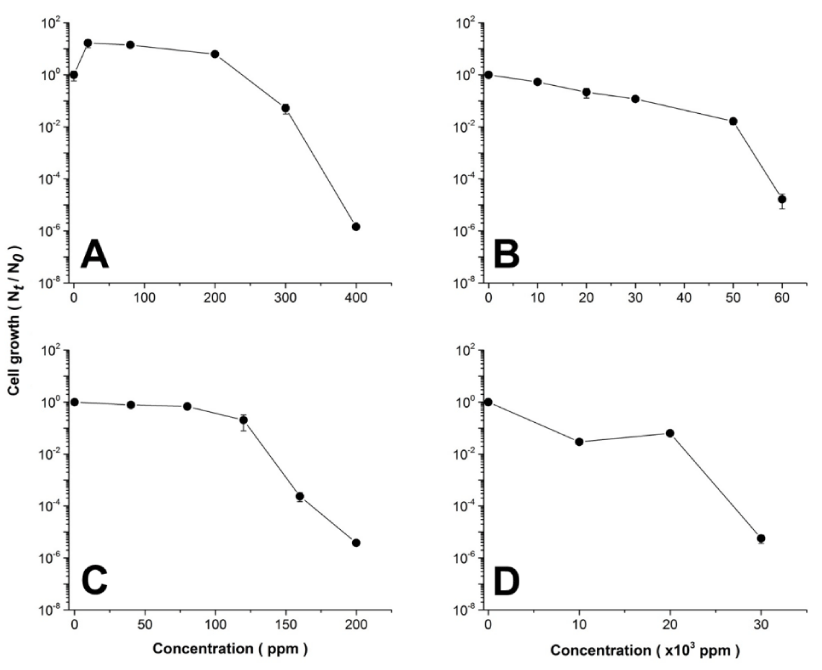
Thus, in order to evaluate the ability of B. megaterium MNSH1-9K-1 to resist diverse concentrations of metals commonly encountered in solid and liquid high metal content residues, namely Al, Mo, Ni, and V, the strain was subjected to grow for 24 h in the presence of each metal at the different concentrations specified for each case, and cell viability was evaluated following the protocol described in Materials and Methods. As it can be observed in figure 1, B. megaterium MNSH1-9K-1 presented metal resistance following the ascendant order of Ni < Al < V < Mo, dictated by the respective median lethal doses of 87 ± 3, 248 ± 1, 1925 ± 247, and 10,400 ± 566 ppms calculated from figure 1A to 1D. Furthermore, Minimum Inhibitory Concentrations (MIC) were also calculated for each metal, and results are shown in table 1, where the metal resistance pattern Ni < Al < V < Mo was corroborated. In the case of Mo, it was not possible to obtain a specific value for Mo, because it was experimentally impossible to dissolve more (NH4)6Mo7O24·4H2O to obtain higher concentrations and reach the MIC value.
The results are in accordance to previous studies, where it has been demonstrated the elevated toxicity of Ni above other metals [21,27,28], since this metal has been directly related to alterations in DNA repair mechanisms, epigenetic effects, and carcinogenesis [29], besides the generation of reactive oxygen species (ROS), which has been broadly related to metal toxicity [30, 31]. It is interesting to note that an enhanced growth was observed when cells were subjected to 25, 75, and 200 ppm of Al. In this respect, it has been reported that many metals may have biological activities [32], participating in chemical processes, cell structures, and biological functions [33], so it may be the case that Al at these three concentrations is promoting B. megaterium growth, possibly by participating somehow in an up to date non specified metabolic function in this microorganism.
