Open Journal of Analytical and Bioanalytical Chemistry
Dissipation kinetics and the pre-harvest residue of chlorantraniliprole in pigeon pea Cajanus cajan L. succulent pods Using Ultra-High-Performance Liquid Chromatography with Photodiode array detector (UHPLC-PDA)
Murali Krishna T1, Devaki K1*, Kiran Kumar K2 and Prasanthi L3
2Project Associate, Pesticide Residue Testing Lab, IFT, RARS, Tirupati, India
3Associate Director of Research, RARS, Tirupati, India
Cite this as
Murali Krishna T, Devaki K, Kiran Kumar K, Prasanthi L (2022) Dissipation kinetics and the pre-harvest residue of chlorantraniliprole in pigeon pea Cajanus cajan L. succulent pods Using Ultra-High-Performance Liquid Chromatography with Photodiode array detector (UHPLC-PDA). Open J Anal Bioanal Chem 6(1): 013-017. DOI: 10.17352/ojabc.000025Copyright License
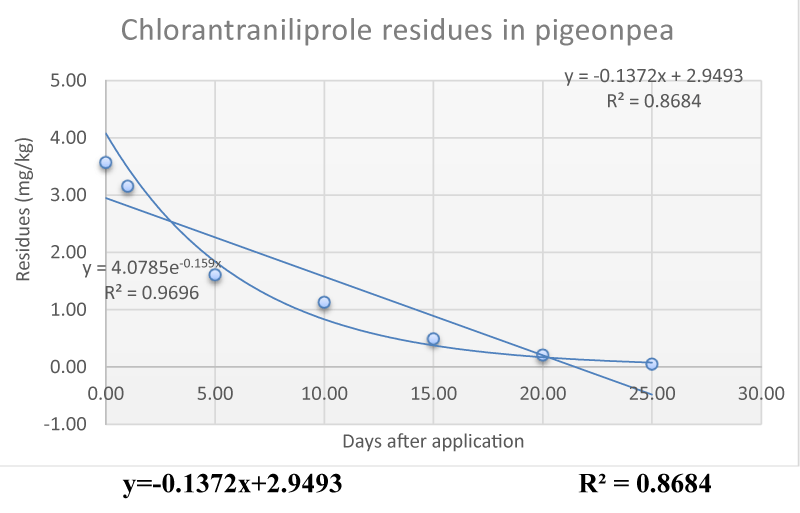
© 2022 Murali Krishna T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Studies were conducted to evaluate insecticide residues of Chlorantraniliprole in pigeon pea succulent pods after foliar application. Chlorantraniliprole was sprayed at 0.6ml/l on pigeon pea crop at the pod formation stage to control pod borers like Helicoverpa armigera and Maruca vitrata. Samples were drawn at 0, 1, 5, 10, 15, 20, 25, and 30 days after spray. A validated liquid chromatography method with a Photodiode Array (PDA) detector was used for the residue analysis of chlorantraniliprole. Separation was achieved through the C18 column in the reverse phase. The calibration graphs of chlorantraniliprole in acetonitrile solvent or five blank matrices were linear within the tested interval of 0.1 to 1ppm with a coefficient of determination over 0.9990. Dissipation of chlorantraniliprole was studied in first-order kinetic models (for which the coefficient of determination, R2 was 0.8684). Residues of chlorantraniliprole was 3.57, 3.16, 1.61, 1.13, 0.49, 0.21, 0.05 and 0.00mg/kg at 0, 1, 5, 10, 15, 20, 25 and 30 days after spraying. The residue level reached below the tolerance limit (MRL) (2mg/kg BW) of Codex Alimentarius Commission (CAC) on Pesticide Residues after 5 days of spray and below the detectable level after 25 days of spray in succulent pods and recorded a biological half-life of 4.36 days.
