Open Journal of Analytical and Bioanalytical Chemistry
Optimization and validation of online column-switching assisted HPLC-spectrometric method for quantification of dansylated endocannabinoids and neurotransmitters in animal models of brain diseases
Maria Baranyi* and Beata Sperlagh
Cite this as
Baranyi M, Sperlagh B (2019) Optimization and validation of online column-switching assisted HPLC-spectrometric method for quantification of dansylated endocannabinoids and neurotransmitters in animal models of brain diseases. Open J Anal Bioanal Chem 3(1): 083-093. DOI: 10.17352/ojabc.000016In scientific research, animal modelling of human disease is pivotal in both studying the mechanisms of the disease and developing potential therapies. An imbalance in the interaction between the endocannabinoid (eCB) and Neurotransmitter (NT) systems may play a role in the pathogenesis of neurological diseases, such as Parkinson’s Disease (PD). The major limitation of current neurochemical practice is that different assays are used to measure each class of NTs. We present a liquid chromatography-spectrometric method that utilizes 5-dimethylamino naphthalane-1-sulfonyl (dansyl) chloride derivatizing reagent for the simultaneous measurement of nine different types of neurotransmitters. In order for our method to achieve the highest possible sensitivity, we tested the following reaction parameters: reaction medium and pH; the effect of reagent concentration; reaction temperature and time, both of which influence the efficiency of dansyl derivative formation of eCBs; and Amino Acids (AAs) and Monoamines (MAs) that are present in the sample together. To achieve the required analytical sensitivity, online solid phase extraction techniques were used to purify and enrich the dansylated sample.
The optimal sample enrichment and clean-up time were calculated from the breakthrough volume of dansyl hydroxide, taken on a capture column (ACE Ultra Core Super C-18), when the mobile phase contained 1.9%(v/v) of acetonitrile and 1.1%(v/v) of methanol in formic acid ammonium salt buffer at a flow rate of 0.3 ml/min. The linear range of calibration was between 50-1575 pmol/ml for all the analytes (aspartic acid, glutamic acid, glycine, γ-aminobutyric acid, dopamine, norepinephrine, serotonin, N-arachidonylethanolamide, or anandamide, and 2-arachidonylglycerol). The Limit of Detection (LOD) and Quantification (LOQ) ranged between 15.75-118.5 pmol and 26.5-196.5 pmol respectively. The precision (repeatability) was in the range of 5.7%-9.9% for each analyte. This method was applied to investigate neurochemical changes in a mouse model of Parkinson’s disease in the presence and absence of P2x7 and P2Y12 purinergic receptors.
In conclusion, the present method shows acceptable precision and adequate sensitivity in quantifying the basal levels of the endocannabinoids, and it is well suited for the simultaneous determination of neurotransmitters and endocannabinoids.
Introduction
The chemical messengers that act as conventional neurotransmitters are stored in synaptic vesicles and are released when Ca2+ enters the axon terminal. These messengers act by binding to receptors on the membrane of the postsynaptic cell. The conventional neurotransmitters can be divided into two main groups: small molecule neurotransmitters, and neuropeptides. The small molecule neurotransmitters are various types of small organic molecules. They include amino acid neurotransmitters, such as glutamate, GABA (γ-aminobutyric acid) and glycine, and the biogenic amines (dopamine, norepinephrine, epinephrine and serotonin). The unconventional transmitters, like endocannabinoids, are synthesized on-demand, are not stored in synaptic vesicles, and may carry messages from the postsynaptic neuron to the presynaptic neuron. Parkinson’s Disease (PD) is a chronically progressive neurodegenerative disorder, the major neurochemical problem of which is the lack of the dopamine neurotransmitter. Clinically, PD patients have higher levels of glutamate, gamma-aminobutyric acid, acetylcholine and other neurotransmitters. To understand the pathogenesis of PD, it is necessary to reproduce its biochemical, physiological and morphological features in animal models. In mice, PD is classically modelled with the application of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [1]. Acute injection of MPTP causes a PD-like syndrome with a massive loss of both nigral dopaminergic neurons and striatal dopamine [2]. However, only the chronical administration of MPTP produces progressive behavioural changes, triggering the formation PD-hallmarks [3]. eCBs can regulate a number of brain functions, including cognition, motor control, emotion, and reward and feeding behaviour. Increasing evidence suggests that the cannabinoid signaling system has a prominent modulatory function in the basal ganglia by modulating both excitatory and inhibitory synaptic signaling. Both major derivatives of arachidonic acid, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) [4], are ubiquitous in the brain. For example, striatal concentration of 2-AG is nearly 150 times greater than that of AEA [5]. Within the nucleus striatum, the eCB, glutamatergic, GABAergic and dopaminergic signalling systems, bidirectionally interact in order to modulate basal ganglia neural network dynamics and long-term forms of synaptic plasticity [6,7]. In preclinical studies, cannabinoid 2-AG have been shown to be effective against excitotoxicity, oxidative stress, inflammation, and motor complications that are associated with PD [8,9].
Many assays for NTs have been developed using chromatographic systems, such as capillary electrophoresis [10], gas chromatography [11] and high-performance liquid chromatography (HPLC) [12-15]. The liquid chromatographic separation of these small molecules is often used in conjunction with UV absorption [16], fluorescence [17] and electrochemical detection [10,18,19], and, more recently, mass spectrometry (MS) [12,20-22]. Electrochemical detection systems have proven to be successful for determining MAs [10,18,19]. Amino acid NTs, which play an important role in neurochemistry, are generally small aliphatic molecules that have neither strong absorbance, nor fluorescence, in the ultraviolet or visible region. In order to improve both the selectivity and sensitivity of amino acid-detection of biological samples, the use of derivatization reactions is required. The most commonly used reagents of the amino group are as follows: dansyl chloride, phenyl-isothiocyanate, ortho-phtaldialdehyde, and benzyl chloride. These reagents are used to convert native amino acids into highly fluorescent derivatives [23-27].
Due to an increasing interest in the biological significance of endocannabinoids, several lipidomics approaches have been developed in order to identify and quantify endocannabinoids in various biological tissues. Because of their trace amounts and structural variability, the quantification of eCBs is not an easy task. The hydrophobic eCB molecules readily solubilize in organic solvents, and they are well suited for mass spectral analysis. Various mass spectrometric approaches, such as GC-MS procedures [5,28,29] and LC-MS methods [30,31], enable the separation, structural identification and sensitive detection of eCBs from biological samples. Moreover, the chemical derivatization step before separation give analytes thermal stability, making them suitable for spectrophotometric detection with an on-column sensitivity in the femtomole range. In accordance with the first published quantitative GC-MS analytical method [32], both functional groups of arachidonoyl ethanol amide are involved in the formation of bis-pentafluorobenzoyl derivative. Alternatively, Wang, et al., [33], used 4-(N-chloroformylmethyl-N-methyl) amino-7-N, N-dimethylaminosulphonyl-2,1,3-benzoxadiazole (DBD-COCl), and both AEA and 2-AG derivatization were possible. Furthermore, Yagen and Burstein described a method for the detection of AEA as its dansyl derivative [34]. Dansyl esters of AEA can be generated by heating AEA with excess dansyl chloride and dimethylaminopyridine in acetone, and the product was detected by UV light at 365nm.