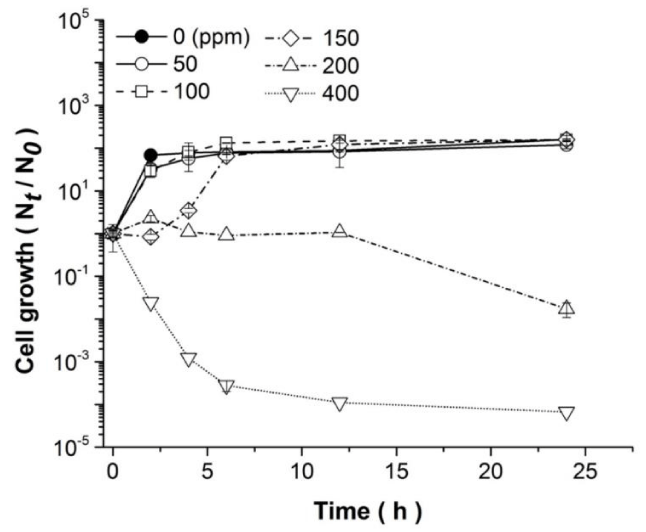
Due to Ni enhanced toxicity to living organisms, and hence the need of developing efficient approaches for its removal, B. megaterium MNSH1-9K-1 Ni resistance and removal abilities were studied in further detail. First, MNSH1-9K-1 growth kinetics were determined in the presence of five different Ni concentrations, comparing them with the growth behavior without the metal. To this end, the strain was subjected to grow for 24 hours in the presence of 50, 100, 150, 200, and 400 ppm of Ni at 37 °C and 200 rpm in 125 ml Erlenmeyer flasks containing LB liquid medium at a final volume of 10 mL, and the results are shown in figure 2. Microorganism growth was observed very similar to the control when 50 and 100 ppm of Ni were added to the medium. However, a lag phase of approximately 2 hours was observed when the strain was exposed to 150 ppm of the metal, although a subsequent growth recovery was achieved. Contrastingly, the strain was only able to tolerate 200 ppm of the metal, but not capable of growing under the stress generated by this Ni concentration, and a 50-fold decrease in cell viability was observed after 24 h of growth. Furthermore, B. megaterium MNSH1-9K-1 had an evident viability loss since the beginning of the experiment when exposed to 400 ppm of Ni, reaching up to a 1600-fold decrement in cell survival.
Previous studies show that Bacillus megaterium strain MNSH1-9K-1 possesses the ability to tolerate up to 200 ppm of Ni contained in a liquid Ni-V mixture [12]. The results presented here demonstrate the ability of MNSH1-9K-1 to grow in the presence of up to 150 ppm of Ni, and support the fact of this strain Ni tolerance at 200 ppm, evidencing also the elevated viability loss at 400 ppm of the metal.
It has been documented that the B. megaterium genome contains several open reading frames of genes involved in stress responses, including oxidative stress, osmotic stress, heat shock, and detoxification [34], which activations, at least in its most studied partner B. subtilis, overlap between general stress responses and more specific pathways, and confer the microorganism with efficient adaptation mechanisms [35]. In this regard, extracytoplasmic sigma (σ) factors regulate several overlapped stress responses. For example, σW regulon is induced by various cell wall antibiotics, alkaline shock, and other stresses affecting the cell envelope, and σM regulon activation may confer advantages to diverse stress factors, including high salinity, ethanol, heat, acid, phosphate starvation, superoxide stress, and exposure to cell wall antibiotics such as bacitracin, vancomycin, and cationic antimicrobial peptides [36]. In the case of B. megaterium, it has been reported that strain uyuni S29 presents a high capability to produce poly [(R)-3-hydroxybutyrate] (PHB) at excessive salinity conditions [37]. Hence, the differences in cell survival when B. megaterium is exposed to diverse Ni concentrations may be due to the diverse molecular mechanisms activated under the stress caused by each Ni concentration. Particularly, the strain was able to adapt at 150 ppm of Ni and resume growth, being evident that this adaptation ability is exceeded at 200 and 400 ppm of Ni under the conditions tested.