Introduction
Chlorantraniliprole [3-bromo-N-[4-chloro-2-methyl-6- [(methyl-amino) carbonyl] phenyl]-1-(3-chloro-2-pyridinyl) -1H-pyrazole-5-caroxamide] is a novel insecticide that belongs to the Anthranilic diamide class. It is a potent and selective activator of insect ryanodine receptors, which are critical for muscle contraction [1-3]. Activation of ryanodine receptors in insects affects calcium homeostasis by the unregulated release of internal calcium in the cell, leading to the cessation of larval feeding, lethargy, muscle paralysis, and ultimately death of the insect [4]. Chlorantraniliprole is extremely potent against lepidopterous pests, including those resistant to neonicotinoid and pyrethroid insecticides [5]. It is also effective against dipteran, hemipteran, and coleopteran pests [6]. The higher efficacy of chlorantraniliprole has been reported against pod borers in pulses [7-10]. Besides, bio-efficacy studies about residues, dissipation patterns, biological half-life, and safe waiting periods have also been conducted in various fields, including horticultural crops. Even though it is the most commonly applied insecticide in legume crops like pigeon pea against borer pests, viz., Helicoverpa armigera, Maruca vitrata, etc., However, no study of its contributions to environmental degradation of Chlorantraniliprole has been reported so far. The studies related to environmental degradation of chlorantraniliprole in pulse crops like pigeon pea, black gram, green gram, field bean, and cowpea are not conducted which are being consumed as both succulent pods and as well dried grains. Keeping this in view, the present studies were conducted at the Department of Entomology, Institute of Frontier Technology, Regional Agricultural Research Station, Tirupati, Chittoor District, Andhra Pradesh, India during the 2017-18 cropping season.
Materials and methods
Chemicals and reagents
Technical grade standards of chlorantraniliprole (purity: 99.8%, Sigma-Aldrich), HPLC grade acetonitrile, n-Hexane, and water Merck make, QuEChERS extraction salts like sodium sulphate anhydrous, sodium acetate, magnesium sulfate anhydrous were purchased from local vendors. Primary Secondary Amine GCB was also obtainedSigma-Aldrich make. Formulation of chlorantraniliprole (18.5%SC) for a field experiment was purchased from a local agricultural pesticide market.
Instrument
Ultra High-Performance Liquid Chromatography (UHPLC) Shimadzu make, with SPD-M20A, PDA detector. Deuterium (D2) and tungsten (W) lamps, which can read a wavelength of 190 to 800nm were used for analysis.
Instrumentation
A UHPLC system equipped with the following specifications was used for standardizing the method for estimating chlorantraniliprole followed by detecting and quantification of chlorantraniliprole residues from pigeon pea succulent pods at different intervals of spray i.e., 0, 1, 5, 10, 15, 20, 25 and 30 days. Chromatographic separation was achieved XR-ODS II (150mm X 2.0mm), The column oven temperature was set at 40oc. The injection volume was 10µl, the pump was LC-30AD which was set at a low-pressure gradient equipped with a flow rate of 0.8µl/min. The mobile phase was set with Pump A- Water (40%), Pump B: Acetonitrile (60%) at a Pump Flow of 0.8ml/min. The detector was a Photodiode array (PDA) detector in the SPD-M20A model consisting of a D2 (Deuterium) lamp. The conditions pertaining to LC conditions were presented in Table 1.
Preparation of solvents for HPLC: Acetonitrile 60 percent was prepared by adding 600ml of acetonitrile in 400ml of HPLC grade water.
Preparation of standard solution: An accurately weighed amount of 100mg of chlorantraniliprole was dissolved in a minimum amount of acetonitrile and made up of 100ml with acetonitrile to obtain 1000µg/ ml of chlorantraniliprole solution. The stock solutions were further diluted to get linear concentrations (0.1 to 1.0µg/ml) and a calibration curve was plotted.
Quantitative analysis of chlorantraniliprole
Method development: The following parameters were validated in method development (based on the external standard method).
- Linearity and range
- Limit of detection (LOD) and Limit of Quantification (LOQ)
- Accuracy
- Precision
- Specificity.
