Open Journal of Analytical and Bioanalytical Chemistry
Fast multi-residue method for determination of nineteen benzimidimidazoles in meat tissues by liquid chromatography tandem mass spectrometry
Milena Funeva-Peycheva* and Georgi Stoev
Cite this as
Peycheva MF, Stoev G (2019) Fast multi-residue method for determination of nineteen benzimidimidazoles in meat tissues by liquid chromatography tandem mass spectrometry. Open J Anal Bioanal Chem 3(1): 065-071. DOI: 10.17352/ojabc.000013A fast, sensitive and selective method has been developed for quantitative determination of residues of nineteen benzimidazoles in meat by speeding the productivity of the conventional liquid chromatographs. The analytes are extracted with phosphate buffer and acetonitrile. Extract is purified by Strata X cartridges. Benzimidazoles are separated within 9 min with a conventional liquid chromatograph. The residues are detected by mass spectrometry at Selected Reaction Monitoring SRM. The separation of the compounds is done on a reversed phase RP-PFP column (50×2,1mm) with 2.6μm core-shell particle size at 50oC. The maximal pressure during analysis is 250 bars. The calculated decision limits (CCα) are between Maximum Residue Limits (MRL)+8% MRL and MRL+33% MRLs. Detection Capability (CCβ) values are in the range MRL+13% and MRL+66%. The detection capabilitys (CCβ) are between MRL and 8% MRL and MRL+66% MRL for the range of investigated benzimidazoles. The results of three separate assays (n= 3×6) show the mean recovery between 80% and 110%. Linearity of the method, assessed by coefficient of correlation r2, is between 0.990 and 0.995. The accuracy at MRL level of benzimidazoles is <10% with precision below 15%, expressed as Relative Standard Deviation (RSD).
Introduction
Benzimidazoles are anti-parasitic agents used against endo parasites and anthelmintics, widely used when raising animals from which food is produced. Benzimidazoles are used as fungicidal agents too [1]. Some representatives of this drug’s class have teratogenic and embryotoxic effects [2]. Large number of metabolites in edible animal tissues can be identified, for example up to and more than 19 possible residues [3]. To ensure consumers the European Union has set Maximum Residue Limits (MRLs) for benzimidazoles and their metabolites in foods with animal origin. These limits range from 50 to 225μg/kg depending on compound and the type of matrixes (muscle, liver, kidney and fat) [4], (Table 1). For most of the benzimidazoles, the marker residue is defined as the sum of the parent drug and/or its major (or most persistent) metabolite. A comprehensive review of properties and methodologies for determination of benzimidazole residues in biological matrices is presented by Danaher et al., [5]. Despite similarities in the molecule structures and mode of action, benzimidazoles show differences in lipophilicity and pKa values. Moreover, the parent (marker) drugs and their metabolites possess quite various properties. This result into difference in their chromatographic behavior and limited number of benzimidazoles can be determined simultaneously.
There are many scientific works related to benzimidazoles analysis in animal tissues, milk and plant tissues. Numbers of reported methods relate to analysis of individual benzimidazoles and their metabolites in food products [6-9]. However, because of the large number of benzimidazoles licensed for use, multi-residue methods are more useful for the practice. They are more attractive and challenging for the analytical experts, too. These methods could provide more complete surveillance for the drugs. Methods for analysis of enlarged number of benzimidazole residues in milk [10-14] and tissues [3,15-18] have been developed also. Methods for determination of 10 up to 14 benzimidazoles, using Liquid Chromatography-tandem mass spectrometry (LC–MS/MS) were published in the last decade [3,19-22]. The separation of the enlarged number of benzimidazoles is realized for 30-40 min. Danaher et al., [23], separate 14 benzimidazoles simultaneously in a run-time of 60 min and gradient elution program using Xterra C18 column. Kinsella et al., [24], develops a LC – MS/MS multi-residue method for simultaneous identification and quantification of 38 residues of the most widely used anthelmintic veterinary drugs, including some benzimidazoles. Lugomer et al., analyzed 18 benzimidazoles in milk for 28 min [21]. Recently several papers present