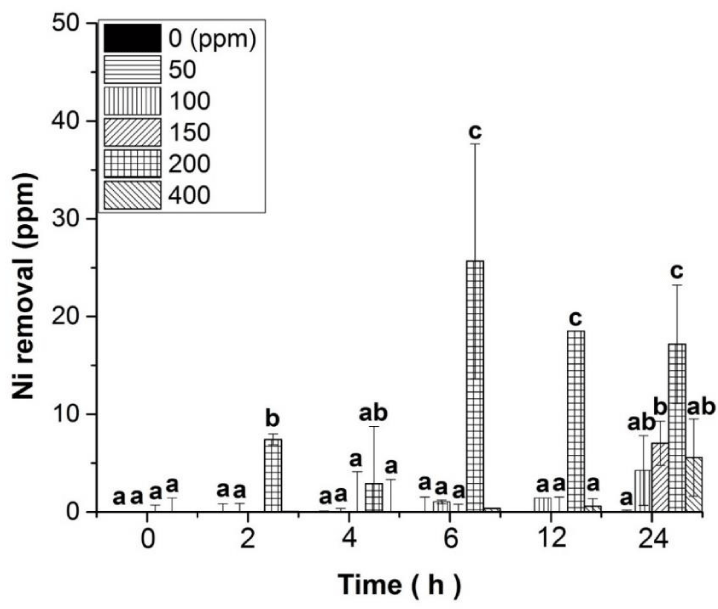
Once B. megaterium MNSH1-9K-1 Ni resistance was determined, analyses were performed to evaluate the ability of this microorganism to remove Ni from liquid medium at the same concentrations tested before (figure 3), observing that Ni removal was significantly obtained when the strain was subjected to grow in the presence of 200 ppm of the metal. This removal was evident since hour 2, and the highest Ni removal of up to 26 ppm was observed from hour 6 and beyond.
Reports have previously shown that B. megaterium possesses high metal sorption capacity [1], and also Ni specific response genes like nccA and smtAB have been correlated to Ni removal by MNSH1-9K-1 [12]. Hence, as the uppermost Ni removal was accomplished at 200 ppm of Ni, even though cell population was 100-times less than in 50 to 150 ppm, the results strongly suggest that the removal of this metal may be accomplished by metabolism components, mainly activated by the stress generated by 200 ppm of Ni.
To date, little is known about the molecular mechanisms involved in the removal of Ni or other toxic metals from high content metal residues [12]. In consequence, transcriptome, proteome, and metabolome approaches are necessary perspectives to further understand the mechanisms involved in B. megaterium Ni removal capability, to enhance and further promote the use of this microorganism for the biotreatment of high Ni content residues.
Conclusion
The current study shows that B. megaterium MNSH1-9K-1 presents a metal resistance pattern of Ni < Al < V < Mo. Besides, the results strongly suggest that this microorganism presents the potential to be used for the biotreatment of high Ni content residues.
This project was supported by Grant 131203 from CONACyT, Mexico.
Conflict of interest
The authors state that they have no conflict of interest in connection with the publication of this article.
The authors declare that there are no sources of funding for this work.