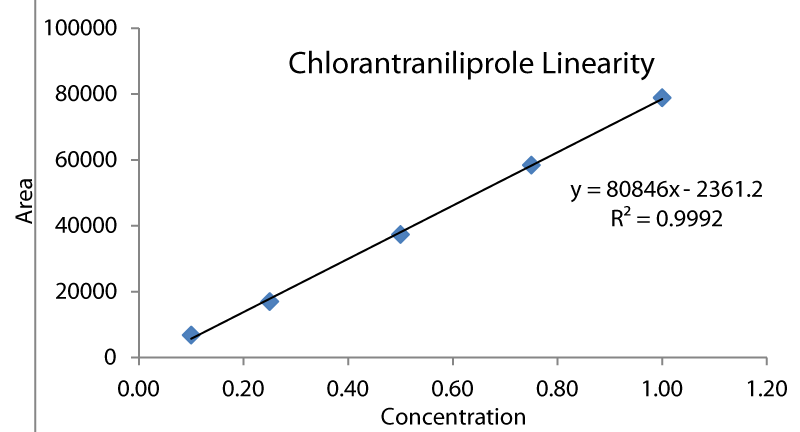
Linearity, range, and calibration: Calibration of instruments is essential in obtaining accurate analysis. Linearity of an analytical procedure is its ability to obtain test results within a given range that is directly proportional to the concentration of an analyte in the sample. It is evaluated by linear regression analysis of the plot of signals as a function of analyte concentration with a minimum of five linear concentrations. The correlation coefficient and slope of the regression line should be satisfactory. A stock solution of chlorantraniliprole (1000µg/ml) pipetted out and was diluted with acetonitrile to give different concentrations and injected into UHPLC-PDA. The peak areas were measured and tabulated. The detector response in terms of peak areas by standard solutions of chlorantraniliprole (0.1 to 1µg/ml in mobile phase) was measured.
Limit of detection (LOD) and Limit of Quantification (LOQ): Chlorantraniliprole was considered the concentration that produced an S/N ratio of 3:1 and the LOQ was defined on an S/N ratio of 10:1. These values were estimated from the chromatogram corresponding to the lowest concentration that could produce a response of 3:1 from different concentrations injected.
Precision was arrived at by injecting the chlorantraniliprole 1ppm concentration in six replicates.
Sample collection and storage
A field evaluation trial was conducted for evaluating the efficacy of chlorantraniliprole against pod borers in pigeon pea at the Regional Agricultural Research Station, Tirupati. The trial was sown during kharif, 2017 (rainy season) with normal agronomic practices recommended by ANGRAU, Guntur. Spraying of chlorantraniliprole @ 0.6ml/l (300ml/ha) was done by using a knapsack sprayer at peak flowering to the pod formation stage. A random sampling method was adopted for sampling and approximately one kilogram (kg) of green pods were collected at 0, 1, 5, 10, 15, 20, 25, and 30 days after spray in sealed covers under refrigerated conditions and brought to the laboratory and stored in deep freezer @ -20oc for further analysis.
Quantitative Analysis of Chlorantraniliprole in pigeon pea succulent pods: About 5g of sample was weighed followed by extraction and clean up process.
Sample extraction
Extraction of chlorantraniliprole from red gram succulent pods was done by following the QuEChERS method suitable for UHPLC with a PDA detector [11]. Five grams of the homogenized pigeon pea pod sample were taken into a 50ml centrifuge tube and added to 30ml of 1 percent acetic acid in acetonitrile. The contents were homogenized, followed by centrifugation at 15000rpm for 3min intermittently followed by the addition of 6g of Na2SO4 and 1.5g of sodium acetate. The contents were vortexed for 1min and centrifuged for 2min at 2500rpm to separate the organic layer. After centrifugation 9 ml of the upper organic layer was transferred into a 15ml centrifuge tube, to which 1.4g magnesium sulfate and 0.45g Primary Secondary Amine (PSA) were added and centrifuged at 2500rpm for 2min. After centrifugation 2ml of the supernatant layer was pipetted out into a 15ml centrifuge tube and filtered through a PTFE filter (0.2µm) and 1ml filtrate was taken into an LC vial to fed directly into UHPLC autosampler.
The residue was calculated by the following formula
Area of sample Conc. Of Standard in µg/ml Residue(µg/gm)= ------------------------- X ------------------------------------- X Dilution factor Area of standard Weight of sample(g)
Method validation
After a 5-g sample of finely blended pigeon pea was placed in a conical flask, an appropriate volume of chlorantraniliprole stock solution of standard (250 and 500μg/ml in acetonitrile) was added. This spiked sample was blended and allowed to air dry. Sample extraction, cleanup, and CE quantitation were done as usual. Recovery was obtained by comparing the amounts that were found with the amounts that were added (based on the external standard method).