multiresidue screening methods, based on the increased efficiency of Ultra-High Performance Liquid Chromatography (UHPLC) and High Resolution Mass Spectrometry (HRMS) [25-28]. The reliability of the methods using other types of mass spectrometry as TOF HRMS is higher than that of the Low Resolution Mass Spectrometry (LRMS) and Selected Reaction Monitoring (SRM) mode. These techniques are not applicable and suitable for confirmatory purposes. The beneficial effects of UHPLC with SRM-LRMS have been applied for determination and confirmation of residues of 24 benzimidazoles in meat [29], where the samples are analyzed within 60 min by two runs at positive and negative ionization, respectively. UHPLC with sub-2μm particle size columns become as an attractive technique for improving the separation and/or reducing the time of analysis. The increased back pressure – above 500 bars - requires new generation of Liquid Chromatography (LC) equipment. In order to realize high efficiency the equipment must possesses reduced delay and death volumes. This equipment, however, is more expensive. Horne et al., and later Kirkland, proposed core-shell particles with porous adsorption layer as a packing material for LC column for increasing the efficiency [30,31]. Many companies have already produced columns with such type of particles, an internal diameter of 1.7 or 2.6μm and 0.23–0.35μm porous layers like C18, HFP or HILIC as packing materials. [32-34]. Comparing the separation ability and efficiency of columns with porous particles (1.7μm) and this one with core - shell particles (2.6μm), it was established that the last ones possess an equal or higher efficiency than one of the columns with sub-2μm porous particles. Applying these columns to analysis of multi compound mixtures of nonsteroidal anti-flamatory drugs, pesticides and benzimidazoles with conventional liquid chromatographs, it can be realized fast separation at 50oC and an acceptable back pressures fewer than 250 bars. These results give us an opportunity to use the convenient liquid chromatographs. So it can be developed fast multi-residue SRM method for confirmation and quantitative determination of an extended range of benzimidazoles instead the expensive UHPLC instrumentation.
Experimental
Standards: The reference standards and their deuterated analogs Hydroxythiabendazol (TBZ-OH), Albendazolsulfonamine (NH2-ALBZ-SO2), Thiabendazol (TBZ), Albendazolsulfoxid (ALB-SO), Albendazolsulfon (ALB-SO2), Oxfendazol (OFEB), Hydroxymebendazol (ОH-MBZ), Aminomebendazol (NH2-MBZ), Oxibendazol (OBZ), Fenbendazolsulfon (FEB-SO2), Aminoflubendazol (NH2-FLU), Mebendazol (MBZ) Albendazol (ABZ), Flubendazol (FLU), Triclabendazol (TKLBZ), Ketotriclabendazol (Keto-TKLBZ), Triclabendazolsulfoxid (TKLBZ-SO), Triclabendazolsulfon (TKLBZ-SO2), Fenbendazol (FEB),Albendazol-d3 (ABZ_d3), Albendazolsulfoxid_d3 (ALB-SO_d3), Albendazolsulfon-d3 (ALB-SO2_d3), Oxfendazol-d3 (OFEB_d3), Oxibendazol-d7 (OBZ_d7), Fendazolsulfon_d3 (FEB- SO2_d3), Mebendazol-d3 (MBZ_d3), Flubendazol_d3 (FLU_d3), Triclabendazol-d3 (TKLBZ_d3) and Fenbendazol-d3 (FEB_d3) (Table 2) with purity above 99% were purchased from Witega (Berlin, Germany) and Sigma Aldrich (St. Louis, MO, USA).
Reagents and materials: HPLC-grade acetonitrile and methanol were acquired from J.T. Backer Chemical Co (Philipsburg, NJ). Ultra-purified water was prepared by using a Millipore Mili-Q system (Milford, CT, USA.) water purification system. Sodium bicarbonate (NaHCO3), di-Potasium hydrogen orthophosphate anhydrous (K2HPO3), formic acid, Dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solid Phase Extraction cartridges (SPE)- StrataTM-X 33µm Polymeric Reversed Phase, 200mg/3ml, tubes were purchased from Phenomenex (Inc. United States). Solid phase extraction was performed using Supelco SPE manifold.
Stock standard solutions of benzimidazoles: Stock standard solutions of benzimidazoles and their deuterated analogs (1000µg.mL-1). were prepared by dissolving 10.00mg of each compound in 10ml DMSO.
Working standard solutions (10µg.mL-1) of benzimidazoles and working standard solutions (10µg.mL-1) of deuterated ones: prepare by dilution of 100 µl from the stock standard solutions of and, respectively, in methanol using 10 ml volumetric flasks.
Samples: Drug free muscles with different types as cattle, pig, sheep and chicken and origin are being used for the experiments. Muscle samples (100g approximately) were homogenised and kept at-20oC.