Previously using a rotenone PD model, we showed that dopamine and its oxidative metabolites provide an additional source of highly reactive free radicals during their breakdown, thereby potentially reinforcing the harmful effects of oxidative stress in striatal dopaminergic neurons [35,36]. We have also evaluated the efficacy of novel (hetero)aryl alkenyl propargylamine compounds in MPTP-induced acute and subacute mouse models of PD [37]. Endocannabinoids primarily act in retrograde, facilitating short-and long-term plasticity, both at excitatory and inhibitory synapses, while also interacting with the dopaminergic system [38]. Concurrent striatal changes in endocannabinoids and amino acids, and their association with dopamine depletion, have not been studied in the animal model of PD when using a different regimen of MPTP treatments. Although general procedures have been employed for lipid extraction, it is clear that many methodological factors have a large impact on the extraction and purification of eCBs from biological matrices. However, the sample preparation procedure used by [34], may not apply because eCBs, AAs, as well as Mas, are present together in an aqueous medium in the biological fluids.
Therefore, the main objectives of the present study were: (i) to develop a stable, reliable and sensitive analytical method for simultaneously monitoring AAs, MAs and eCBs; (ii) to expand the automation of sample preparation by applying an on-line solid phase extraction (SPE) method to analyse trace levels of dansylated eCBs in biological samples. The present method and analysis results allow the further study of the MPTP-induced neurotoxicity with the consequent changes in the interaction between the eCB- and NT-systems.
Experimental
Chemicals and reagents
Amino acid standards were obtained from Pierce (Roxford, Illinois, USA.). Other chemicals: monoamines, endocannabinoids and theophylline, dansyl chloride (DNS-Cl), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, ammonium formate salt, sodium carbonate, Chroma Solv HPLC grade methanol, acetonitrile, formic acid and perchloric acid were purchased from (Sigma-Aldrich, Germany). The standard solutions were prepared monthly at a concentration of 1mg/ml and stored at -80°C. The endocannabinoid standards were dissolved in ethanol and the mixture was diluted from stokcs with filtered HPLC water.
Sample preparation
Murine model of parkinson’s disease: Experiments were conducted in accordance with the procedures outlined in the NIH Guide for the Care and Use of Laboratory animals and were approved by the local Animal Care Committee of the Institute of Experimental Medicine (Budapest, Hungary, ref. No. 22.1/3671/003/2008). About 70 and 90 days old male C57BL/6J or P2X7 receptor knockout (P2X7 R ko) and lack of P2Y12 (P2Y12 R ko) receptor type mice were housed. All animals received intraperitoneally sterile saline or MPTP solution according to vehicle or PD model treatment. In the acute treatment mice received 4x20mg/kg MPTP at 2-hour intervals, while the subacute treatment MPTP was given at the same dose daily for five days.
Brain tissue preparation: Mice were decapitated 3 or 21days after the last MPTP and vehicle treatment and the striatum was dissected on ice than was frozen by liquid nitrogen. The weighed frozen tissue was homogenized in an appropriate volume of ice-cold 0.1M perchloric acid (PCA) which contained 0.1% w/v sodium metabisulphite and 10μM theophylline (Theo, internal standard). One part of the samples was used for the dansyl derivatization, while the second part was used to measure the content of nucleotides and monoamines as described in the method [35]. The pellet was saved for protein measurement according to Lowry, et al., [39].
Monitoring of extracellular level of NTs
Adult male C57BL/6J or 6NCrl type of mice (Charles River Laboratories Inc.) was used for micro dialysis experiments eight weeks after the AAV virus construction administration. Special (EICOM Cx-I) micro dialysis probe was implanted into the Median Raphe Nucleus (MRN) or prefrontal cortex (PFC). The micro dialysis probe was flushed artificial cerebrospinal fluid (ACSF) at a flow rate of 2µl/min using a syringe pump. Dialysate sample were diluted and one part (240µl) was used for monoamines measurement as described in [35] and the left, 50µl was dansylated and used for amino acid analysis. The experimental results were published in the following journals [40,41].
Derivatization process
Pre-column derivatization was performed in a 1.5ml Eppendorf sample tube by mixing (50µl) tissue extract or (50µl) micro dialysate solutions. One hundred microliters of 2.7M Na2CO3, which contained 30 nmol/ml nor-Valine as Internal Standard (IS) were added and mixed for 1min. Then (100µl) of DNS-Cl (5.4 mg/ml in acetonitrile) solution were added and mixed again. Subsequently the mixture was incubated for 15min at 70°C temperature in a dark oven. The reaction was terminated by acidification in a ratio of 1 part of sample mixture and 1-part volume of “washing buffer” (the composition can be found in part the Online solid phase extraction).
HPLC analysis
The liquid chromatographic system was a Shimadzu (Analytical & Measuring Instruments Division, Kyoto, Japan) Nexera xR HPLC. For separation of analytes ACE UltraCore Super Phenyl-Hexyl (7.5cmx2.1mm I.D., 5µm particle size) and ACE UltraCore Super C-18 (150x2.1mm I.D., 5μm particle size) columns from A.C.T.L. (Scotland) were used. The columns was kept at room temperature (about 22-25°C). A flow rate 0.385 ml/min was used throughout the separation. The mobile phase “A” consisted of 15 mM FAA buffer with 21.5 % (v/v) MeOH-ACN in a ratio of (1:3.5). The mobile phase “B” was 15mM FAA buffer with 93% (v/v) MeOH-ACN in a same ratio, and the pH was adjusted to 3.7 with FA. The increments of mobile phase “B” in the gradient program were as follows: 98.6%, 100%, at times of 0-33 and 48.0min respectively, and keeping “B” at 100% for 5min and then decreased linearly for 2.0min to the initial conditions. To establish a new equilibrium of the column the buffer “A” was maintained for 29min prior to the next injection. Mobile phases were filtered through a 0.45μm Nylon membrane and degassed by sonication prior to use. The effluent was monitored by a filter fluorimeter (Gilson Model 121) wavelength range of excitation at 310-410nm and 480-520nm of emission. The variable wavelength UV detector (Agilent 1100 series, Waldbronn, Germany) connected in a cascade line and worked at 318nm. Peak area of analytes was determined by Shimadzu LC-Solution and Agilent data acquisition system. The automatic integration was individually checked.