Dissipation kinetics of chlorantraniliprole residues in pigeon pea
The dissipation kinetics of insecticide residues were determined by plotting the residue concentration against the time elapsing from treatment (in days); then, curves of best-fit equations were determined, for maximum coefficients of determination (R2). It was identified that an exponential relationship existed for the chlorantraniliprole dissipation in pigeon pea, corresponding to the general first-order kinetic (Table 2, Figure 1).
Rt ¼ R0e−kt
Where Rt represents a concentration of pesticide residues at any time t,
R0 is an initial residue concentration and k is the constant rate of the pesticide disappearance per day.
From this equation, the dissipation half-life period (t1/2 = ln(2)/k) of the pesticide and the time that must elapse until its residues reach the concentration level of 0.01 mg/kg (tR=0.01 = ln(0.01/R0)/(−k) were calculated.
Statistical analysis
The dissipation pattern of chlorantraniliprole in pigeon pea pods over time was expressed by the following first-order kinetics, which is a function of exponential decay. The biological half-lives, which means the time required for the initial residue to decrease by ½, were also calculated as follows [12,13] Table 3.
Ct = C0 × e-kt, DT50 = ln2/k (1) ijvsr 236
Where C0 is the initial residue concentration of pesticides from field experiments ‘t’ is the days after pesticide application and k is the rate constant of dissipation.
According to the dissipation pattern data and MRL, the PHRLs on the day before harvest that is the allowable limit of the pesticide residues before the harvest was estimated from 0 to15 days before harvest based on the following equation.
PHRLd = MRL _ ekmin _ d (2)
Results
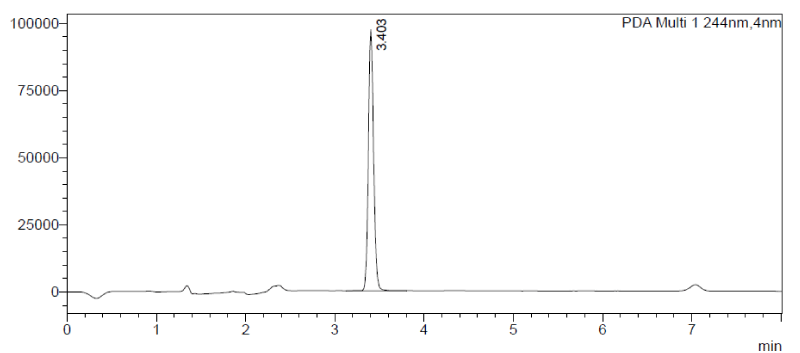
The Chlorantraniliprole retention time was observed at 3.403 min for the standard solution at five different concentrations. Linear concentrations were 0.10, 0.25, 0.50, 0.75 and 1.00ppm.
Standardization of the chlorantraniliprole was done at five different concentrations, 0.10, 0.25, 0.50, 0.75, and 1.00ppm, and found retention time at 3.402 min Simultaneously blank was run with acetonitrile for comparison (Figures 2-4).
Calculation of linearity and instrument sensitivity (LOQ & LOD)
The limit of quantification (LOQ) was defined as the minimum concentration that gives a sufficient signal-to-noise of 10 in the chromatographic signal. The standards treated with the sample preparation method described above were injected into the ultra-high performance liquid chromatography (UHPLC) instrument to compare the signal-to-noise ratios. The LOQ was estimated to be 0.07µg/mL in chlorantraniliprole and LOD was estimated to be 0.02µg/mL.
The results of the present study indicated that residues of the chlorantraniliprole from pigeon pea succulent pods were 3.57 at 0 days (2h) after spray, followed by 3.16, 1.61, 1.131, 0.49, 0.21and 0.05mg/kg bw at 1, 5, 10, 15, 20, and 25 days after spray. It reached below the detectable level 30 days after spray as chlorantraniliprole dissipated from pigeon pea pods. The residues of chlorantraniliprole reached below the tolerance limit by the 5th day (given by Codex Committee on Pesticide Residues in succulent pods) and a biological half-life of 4.36 days for chlorantraniliprole was observed for pigeon pea succulent pods.