Sample preparation: Samples were prepared according to the procedure of Federal Office of Consumer Protection and Food Safety BVL Berlin [29]. Portions of 2g of homogenised muscles and 10ml phosphate buffer, pH 8, were mixed with vortex for 1 min in 50ml centrifuge tube. 20ml acetonitrile was added, the mixture was shaken for 10 min and centrifuged for 10 min at 4000rpm. The superenatant was decanted, transferred into another 50ml centrifuge tube and then evaporated to dryness under nitrogen at 40oC. The dryed extract was dissolved in mixture 1ml 0.1M KH2PO4 and 5ml 0.5M NaHCO3 with vortex for several seconds.
The Strata X cartridge was pre-conditioned with 5ml methanol and 5ml water. The extract was loaded on the cartridge, was washed with 3ml water and the cartridge was dried by air at 600 mbar for 10 min. The analytes were eluted with 5ml methanol, evaporated to dryness under nitrogen at 40oC, reconstruted in 500µL 5% of MeOH in water with vortex for 1 min and transferred into Eppendorf vials. Sample of 50µl was injected into the chromatograph.
LC-MS-MS system: The benzimidazole determination is carried out in a LC–MS/MS System TSQ Quantum Discovery MAX (Thermo Electron Corporation). The electrospray ionization–tandem mass spectrometry (ESI–MS/MS) detection of the benzimidazoles is achieved using a triple stage quadrupole instrument. The negative and positive ionization mode is used, and the ions are monitored in the Selecting-Reaction Monitoring (SRM) mode. The ESIMS/MS conditions are the following: spray voltage (-4) kV; sheath gas (N2, >95%) 50 (arbitrary units); auxiliary gas (N2, >95%) 10 (arbitrary units); capillary offset/voltage (-5) V, capillary temperature 350oC. The dwell time was 100ms/transition. Two transitions are followed for identification but only one was used for quantitation (Table 2). In depends on the retention times of the analytes they are grouped in several time segments and for everyone is constructed scan event with its characteristic ions (Table 2). The analytes, which are ionized in a negative mode (TKLBZ, Keto-TKLBZ, TKLBZ-SO, TKLBZ-SO2 and TKLBZ_d3), are analyzed in the last segment (Table 2). The LC effluent was diverted from the mass spectrometer for the first 1.5 min, and again at 8.5 min. Instrumental control and data analysis were performed by Qualbrauser application software from Thermo Electron Corporation. The chromatographic column is Kinetex RP-PFP column (50×2.0mm i.d., 2.6μm pore diameter, Phenomenex) equipped with the pre-column type Gemini (3.0mm×2.0mm i.d., 5μm particle sizes, Phenomenex).
A gradient elution mode with mobile phases consisting of 0.1% formic acid in water (A), 0.1% formic acid in acetonitrile (B), and 0.1% formic acid in methanol (C) is used. The flow rate of 0.75ml/ min is applied to separate the analytes at 50oC (Table 3).