- Monachese M, Burton JP, Reid G (2012) Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl Environ Microbiol 78: 6397-6404. Link: https://tinyurl.com/y8gfmtdc
- Akcil A, Vegliò F, Ferella F, Okudan MD, Tuncuk A (2015) A review of metal recovery from spent petroleum catalysts and ash. Waste Manage 45: 420–433. Link: https://tinyurl.com/yc9ko4gu
- Ameer Basha S, Rajaganesh K (2014) Microbial bioremediation of heavy metals from textile industry dye effluents using isolated bacterial strains. Int J Curr Microbiol App Sci 3: 785–794. Link: https://tinyurl.com/yaggpgez
- Coelho LM, Rezende HC, Coelho LM, de Sousa PAR, Melo DFO, et al. (2015) Advances in bioremediation of wastewater and polluted soil. In: Shiomi, N., editor. InTechOpen, Rijeka, Croatia 1-274. Link: https://tinyurl.com/ya3gyttr
- Mejáre M, Bülow L (2001) Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19: 67–73. Link: https://tinyurl.com/ydg8t8y8
- Perpetuo EA, Souza CB, Nascimento CAO (2011) Engineering bacteria for bioremediation. In: Carpi, A., editor. Progress in molecular and environmental bioengineering - from analysis and modeling to technology applications. InTechOpen, Rijeka, Croatia: 605-632. Link: https://tinyurl.com/y8srokcs
- Salgaonkar BB, Mani K, Braganca JM (2013) Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J Appl Microbiol 114: 1347–1356. Link: https://tinyurl.com/yaksthrg
- Pal KK, Dey R, Sherathia D, Vanpariya S, Patel I, et al. (2014) Draft genome sequence of a moderately halophilic Bacillus megaterium strain, MSP20.1, isolated from a saltern of the little rann of Kutch, India. Genome Announc 2: 01134-13. Link: https://tinyurl.com/y93rtjn5
- Narita M, Chiba K, Nishizawa H, Ishii H, Huang CC, et al. (2003) Diversity of mercury resistance determinants among Bacillus strains isolated from sediment of Minamata Bay. FEMS Microbiol Lett 223: 73–82. Link: https://tinyurl.com/yb7pkko8
- Rajkumar M, Ma Y, Freitas H (2013) Improvement of Ni phytostabilization by inoculation of Ni resistant Bacillus megaterium SR28C. J Environ Manage 128: 973–980. Link: https://tinyurl.com/y9ykgqml
- Chien M, Nakahata R, Ono T, Miyauchi K, Endo G (2012) Mercury removal and recovery by immobilized Bacillus Megaterium MB1. Front Chem Sci Eng 6: 192–197. Link: https://tinyurl.com/y7ojglsw
- Fierros-Romero G, Rivas-Castillo AM, Gómez-Ramírez M, Pless RC, Rojas-Avelizapa NG (2016) Expression analysis of Ni- and V-associated resistance genes in a Bacillus megaterium strain isolated from a mining site. Curr Microbiol 73: 165–171. Link: https://tinyurl.com/y8qwvkdh
- Stefanescu IA (2015) Bioaccumulation of heavy metals by Bacillus megaterium from phosphogypsum waste. Scientific Study and Research 16: 93–97. Link: https://tinyurl.com/yanvqano
- Gómez-Ramírez M, García-Martínez L, Fierros-Romero G, Rojas-Avelizapa NG (2016) Evaluation and identification of microorganisms able to remove Ni and V from spent catalyst. In: Biohidrometallurgy Proceedings. Falmouth, Cornwall, UK. Link: https://tinyurl.com/y8u83ldx
- Arenas-Isaac G, Gómez-Ramírez M, Montero-Álvarez LA, Tobón-Avilés A, Fierros-Romero G, et al. (2017) Novel microorganisms for the treatment of Ni and V of spent catalysts. Indian J Biotechnol 16: 370-379. Link: https://tinyurl.com/y9dnt7dl
- Sigel A, Sigel H, Sigel RKO (2007) Nickel and its surprising impact in nature. In: Sigel A, Sigel H, Sigel RKO, editors. Metal ions in life sciences, Volume 2. Wiley InterScience, New Jersey, United States: 1-728. Link: https://tinyurl.com/y8zhnpvp
- Bartha R, Ordal EJ (1965) Nickel-dependent chemolithothrophic growth of two hydrogenomonas strains. J Bacteriol 89: 1015–1019. Link: https://tinyurl.com/y9znbcy9
- Olson JW, Mehta NS, Maier RJ (2001) Requirement of nickel metabolism proteins HypA and HypB for full activity of both hydrogenase and urease in Helicobacter pylori. Mol Microbiol 39:176–182. Link: https://tinyurl.com/yam9n595
- Mulrooney SB, Hausinger RP (2003) Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev 27: 239–261. Link: https://tinyurl.com/yc9wk76t
- Cempel M, Nikel G (2006) Nickel: A review of its sources and environmental toxicology. Polish J Environ Stud 15: 375–382. Link: https://tinyurl.com/yap7vpyg
- Latvala S, Hedberg J, Di Bucchianico S, Möller L, Odnevall Wallinder I, et al. (2016) Nickel release, ROS generation and toxicity of Ni and NiO micro- and nanoparticles. PLoS ONE 11: 0159684. Link: https://tinyurl.com/yc7z5x54
- Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, et al. (2017) The health effects of aluminum exposure. Dtsch Arztebl Int 114: 653–659. Link: https://tinyurl.com/y75klwjb
- Nicholson WL, Setlow P (1990) Sporulation, germination and outgrowth. In: Harwood C.R., Cutting S.M., editors. Molecular biological methods for Bacillus. John Wiley and Sons. Chichester, U.K 391-450.