Discussion
The present studies are supported by the earlier findings of rapid degradation of chlorantraniliprole with half-lives of 4.9–5.4 days in corn straw [5], 1.25 and 1.36 days in cauliflower [14], 2.7 days in grape (Malhat, 2014), 3.3 days in tomato, 0.93–1.33 days in berseem leaf, 0.48 to 1.47 days at 30 and 60g a.i. ha–1 in pigeon pea [15]. This is supported by the statement of Fritz and Hoffmann [16] who reported that atmospheric effects on the fate of agricultural sprays and found that no single meteorological factor dominated the downwind transport of aerially applied sprays. Generally, lower relative humidity decreased the amount of downwind deposition due to evaporative effects. Increasing wind speeds decreased deposition and increased the amount of mass that could not be accounted for in the experiment. Similarly [17-22] reported the factors affecting the degradation behavior of pesticides applied to crops to include the physicochemical characteristics of a given pesticide (viz., vapor pressure, water solubility, hydrolysis), characteristics of the crop commodity (ex, growth rate, ease of penetration, translocation, excretion), microbial activity, and environmental factors (ex., rainfall, temperature, sunlight, humidity).
Contrary to the present studies, the slower dissipation tendencies of chlorantraniliprole were observed and relatively long half-lives of chlorantraniliprole were reported by several other researchers. He, et al. [23] reported 9.0–10.8 days in maize straw, Szpyrka, et al. [24] identified 16-17 days in apple. Similarly, Jonghwa, et al. [25] and Rohan, et al. [26] noticed 15.2 and 30 days in kimchi cabbage and in pigeon pea, respectively [27-29].
Conclusion
The present investigations were carried out on the Dissipation pattern of chlorantraniliprole residues in an open-field experimental system from pigeon pea succulent pods. To determine the residues, simple and rapid analytical methods were developed and analyzed using a UHPLC. In the field study, the dissipation dynamics of chlorantraniliprole in the pigeon pea succulent pods were identified with a biological half-life of 4.36.
- Teixeira L AF, Gut LJ, Wise JC, Isaacs R (2009) Lethal and sublethal effects of chlorantraniliprole on three species of Rhagoletis fruit flies (Diptera: Tephritidae). Pest Manag Sci 65: 137–143. Link: https://bit.ly/3LmE8KN
- Bassi A, Molnar I, Zielinski D, Savulescu I, Shulgan V, et al. (2008) Proceedings of the 17th Triennial Conference of the EAPR – International Potato Conference 475.
- Selby TP, Lahm GP, Stevenson TM (2017) Pest Management Science 73: 658.
- Cordova D, Benner EA, Sacher MD, Rauh JJ, Sopa JS, et al. (2006) Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic Biochem Phys 84: 196-214. Link: https://bit.ly/3keX1U7
- Dong F, Xu J, Liu, X, Li J, Li Y, et al. (2011) Chromatographia 74: 399.
- Sharma AK, Zimmerman WT, Singles SK, Kalumbu M, Swain S, et al. (2014) Photolysis of Chlorantraniliprole and Cyantraniliprole in Water and Soil: Verification of Degradation Pathways via Kinetics Modeling. J Agric Food Chem 62: 6577–6584. Link: https://bit.ly/3xVRA4g
- Patange NR, Chiranjeevi B (2017) Bioefficacy of newer insecticides against pigeonpea (Cajanus cajan L. Millsp.) pod borers. J Entomol Zool Stud 5: 28-31. Link: https://bit.ly/3Ky2d05
- Swami HOP, Lekha A (2017) Bioefficacy of novel insecticides against pod borer, (Helicoverpa armigera Hubner) in pigeonpea. Legume Research 40: 756-761. Link: https://bit.ly/3kd63Rs
- Dodia DA, Prajapati BG, Acharya S (2009) Efficacy of insecticides against gram pod borer, Helicoverpa armigera Hardwick, infesting pigeonpea. Journal of Food Legumes 22: 144-145. Link: https://bit.ly/3vtsloB
- Patel SA, Lanjewar A (2013) An International E-Journal 2: 398.