Method validation: The validation of the method is carried out according rules of Commission Decision 2002/657/EC [35]: Specificity, linearity, recovery, repeatability, accuracy, decision limit (CCα) and detection capability (CCβ).
Results and Discussion
Optimization of LC-MS conditions
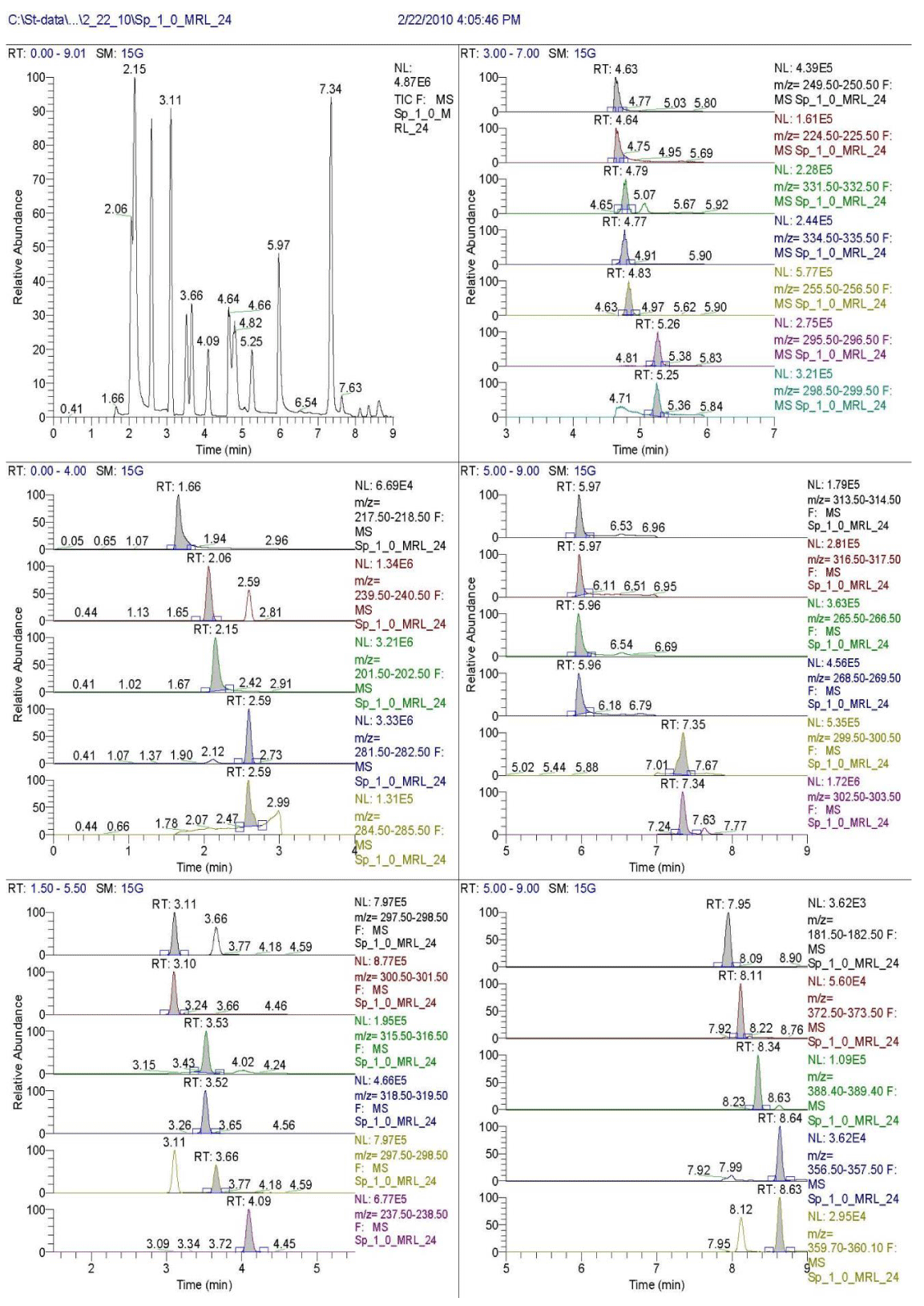
Many difficulties are arisen during separation of large number of compounds. Despite the long analyses time at the multi-residue methods, considerable number of the analytes can be co-eluted [25-29]. Kinetex RP-PFP column (50×2mm, 2.6µm) demonstrates a high efficiency to benzimidazoles (Figure 1). Limits of 250 bars are accepted as a medium value of the pressure at which pumps still work without risk of damages. Another approach applied for reducing the back pressure is to decrease the viscosity of the mobile phase increasing the temperature. Carrying out the separation of benzimidazoles at 50oC, which is also a practically acceptable value, the goal can be achieved. Combination of these two factors gives the possibility to realize acceptable separation and effective resolution of the analytes within 9 min at maximal back pressure of 250 bars. Five analytes can’t be fully separated because of mobile phase composition and molecule structure: triclabendazoles needs negative ionization mode. They can be eluted in the end of the run with one change of positive and negative modes in this segment. Despite the low resolution, ignoring it, it can be obtained reliable SRM mass spectra and quantitative data for all analytes. The SRM scan event for every one of the analytes is combined in six segments (Table 2). The peak shapes has correct Gaussian form. By this way the required three identification points, according to 2002/657/EC as confirmatory criteria for Group B substances are exceeded.
With more than thousands runs of standards, fortified samples, samples of routine laboratory practice, the column retains its efficiency and selectivity (Figure 1). The reproducibility of the relative retention times of analytes, assessed by RSD, is below 2.0%. Both, the positive and negative ionization modes (ESI+/ESI−), are used. Triclabendazoles show much higher [M-H]- abundance under a negative (ESI-) mode. To obtain higher sensitivity, ESI and MS/MS parameters, voltage and temperature of capillary, scan width and scan time, Collision Energy (CE), isolation width and pressure of the collision gas are optimized for each benzimidazoles (Table 2). Because 19 benzimidazoles and their 10 internal standards require large number of scan events, the whole scanning time (9 min) is divided into six segments for acquisition of 15 to 20 points of each one of chromatographic peaks. This ensures accuracy of quantitative analysis, because a signal to noise criterion, S/N, - 10 and 20 for LOD and LOQ, respectively, is used. So the false positives signals during determination at levels close to limits of detection were minimizised (Table 1).