- Yilmaz E (2003) Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Res Microbiol 154: 409–415. Link: https://tinyurl.com/yalrerjt
- Singh SK, Tripathi VR, Jain RK, Vikram S, Garg SK (2010) An antibiotic, heavy metal resistant and halotolerant Bacillus cereus SIU1 and its thermoalkaline protease. Microb Cell Fact 9: 1-11. Link: https://tinyurl.com/y8suzkzp
- Elsilk SE, El-Raheem A, El-Shanshoury R, Ateya PS (2014) Accumulation of some heavy metals by metal resistant avirulent Bacillus anthracis PS2010 isolated from Egypt. Afr J Microbiol Res 8: 1266–1276. Link: https://tinyurl.com/y8t6ddc2
- Amer AM (2002) Processing of Egyptian boiler-ash for Extraction of vanadium and nickel. Waste Manage 22: 515–520. Link: https://tinyurl.com/yc3688fv
- Caicedo M, Jacobs JJ, Reddy A, Hallab NJ (2008) Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni2+ and V3+ are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+. J Biomed Mater Res A 86: 905–913. Link: https://tinyurl.com/y92n8n4e
- Macomber L, Hausinger RP (2011) Mechanisms of nickel toxicity in microorganisms. Metallomics 3: 1153–1162. Link: https://tinyurl.com/ybebvwjt
- Stohs S, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radical Bio Med 18: 321–336. Link: https://tinyurl.com/ychuxxh7
- Geslin C, Llanos J, Prieur D, Jeanthon C (2001).The manganese and iron superoxide dismutases protect Escherichia coli from heavy metal toxicity. Res Microbiol 152: 901–905. Link: https://tinyurl.com/ybbvcve5
- Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122: 109-119. Link: https://tinyurl.com/ycmx993s
- Shilev S, Naydenov M, Sancho-Prieto M, Vassilev N, Sancho ED (2012) PGPR as inoculants in management of lands contaminated with trace elements. In: Maheshwari D., editor. Bacteria in Agrobiology: Stress Management. Springer, Heidelberg, Germany: 259-277. Link: https://tinyurl.com/y93na7p6
- Liu L, Li Y, Zhang J, Zou W, Zhou Z, et al. (2011) Complete genome sequence of the industrial strain Bacillus megaterium WSH-002. J Bacteriol 193: 6389–6390. Link: https://tinyurl.com/ya4npuz5
- Young JW, Locke JC, Elowitz MB (2013) Rate of environmental change determines stress response specificity. Proc Natl Acad Sci USA 110: 4140-4145. Link: https://tinyurl.com/y8fyemh6
- Mascher T, Hachmann AB, Helmann JD (2007) Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function factors. J Bacteriol 189: 6919-6927. Link: https://tinyurl.com/ydxdgje8
- Rodríguez-Contreras A, Koller M, Braunegg G, Marqués-Calvo MS (2016) Poly [(R)-3-hydroxybutyrate] production under different salinity conditions by a novel Bacillus megaterium strain. New Biotechnol 33: 73-77. Link: https://tinyurl.com/yc4uk6ay

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