Online solid phase extraction and column switching
The online solid phase extraction (SPE) was carried out on an ACE UltraCore Super Phenyl-Hexyl (7.5cm×2.1mm I.D., 5µm particle size) column. It was connected to a high-pressure column switching valve (FCV-32AH, Shimadzu) and conditioned with “washing buffer”: 15mM FAA buffer which contained 3% (v/v) organic components, ACN-MeOH in a same ratio (3.5:1). The apparent pH was 3.0 and 0.3 ml/min flow rate was applied by Shimadzu Nexera×R pump. Five hundred µl of sample derivative was delivered onto the capture column (C1) by SIL-AD VP automatic injector (Shimadzu Corporation, Analytical & Measuring Instruments Division, Japan). Processing for maximum of sample volume (1.5ml) the enrichment (3x4.5min) and subsequent purification (1×4.0min) required duration of 17.5minutes.
Method validation
The method validation were carried out in accordance with the Good Laboratory Practice (GLP) and Eurachem Guides [42], regulations. Validation was performed by taking into account selectivity, linearity, precision and accuracy, limit of detection and limit of quantification.
Selectivity was evaluated in order to identify potential interferences by other analytes or other compounds at the chromatographic region of interest Analytical standards (Asp, Glu, Gly, Gaba, DA, NE, 5-HT, AEA and 2-AG) and tissue extracts or micro dialysate were analysed to assess the selectivity of the method at the retention times of NTs and eCBs within the analytical conditions established.
The linearity of the method was determined by calculating the peak area ratio between the analytes and the Internal Standard (IS). To prepare the calibration curve, a sample matrix solution was used, which was Krebs bicarbonate buffer solution or tissue extraction solution. To create the appropriate calibration ranges the following concentrations were prepared from (1.0 mg/ml) stock solutions of standards: (395-1575 pmol/ml) for AEA; (105-420 pmol/ml) for 2-AG; (60-120 pmol/ml) for Asp and Glu; (80-315 pmol/ml) for GABA and Gly; (55-210 pmol/ml) for NE and DA, and (50-200 pmol/ml) for 5-HT. Three determinations (n=3) were carried out for each solution and each calibration point was fitted by linear regression. First-order calibration curve equations (y=mx+b), coefficients of determination (r2) for slope and intercept were calculated.
The precision of the method was assessed for each analyte as repeatability (intra-day precision) and intermediate precision (between days and concentration levels) during two different days and two concentration levels (35 and 135%). Five replicates (n=5) were performed for each working solution. The results were expressed as percentage relative standard deviation (% RSD). The HoRat quotient was used to evaluate intra-laboratory precision, according to Horwitz equation: RSD=2 exp (1-0.5 log C) where C is the concentration as a decimal fraction [43]. RSDr is the expected coefficient of variation under repeatability conditions; RSDR is the expected coefficient of variation under electrochemical method [35] conditions.
Accuracy was determined by recovery (%) at two concentration levels (35 and 135%) in triplicate (n=3). The experiment was performed by adding known quantities of analytical standards (AAs, MAs and eCBs) to tissue extracts prepared at 50% of center point of linear range.
The Limit of Detection (LOD) and the Limit of Quantification (LOQ) were estimated based on calibration curves containing the analytes spiked to the sample extracts. The standard deviation of the intercept was used to express the experimental error. Additionally, mathematically estimated at the lower concentrations with appropriate signal/noise ratio (S/N>2-3.5) and precision (% RSD <5.0) were adopted LOD and LOQ for each analyte, respectively.
Statistical analysis was performed using the TIBC Statistical Program. Multiple comparisons of variance Non-parametric analysis (Kruskal-Wallis p test) was used to compare the differences between chromatographic UV and FL signals (peak areas) derived from the study of the effect of certain parameters that influence the derivatization process of each component. The null hypothesis of the Kruskal–Wallis test is that the medians of the groups are equal this means that the tested parameter does not affect the efficiency of the derivatization, the area under the curve. If p<0.05 then the null hypothesis was rejected.
Data processing, calculations and graphic plotting were performed using Microsoft Office Excel 2010. Data are expressed as a mean ± standard error of mean. To assess normality all continuous variables of measurements the Kolmogorov-Smirnov test was used and performed for each individual repeated measurement. Where the measured variables met the normality assumption there was performed two-way factorial measures (FM ANOVA) analysis on all striatal data followed by post-hoc Tukey test to test the pairwise difference between every MPTP and vehicle treated animals. The threshold for statistical significance was set at p<0.05.
Results and Discussion
Optimization of reaction conditions for derivatization
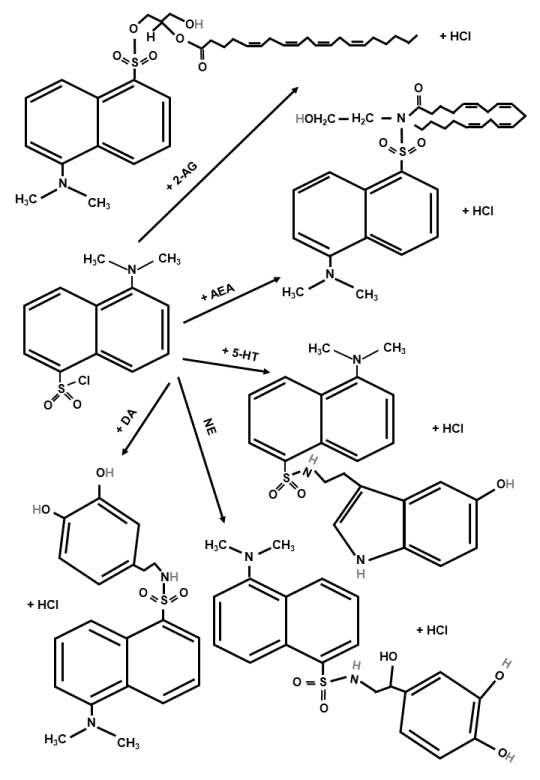
Dansyl chloride is the most commonly used derivatizing reagent because of its chemical structure, with its naphthalene backbone having fluorescent properties. Derivatization of the sample analytes can be described by the following chemical reactions:
(Amino acid and monoamine analytes -NH2 + DNS-Cl = analytes -HN-DNS + HCl)
(Anadamide analytes -NH + DNS-Cl = analytes -N-DNS + HCl)
(2-AG, AEA and monoamine analytes -OH + DNS-Cl = analytes -O-DNS + HCl).