- Malhat FM, Loutfy N, Ahmed MT (2015) Validation of QuEChERS Based Method for Determination of Flusilazole Residues in Grape by high performance liquid chromatography with photodiode array detector. Toxicol Environ Chem. Link: https://bit.ly/3vMC9J5
- Farha W, Abd El-Aty AM, Rahman MM, Kabir MH, Chung HS, et al. (2018) Biomedical Chromatography 32: 4092.
- Hwang KW, Moon JK (2018) Translocation of chlorpyrifos residue from soil to Korean cabbage. Appl Boil Chem 61: 145-152. Link: https://bit.ly/3OyuTZQ
- Kar A, Mandal K, Singh B (2013) Environmental fate of chlorantraniliprole residues on cauliflower using Quenchers technique. Environ Monit Assess 185: 1255-1263. Link: https://bit.ly/3OyuTZQ
- Chawan R, Naik RH, Pallavi MS, Rachappa V, Pramesh D, et al. (2020) Lc-esi-ms/ms method for determination of chlorantraniliprole residue and its dissipation kinetics in pigeonpea. Pesticide Research Journal 32: 96-106. Link: https://bit.ly/3KpO0St
- Fritz BK, Hoffmann WC (2008) Agric Eng Int 10: PM08 008.
- Tewary DK, Kumar V, Ravindranath SD, Shanker A (2005) Dissipation behavior of bifenthrin residues in tea and its brew. Food Control 16: 231-237. Link: https://bit.ly/3LoydVp
- Fujita M, Yajima T, Iijima K, Sato K (2012) Comparison of the Variability in the Levels of Pesticide Residue Observed in Japanese Cabbage and Grape Units. J Agric Food Chem 60: 1516–1521. Link: https://bit.ly/38rga2i
- Ashok KS, William TZ, Suzanne KS, Kalumbu M, Scott S, et al. (2014) Journal of Agricultural and Food Chemistry 62: 6577-6584.
- Sharma N, Mandal K, Kumar R, Kumar B, Singh B (2014) Persistence of chlorantraniliprole granule formulation in sugarcane field soil. Environ Monit Assess 186: 2289-2285. Link: https://bit.ly/3vk66kI
- Paramasivam M (2021) Dissipation kinetics, dietary and ecological risk assessment of chlorantraniliprole residue in/on tomato and soil using GC–MS. J Food Sci Technol 58: 604-611. Link: https://bit.ly/3vPO094
- Sun C, Bei K, Xu Y, Pan Z (2021) Effect of Biochar on the Degradation Dynamics of Chlorantraniliprole and Acetochlor in Brassica chinensis L. and Soil under Field Conditions. ACS Omega 6: 217−226. Link: https://bit.ly/3EUmKuA
- He M, Song D, Jia HC, Zheng YJ (2016) Environ Sci Health Part B 51: 594.
- Szpyrka E, Matyaszek A, Słowik-Borowiec M (2017) Dissipation of chlorantraniliprole, chlorpyrifos-methyl and indoxacarb—insecticides used to control codling moth (Cydia Pomonella L.) and leafrollers (Tortricidae) in apples for production of baby food. Environ Sci Pollut Res 24: 12128-12135. Link: https://bit.ly/399FV7C
- Lee J, Kim BJ, Kim E, Kim JH (2019) Dissipation Kinetics and the Pre-Harvest Residue Limits of Acetamiprid and Chlorantraniliprole in Kimchi Cabbage Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 24: 2616. Link: https://bit.ly/3EPrnpF
- Rohan VK, Vanrajsinh HS, Singh S, Digvijaysinh C, Heliyon 7: e06377.
- Malhat FM (2012) Determination of Chlorantraniliprole Residues in Grape by High-Performance Liquid Chromatography. Food Anal Methods 5: 1492-1496. Link: https://bit.ly/3xWwjHS
- Report of the 45th Session of the CODEX Committee on Pesticide Residues, Beijing, China. Link: https://bit.ly/3KrUpNm
- Zongmao C, HaibinW (1988) Factors affecting residues of pesticides in tea. Pestic Sci 23: 109-118. Link: https://bit.ly/3y0sGR5

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