The monoamines and endocannabinoids derivatization reaction mechanism is outlined in Figure 1. The derivatization reaction can be carried out as a pre-column process. The yields of target analytes (amino acids, monoamines and endocannabinoids) were tested as a function of sample solvents, reaction temperature and time, pH of medium, and concentration of reagent.
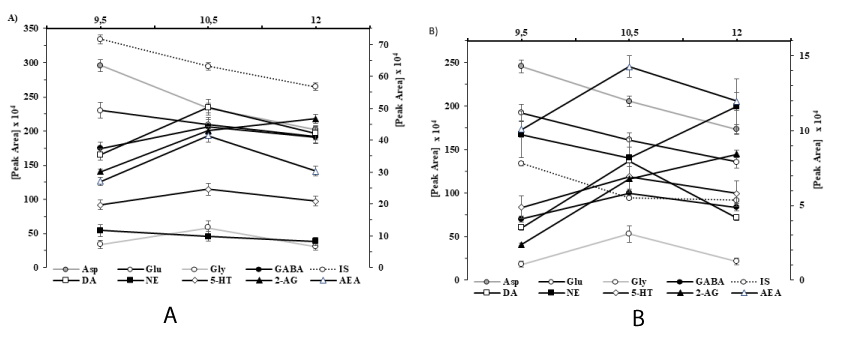
Effect of the sample solvent on the efficiency of dansyl derivative formation: The preparation for tissue eCB analysis typically consists of sample homogenization in an organic solvent, which is then followed by a lipid extraction. Biological fluids are aqueous and, in the experimental procedure, small peptides, phospholipids, and other impurities are included in the sample, all of which may affect the derivatization process. First, we investigated the impact of solvents, particularly where the tissue sample is extracted and used to dissolve the standards and reagent. We studied the effect of the following solvents: water, as a general biological fluid; Krebs bicarbonate buffer (a general buffer solution for in vitro tissue technology); 0.1M PCA (a tissue extraction solution); and acetonitrile (ACN), a solvent for the derivatizing reagent. Acetonitrile is a good solvent for both dansyl chloride and the eCBs [33]. According to experimental data, water may be the preferred solvent for the reaction mixture of amino acids and monoamines. The Kruskal-Wallis test was used to test the differences between the peak areas that are acquired for each analyte when different solvents and different types of spectrometric detection were used. (The results are summarized in part the Method validation and Table 1). Based on the UV detector signal, no significant differences were found in the efficiency of derivatization of the different types of solvents. The solvent effects test data indicated that the fluorescent detector signal (peak areas in Figures 2A,B) were smaller in the case of Krebs-bicarbonate buffer and tissue extraction solution than those in the acetonitrile solvent. This effect was significant (H3,12=9.667), and not only in the case of the eCBs, but also in the case of NE (H3,12=9.462), as shown by the p values in Table 1. The differences found in the fluorescence detection are due to the excitability of the dansyl derivatives. It can be concluded from the solvent effect study that changing the medium of the tissue extract to an organic solvent does not increase the efficiency of the derivatization reaction; rather, it causes a loss of the sample.
Effect of reaction and heating time on the efficiency of dansyl derivative formation: Other important factors in the dansylation are the reaction time and temperature, which should be the most appropriate given the thermal sensitivity of MAs and eCBs. Because of the numerous data are available in the literature [12,34,44,45], derivatization was only studied at higher temperature values in order to use the shortest reaction time. In our investigations, the yields of the derivatives (areas under the curve) did not change beyond the range of 70 and 90°C, so the optimum temperature for dansylation was assumed to be 70°C. To ensure sample stability, our goal was to choose the shortest reaction time. During the test, the application of 10minutes of reaction time resulted the highest yield in dansylation (maximum area under the curve), which did not increase when 30minutes was used. Conversely, reducing the reaction time to 5minutes caused poor repeatability. This was due to the possible variability of time delays between the individual steps of sample derivatization.
Effect of buffer pH on the efficiency of dansyl derivative formation: The sulfonyl chloride group of DNS-Cl can react with the nucleophilic groups of analytes. Also, the acidity of these groups plays an important role in the dansylation reaction. The rate of hydrolysis of dansyl chloride to pH 9.5 is low and constant, and then increases rapidly with alkaline pH. The reaction can be accelerated by the alkaline pH, but the high pH shifts the reaction between dansyl chloride and water (H2O+DNS-Cl=DNS-OH+HCl) formation to dansyl hydroxide. AEA, the amide derivative of arachidonic acid, has the capacity to react with dansyl chloride by secondary amine and primary hydroxyl groups. The amide nitrogen is not a good nucleophile, meaning that a strong base is required to deprotonate the amide. 2-AG, a glycol ester derivative of arachidonic acid, is capable of reacting with dansyl chloride with its primary and secondary hydroxyl groups. The pH of the reaction mixture affects the efficiency of derivatization due to the degree of dissociation of nucleophilic groups of analytes [Asp-N+H3 9.9; Glu-N+H3 9.5; Gly-N+H3 9.8; GABA-N+H3 10.4; (IS) Nor-Val-N+H3 9.8; DA-N+H3 10.3; NE-N+H3 10.3; 5-HT-N+H3 10.0] aromatic-OH of [MAs 8.4-8.7 and 10.3-10.9] and the alcoholic-OH of [ECBs 13.5-15.4] and conversion to di-[5-HT and 2-AG] or three-[NE and DA]-dansyl derivatives, and alkaline pH accelerates the hydrolysis of dansyl derivatives. In order to determine the effect of pH, our experiments were carried out as follows. Sodium carbonate was used at a concentration of 2.7 M, and the pH of the biological media (extracellular buffer and tissue extract) was adjusted to 9.5 to 12.0 by varying the volume of the solution. The pH of the medium significantly influenced the yield of derivatization, which was also confirmed by the Kruskal-Wallis test (H2,9=5.96 to 7.2) for the analytes tested (Table 1). Most significance values were obtained for the 5-HT (H2,9=7.2) and GABA (H2,9=6.49) analytes, which may be due to the similar degree of dissociation of the amino and aromatic hydroxyl groups. Experimental evidence indicates that highly basic media induce autoxidation damage, especially for dopamine analytes, as confirmed by our previous results [35]. The autoxidation side reaction was observed at 150nmol of dopamine at pH 10.5, and its appearance was indicated by the dansylated amino chrome, eluting as a satellite peak after 5-HT. The amount of these by-products was calculated from the regression equation of DNS-DA and DA, expressed as a percentage of the initial mass concentration. Based on our results, the samples were adjusted to pH 10.5 with 100µl of 2.7M sodium carbonate in order to obtain the highest derivative yield. As shown above, and as illustrated in Figures 3A,B, under strongly basic conditions, the -NH and -OH groups of test compounds react with dansyl chloride, and this is consistent with the results of previous publications [12,13,15,23,34,44,46,47].
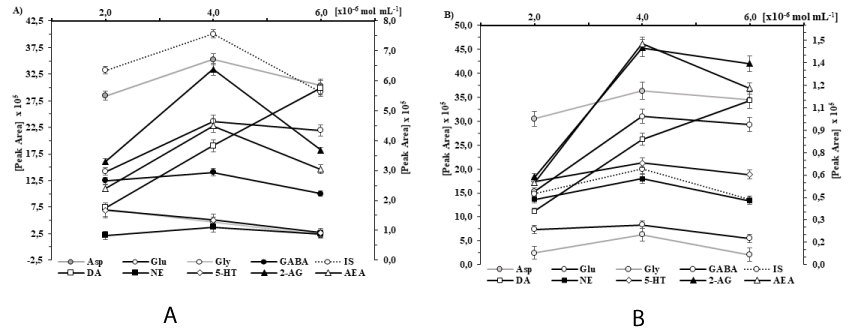
Effect of reagent concentration on the efficiency of dansyl derivative formation: The yield of the derivatives for each component was tested separately depending on the concentration of reagent under the already optimized reaction conditions (solvent, temperature, time and pH). In this assay, NTs and eCBs were used at the following concentrations: 50 nmol/l AA and 50 pmol/l MA and eCB, corresponding to the mouse striatum content. The tissue extract was diluted with water (in a ratio of 1:4) to 250µl. After adding 20 mM of dansyl chloride to acetonitrile solution, the alkalinized sample became cloudy; however, upon stirring and heating the mixture, it turned clear. Increasing the volume of reagent up to 50µl produced a proportional increase in the yield of the reaction product. However, no further increase in the peak area was observed when increasing the volume of the reagent to 150µl. (See Kruskal-Wallis test result in Table 1.) As can be seen in Figures 4A,B DNS-Cl provides the maximum yield of derivatization reaction at a concentration of 4.0 10-6 mol/ml that is not increased with additional surplus. Therefore, 100µl of volume from 2.0 10-2 mol/l of DNS-Cl solution was chosen for the derivatization reaction. In contrast to the generally accepted quenching techniques, the removal of dansyl chloride excess was based on the acidic effect that promotes the formation of the dansyl hydroxide. Initially, 0.5M formic acid was used to terminate the reaction. In order to avoid the strong gas formation (CO2), the derivatization process was stopped by diluting the reaction mixture with the “washing buffer”. The application of this acidification method resulted in a strong increase in the sample volume, but it avoided the formation of an additional derivative.
Optimization of online solid phase extraction and column switching conditions
To optimize the online SPE step, there was necessary to select 1) preconcentration column (type and chemistry of the sorbent), 2) composition of the washing mobile phase (water-organic solvent ratio), 3) flow rate, 4) washing time and 5) maximum volume of sample to be injected for the optimal conditions to keep the analytes firmly in the capture column. Elute the interference matrix component from the column.
The following columns were tested: Guard Cartridge Discovery C-18 (2cm×4.0mm I.D., 5µm particle size) from Supelco, Sigma-Aldrich (Germany); Eurospher C-18 column (7.5cm×2.1mm I.D., 3µm particle size) from Säulentechnik KNAUER (Germany); and ACE UltraCore Super C-18 (7.5cm×2.1mm I.D., 5µm particle size) from A.C.T.L. (Scotland). ACE UltraCore Super C-18 was selected for our study. In the assay, the core type columns provided good retention for the dansylated analytes, and there were no problems in back pressure inconsistency between the extraction column and the analytical column.
In order to remove the hydrophilic dansyl hydroxide: We investigated the effect of the organic modifier composition of the washing mobile phase, with ACN ranging from 0% to 15% (v/v) and MeOH ranging from 0% to 5% (v/v) in the FAA buffer. Increasing the concentration of both ACN and MEOH resulted in a decrease in the peak area of dansyl hydroxide. Moreover, the peak shape of dansyl hydroxide tended to tail on the separation column. Therefore, ACN-MeOH organic mixture was chosen with the FAA buffer because all dansylated analytes were sufficiently retained on the capture column, eluting from the precolumn when using the analytical mobile phase under the gradient conditions. For these reasons, the washing mobile phase contained 1.9% (v/v) ACN and 1.1% (v/v) MeOH in FAA buffer, which was used for further experiments.
In determining the optimum flow rate of the mobile phase: The operating time of the automatic sampler shall also be taken into account, in particular in the case of the multistage injection of the sample. The influence of flow rate in a range from 0.25 to 0.5 ml/min were tested in each injection step. The extraction was carried-out on the ACE UltraCore Super C-18 (7.5cm×2.1mm I.D., 5µm particle size) column with a “washing” mobile phase (1.9% (v/v) of ACN and 1.1% (v/v) of MeOH in 15mM FAA buffer at pH 3.7), and at a flow rate of 0.3 ml/min.
The optimum of sample enrichment and clean-up time: Were calculated from the experimentally measured breakthrough volume of dansyl hydroxide (VB=VR-(Wb/2) U), with VB, breakthrough volume, VR retention volume and Wb peak width of dansyl hydroxide, and U flow rate of “loading”. The breakthrough volume is related to the properties of the first column, and this can also be calculated using mathematical definition with the theoretical parameters of separation: [(VB=(1+kp)(1-(2.3*Vm/√N) where VB, breakthrough volume, kp retention factor of dansyl hydroxide, Vm the hold-up volume and N number of plates of the first column]. Using the above-described parameters, the breakthrough volume of dansyl hydroxide was determined through calculation and experimental methodology, the resulting value being 1500µl. In the PD model study, when 150 or 450µl sample volume was injected into the ACE C-18 (7.5×2.1, 5µm) column by using the “washing” mobile phase composition with a flow rate of 0.3 ml/min, the maximum sensitivity for each of the analytes was obtained by selecting the column-switching time at a value set of 4 or 8minute, respectively.
To improve the sensitivity and peak shape we tested the different injection of sample volumes: The maximum injection of sample volume was analysed at a range from 250 to 2000µl. The experimental measurement of the breakthrough volume was based on the elution of the strong polar property of dansyl hydroxide. A range from 300 to 2000µl of sample volume was injected onto the column, and the peak area of dansyl hydroxide was detected by its UV absorption wavelength (at 318nm). The maximum peak area of dansyl hydroxide was obtained at a volume of 1500µl, and no increase was achieved by increasing the sample volume to 2000µl.
Method validation
Selectivity: Blank solution, authentic standards and tissue extract from mice striatum were analysed under the analytical method conditions explained here (Figure 5,6). The developed method exhibits good selectivity since no interfering peaks are observed at the retention time of the desired analytes (as shown in Figure 1). Its good sensitivity is also evident from the high (r2>0.953) and the low LOD values of anandamide that are given in Table 2,3.
Linearity Nine curves were plotted at different levels within an appropriate concentration range of Asp, Glu, Gly, Gaba, NE, DA, 5-HT, EAE and 2-AG in extraction solution and perfusion solution. As shown in Table 2, all UV and FL curves have a linear response with correlation coefficients (r2) in a range of 0.953-0.998 for all analytes. The intercepts were not significantly different from zero, while slopes were significantly different to zero. Additionally, 95% confidence interval of intercepts include zero, which means that quantification analysis can be performed when based on a single-point calibration approach.
Accuracy: The Accuracy was determined by means of the recovery experiment, with the addition of known concentrations at two concentration levels (35% and 135%). The spiked solution was analysed, and the results expressed a percent recovery (%). It was observed that the accuracy of DA analyte is lower than that obtained by the other analytes. This low value can be attributed to its high sensitivity in strong basic reaction medium. Table 3 shows that the accuracy data at both concentration levels meets the acceptance criteria, as recovery values ranged from 80 to 97%.
The Limit of Detection (LOD) and the Limit of Quantification (LOQ) were estimated based on calibration curves of analytes, constructed in a 50-395 pmol/l range. This method is able to detect and quantify Asp, Glu, Gly, Gaba, NE, DA, 5-HT, EAE and 2-AG in concentrations below that of 26.5 to 196.5 pmol, and 15.75 to 118.25 pmol, respectively (Table 2).
Applications to murine model of parkinson’s disease
Subsequent to the optimization of the dansylation, and the validation of the proposed analytical method, the contents of NTs and eCBs from MPTP treated striatum of mice were assessed. Changes in the concentration of neurotransmitters–such as dopamine, glutamate, GABA, anandamide and 2-AG – are shown in Tables 4,5. The acute MPTP treatment significantly reduced the striatal dopamine content (F1,35=943.54; p<0.000001) when compared to the salt treated mice, and this effect was independent of the genotype of the mice and was consistent with our previous findings [37]. However, when comparing the rate of dopamine reduction, the MPTP effect was significantly lower in subacute treated and in P2Y12 receptor deficient animals.
When MPTP was administered in an acute manner, both amino acid contents significantly increased [GABA (F1,51=91.8; p<0.00001)], [Glu (F1,51=18.85; p< 0.00001)], but this effect was not observed in P2Y12 R-deficient animals. The results of our acute PD model, the increase in striatal Glu and GABA content, was in line with the data of plasma and post-mortem brain samples of human patients [6,7].
The subacute regimen of MPTP treatment induced significant increases in both AEA (F1.51=10.76; p<0.002) and 2-AG (F1,51=28.73; p<0.000003) levels of wild type, and P2X7 receptor deficient, striatum of mice. In contrast, after acute MPTP administration, only the anandamide levels increased (F1,51=9.75; p<0.0032) in the P2X7-ko type mouse striatum. In acute PD model of the P2Y12 purine receptor assay, the 2-AG content was higher in the striatum of salt treated animals. Surprisingly, MPTP treatment did not alter the content of either amino acids or endocannabinoids in the striatum of P2Y12 receptor-deficient mice.
Additionally, it was shown in a human platelet lysate neuroprotection approach that when [the Lund Human Mesencephalic] LUHMES cell culture was treated with MPP+, the viability of the culture was reduced, which could be prevented by the addition of a PLC inhibitor [48]. It can be assumed that MPP+ produced from MPTP by monoamine-oxidase (MAO) also exerts a similar effect in the striatum of living mice, activating PLC. In animals, phospholipase C (PLC) cleaves phosphatidylinositol-4,5-bisphosphate (PIP2) into the second messenger’s diacylglycerol (DAG) and inositol-1,4,5-triphosphate. Several G-protein-coupled receptors (GPCRs), including P2Y receptors, activate PLC. In microglia, for example, it was found that P2X7 receptor induces Ca2+ rise, increases DAG lipase activity, and thus favours the production of the endocannabinoid 2-AG from DAG, which is generated by PLC [49]. Based on all these references, and on our experimental results, it can be hypothesized that the P2X7 receptor, by increasing the level of 2-AG, may contribute to minor damage in the striatum of the P2Y12 receptor-deficient animal.
Conclusions
The purpose of this study was to describe the optimal experimental conditions for maximizing the formation and detection of aqueous media of dansyl derivatives of hydrophilic and lipid-like neurotransmitters, and to validate the appropriate method to simultaneously quantify the analytes – including DA, NE, 5-HT, Asp, Glu, Gaba, Gly, AEA and 2 AG – in biological samples column-switching assisted high performance liquid chromatography, UV, and fluorescence detection. Although there are reports of analytical work on monoamine and endocannabinoid dansylation, previous studies have focused on the optimization of non-aqueous media processes. Therefore, the methodology described in this work includes several novel parameters-such as sample solvent, reagent concentration and pH, among others-combined with statistical tools, in order to determine the optimal dansylation and column-switching conditions and the quantification of NTs and eCBs from different types of biological samples. We chose to use aqueous acidification to quench the dansyl reaction because it can be widely applied; this is due to the advantages it has over traditional approaches. For example, it does not appear that an additional peak in the chromatogram avoids the possibility of interferences. The results of this investigation show that the efficiency of the dansylation reaction is not reduced by the use of an aqueous medium in the case of the lipid component. The two-dimensional techniques made it possible to inject large volumes of the sample, and by controlling the column-switching time, to remove dansyl hydroxide matrix.
The HPLC–UV, FL method showed adequate validation parameters, such as specificity, linearity, precision, accuracy, and limits of detection and quantification on pmol/ml scale. It could be concluded that the HPLC–UV, FL method is reliable and adequate for simultaneously determining of the NTs and eCBs of brain samples; this method is also an important tool for testing animal model of diseases. Our investigation could play a leading role in further research.
This study was supported by research grants from Hungarian Research and Development Found (Grant K116654), Hungarian Brain Research Program [2017-1.2.1-NKP-2017-00002] and the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No. 766124.
Contributions
Maria Baranyi (MsC) conceived and conducted the study including data collection and data analysis, as well as wrote the manuscript.
Prof Beata Sperlagh (MD PhD DsC) designed MPTP research and provided funding for the study. All authors reviewed the results and the final version of the manuscript.
- Sonsalla PK, Youngster SK, Kindt MV, Heikkila RE (1987) Characteristics of 1-methyl-4-(2'-methylphenyl)-1,2,3,6-tetrahydropyridine-induced neurotoxicity in the mouse. J Pharmacol Exp Ther 242: 850-857. Link: http://bit.ly/2Qao4R2
- Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson's disease. Nat Protocols 2: 141-151. Link: http://bit.ly/2sZKTi7
- Fornai F, Schlüter OM, Lenzi P, Gesi M, Ruffoli R, et al. (2005) Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and α-synuclein. Proc Natl Acad Sci U S A 102: 3413-3418. Link: http://bit.ly/35LHYZ0
- Ivanov I, Borchert P, Hinz B (2015) A simple method for simultaneous determination of N-arachidonoylethanolamine, N-oleoylethanolamine, N-palmitoylethanolamine and 2-arachidonoylglycerol in human cells. Anal Bioanal Chem 407: 1781-1787. Link: http://bit.ly/2PNnM3i
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, et al. (1999) Brain Regional Distribution of Endocannabinoids: Implications for Their Biosynthesis and Biological Function. Biochem Biophys Res Commun 256: 377-380. Link: http://bit.ly/2PNGgko
- Buchanan RJ, Gjini K, Darrow D, Varga G, Robinson JL, et al. (2015) Glutamate and GABA concentration changes in the globus pallidus internus of Parkinson's patients during performance of implicit and declarative memory tasks: a report of two subjects. Neurosci Lett 589: 73-78. Link: http://bit.ly/371zfC7
- Yuan YS, Zhou xJ, Tong Q, Zhang L, Zhang L, et al. (2013) Change in Plasma Levels of Amino Acid Neurotransmitters and its Correlation with Clinical Heterogeneity in Early Parkinson's Disease Patients. CNS Neurosci Ther 19: 889-896. Link: http://bit.ly/35QkC4y
- Fernández-Ruiz J, Moreno-Martet M, Rodríguez-Cueto C, Palomo-Garo C, Gómez-Cañas M, et al. (2011) Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol 163: 1365-1378. Link: http://bit.ly/2ENvTxi
- Mounsey RB, Mustafa S, Robinson L, Ross RA, Riedel G, et al. (2015) Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Exp Neurol 273: 36-44. Link: http://bit.ly/395pk0l
- Parrot S, Sauvinet V, xavier JM, Chavagnac D, Mouly-Badina L, et al. (2004) Capillary electrophoresis combined with microdialysis in the human spinal cord: a new tool for monitoring rapid peroperative changes in amino acid neurotransmitters within the dorsal horn. Electrophoresis 25: 1511-1517. Link: http://bit.ly/2PQ0xfF
- Hong JY, Park NH, Oh MS, Lee HS, Pyo H, et al. (2013) Profiling analysis of biogenic amines and their acidic metabolites in mouse brain tissue using gas chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 940: 94-103. Link: http://bit.ly/2sgDIlE
- Cai HL, Zhu RH, Li HD (2010) Determination of dansylated monoamine and amino acid neurotransmitters and their metabolites in human plasma by liquid chromatography–electrospray ionization tandem mass spectrometry. Anal Biochem 396: 103-111. Link: http://bit.ly/2sUDyAu
- Wu Y, Li L (2014) Dansylation Metabolite Assay: A Simple and Rapid Method for Sample Amount Normalization in Metabolomics. Anal Chem 86: 9428-9433. Link: http://bit.ly/376dlO3
- Kumar A, Hart JP, McCalley DV (2011) Determination of catecholamines in urine using hydrophilic interaction chromatography with electrochemical detection. J Chromatogr A 1218: 3854-3861. Link: http://bit.ly/2rjJa6T
- Huang F, Li J, Shi HL, Wang TT, Muhtar W, et al. (2014) Simultaneous quantification of seven hippocampal neurotransmitters in depression mice by LC–MS/MS. J Neurosci Methods 229: 8-14. Link: http://bit.ly/2PQ1gHl
- Wu x, Wang R, Jiang Q, Wang S, Yao Y, et al. (2014) Determination of amino acid neurotransmitters in rat hippocampi by HPLC-UV using NBD-F as a derivative. Biomed Chromatogr 28: 459-462. Link: http://bit.ly/35PjjTz
- Yoshitake T, Fujino K, Kehr J, Ishida J, Nohta H, et al. (2003) Simultaneous determination of norepinephrine, serotonin, and 5-hydroxyindole-3-acetic acid in microdialysis samples from rat brain by microbore column liquid chromatography with fluorescence detection following derivatization with benzylamine. Anal Biochem 312: 125-133. Link: http://bit.ly/2SgEmu2
- Del Pino J, Martínez MA, Castellano VJ, Ramos E, Martínez-Larrañaga MR, et al. (2011) Effects of prenatal and postnatal exposure to amitraz on norepinephrine, serotonin and dopamine levels in brain regions of male and female rats. Toxicology 287: 145-152. Link: http://bit.ly/34Pnpts
- Nguyen AT, Aerts T, Van Dam D, De Deyn PP (2010) Biogenic amines and their metabolites in mouse brain tissue: Development, optimization and validation of an analytical HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci 878: 3003-3014. Link: http://bit.ly/2ZinqVE
- Su F, Wang F, Zhu R, Li H (2009) Determination of 5-Hydroxytryptamine, Norepinephrine, Dopamine and Their Metabolites in Rat Brain Tissue by LC–ESI–MS–MS. Chromatographia 69: 207-213. Link: http://bit.ly/2PQ7KG7
- Tareke E, Bowyer JF, Doerge DR (2007) Quantification of rat brain neurotransmitters and metabolites using liquid chromatography/electrospray tandem mass spectrometry and comparison with liquid chromatography/electrochemical detection. Rapid Commun Mass Spectrom 21: 3898-3904. Link: http://bit.ly/2SkSW3K
- Zhu KY, Fu Q, Leung KW, Wong ZC, Choi RC, et al. (2011) The establishment of a sensitive method in determining different neurotransmitters simultaneously in rat brains by using liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr B 879: 737-742. Link: http://bit.ly/2ENdqKu
- Zhou R, Guo K, Li L (2013) 5-Diethylamino-naphthalene-1-sulfonyl Chloride (DensCl): A Novel Triplex Isotope Labeling Reagent for Quantitative Metabolome Analysis by Liquid Chromatography Mass Spectrometry. Anal Chem 85: 11532-11539. Link: http://bit.ly/394FF5s
- Wilson SF, James CA, Zhu x, Davis MT, Rose MJ (2011) Development of a method for the determination of glycine in human cerebrospinal fluid using pre-column derivatization and LC–MS/MS. J Pharm Biomed Anal 56: 315-323. Link: http://bit.ly/2PNHgFa
- Guo K, Bamforth F, Li L (2011) Qualitative Metabolome Analysis of Human Cerebrospinal Fluid by 13C-/12C-Isotope Dansylation Labeling Combined with Liquid Chromatography Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J Am Soc Mass Spectrom 22: 339-347. Link: http://bit.ly/2QaPSEO
- Song P, Mabrouk OS, Hershey ND, Kennedy RT (2012) Kennedy, In Vivo Neurochemical Monitoring using Benzoyl Chloride Derivatization and Liquid Chromatography – Mass Spectrometry. Anal Chem 84: 412-419. Link: http://bit.ly/2PMD7RQ
- Málly J, Baranyi M, Vizi ES (1994) Change in the concentrations of amino acids in cisternal CSF of patients with essential tremor. J Neural Transm (Vienna) 57: 1012-1013. Link: http://bit.ly/2MnK9KP
- Fontana A, Di Marzo V, Cadas H, Piomelli D (1995) Analysis of anandamide, an endogenous cannabinoid substance, and of other natural N-acylethanolamines. Prostaglandins Leukot Essent Fatty Acids 53: 301-308. Link: http://bit.ly/2tCSaEW
- Giuffrida A, Piomelli D (1998) Isotope dilution GC/MS determination of anandamide and other fatty acylethanolamides in rat blood plasma. FEBS Lett 422: 373-376. Link: http://bit.ly/2QaWvH3
- Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, et al. (1996) Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett 393: 231-235. Link: http://bit.ly/2Mkhiqy
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA (2007) Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem 360: 216-226. Link: http://bit.ly/2SkKbXy
- Kempe K, Hsu FF, Bohrer A, Turk J (1996) Isotope dilution mass spectrometric measurements indicate that arachidonylethanolamide, the proposed endogenous ligand of the cannabinoid receptor, accumulates in rat brain tissue post mortem but is contained at low levels in or is absent from fresh tissue. J Biol Chem 271: 17287-17295. Link: http://bit.ly/2SiCqRZ
- Wang Y, Liu Y, Ito Y, Hashiguchi T, Kitajima I, et al. (2001) Simultaneous measurement of anandamide and 2-arachidonoylglycerol by polymyxin B-selective adsorption and subsequent high-performance liquid chromatography analysis: increase in endogenous cannabinoids in the sera of patients with endotoxic shock. Anal Biochem 294: 73-82. Link: http://bit.ly/2PNnhGw
- Yagen B, Burstein S (2000) Novel and sensitive method for the detection of anandamide by the use of its dansyl derivative. J Chromatogr B Biomed Sci Appl 740: 93-99. Link: http://bit.ly/2rjsuMQ
- Baranyi M, Milusheva E, Vizi ES, Sperlágh B (2006) Chromatographic analysis of dopamine metabolism in a Parkinsonian model. J Chromatogr A 1120: 13-20. Link: http://bit.ly/2tK6UlC
- Hracsko Z, Baranyi M, Csolle C, Goloncser F, Madarasz E, et al. (2011) Lack of neuroprotection in the absence of P2x7 receptors in toxin-induced animal models of Parkinson's disease. Mol Neurodegener 6: 28. Link: http://bit.ly/2Qis7Lc
- Baranyi M, Porceddu PF, Gölöncsér F, Kulcsár S, Otrokocsi L, et al. (2016) Novel (Hetero)arylalkenyl propargylamine compounds are protective in toxin-induced models of Parkinson’s disease. Molecular Neurodegeneration 11: 6. Link: http://bit.ly/2QaDZi1
- Adermark L, Talani G, Lovinger DM (2009) Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci 29: 32-41. Link: http://bit.ly/372Ww6U
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. Link: http://bit.ly/2Q5HZQM
- Goloncser F, Baranyi M, Balazsfi D, Demeter K, Haller J, et al. (2017) Regulation of Hippocampal 5-HT Release by P2x7 Receptors in Response to Optogenetic Stimulation of Median Raphe Terminals of Mice. Front Mol Neurosci 10: 325. Link: http://bit.ly/2ZoceqE
- Balázsfi D, Zelena D, Demeter K, Miskolczi C, Varga ZK, et al. (2018) Differential Roles of the Two Raphe Nuclei in Amiable Social Behavior and Aggression - An Optogenetic Study, Front Behav Neurosci 12: 163. Link: http://bit.ly/2PQ2P8b
- Euracham/CITAC (2017) Guide to Quality in Analytical Chemistry-3rd edition. Link: http://bit.ly/34QmsRD
- Horwitz W, Albert R, Deutsch MJ, Thompson JN (1990) Precision parameters of methods of analysis required for nutrition labeling. Part I. Major nutrients. J Assoc Off Anal Chem 73: 661-680. Link: http://bit.ly/2sRk28a
- Pernica M, Poloucka P, Seifertova M, Simek Z (2015) Determination of alkylphenols in water samples using liquid chromatography-tandem mass spectrometry after pre-column derivatization with dansyl chloride. J Chromatogr A 1417: 49-56. Link: http://bit.ly/395MrI8
- Bartzatt R (2001) Dansylation of hydroxyl and carboxylic acid functional groups. J Biochem Biophys Methods 47: 189-195. Link: http://bit.ly/2tK5VSt
- Honda L, Becerra-Herrera M, Richter P (2018) Liquid chromatography-time-of-flight high-resolution mass spectrometry study and determination of the dansylated products of estrogens and their hydroxylated metabolites in water and wastewater. Anal Bioanal Chem 410: 7909-7919. Link: http://bit.ly/2POVDJh
- Bartzatt R (2001) Fluorescent labeling of drugs and simple organic compounds containing amine functional groups, utilizing dansyl chloride in Na(2)CO(3) buffer. J Pharmacol Toxicol Methods 45: 247-253. Link: http://bit.ly/2PNIksE
- Gouel F, Do Van B, Chou ML, Jonneaux A, Moreau C, et al. (2017) The protective effect of human platelet lysate in models of neurodegenerative disease: involvement of the Akt and MEK pathways. J Tissue Eng Regen Med 11: 3236-3240. Link: http://bit.ly/2ENsNCF
- Witting A, Walter L, Wacker J, Möller T, Stella N (2004) P2x7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci U S A 101: 3214-3219. Link: http://bit.ly/2Zjocl3

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