Validation study
The method is validated using porcine muscle as a model matrix at the specified MRLs of each one of analytes. While most of benzimidazole compounds are expressed as the sum of the marker metabolites (Table 1), validation is provided applying MRL value for each individual marker residue. This is necessary because of the variability in proportions of marker residues which might occur in samples. The concentrations of the analytes are corrected with coefficients, which presents their transformation to metabolites [29]:
Sum of albendazole:
mALBZ=mALB-SO×0.943+mALB-SO2×0.892+
mNH2-ALBZ-SO2-×1.109
Sum of fenbendazole:
mFEB-SO2=mFEB-SO2+mFEB×1.107+OFEB×1.051
Sum of flubenvdazole:
mFLU=mFLU+mNH2-FLU
Sum of mebendazole:
mMBZ=mMBZ+mNH2-MBZ- ×1.244+mOH-MBZ×1.014
Sum of thiabendazole:
mTBZ=mTBZ+mTBZ-OH
Sum of triclabendazole:
mKeto-TCBZ=mKeto-TKLBZ+mTKLBZ×0.916+mTKLBZ-SO×0.878+mTKLBZ-SO2×0.842
Selectivity: To establish the selectivity of the method, five non-fortified samples from every one of matrixes - cattle, pig, sheep and chicken muscle - are processed and analysed according procedures 2.5 and 2.6 respectively. No interfering peaks with the characteristic precursor and two daughter ions were observed at the retention times typical of benzimidazoles (Table 2).
Linearity of the response: The linearity of the chromatographic response is defined with samples fortified with standards at five calibration points in concentration range of 0.5–5.0 MRL for each of benzimidazoles. The regression coefficients (r2) are higher than 0.95 (between 0.990 and 0.995) (Table 1).
Recovery: The recovery of the method is determined using samples of porcine muscle fortified at 0.5 MRL, 1.0 MRL and 1.5 MRL levels for each one of benzimidazoles. Mean recovery (number of studies n = 6) of the analytes, calculated in three separate assays, is between 80% and 110%. The reproducibility, presented as RSD%, is 15% (Table 1).
Decision limit CCα and detection capability CCβ: Both of these parameters are determined using calibration curve procedure. Here blank samples are being fortified around MRL levels in equidistant steps (0.5 MRL, MRL and 1.5 MRL). The concentration at MRL plus 1.64 times the standard deviation at the MRL level, give CCα value for each one of the analytes. The CCβ value is determined as sum of CCα value +1.64 times the standard deviation (Table 3). Calculated CCα values in the range between MRL+8% MRL and MRL+33% MRL. CCβ values are in the range MRL+13% and MRL+66%.
LOD and LOQ: To verify Limits of Detection (LOD) and Limits of Quantification (LOQ) samples are fortifyed with all analytes on 0.100, 0.010 and 0.001 MRL-levels. LOD and LOQ are calculated on the basis of signal to noise ratio S/N=10 for LOD and S/N=20 for LOQ. The higher efficiency of chromatographic column with core-shell particles aids the LOQ for all analytes to be below CCα and CCβ (Table 3). Only LOQ for TKLBZ-SO and TKLBZ-SO2 are around 0.01 MRLs because the degree of the negative ionization is lower.
Accuracy: Eight samples of each type of matrixes (cattle, pig, sheep and chicken) are fortified with all compounds and their metabolites at concentration level of 1.0 MRL. They are analyzed to estimate the accuracy of the method. It is found that the accuracy of the method is between 4 and 10%. The precision is lower than 15%, expressed as RSD%.
The method is applied in National referent laboratory (NRL) routine analytical work. The method is under the scope of accreditation and is useful for control purposes.
Conclusion
The work reported the development and validation a fast RP-HPLC/MS-MS method for determination of residues of 19 benzimidazoles in meat within 9 min. Benzimidazoles compounds were successfully separated by HPLC. The matrix effect are eliminated effectively by employing the appropriate SPE cleanup procedure. The method has satisfactory validation characteristics according to Commission Decision 2002/657/EC for all analytes. The method is suitable for food quality and food safety control as confirmatory method.
- Li B, Zhao B, Yang GY, Wang Q, Niu LL, et al. (2012) Mebendazole in the treatment of Hymenolepis nana infections in the captive ring-tailed lemur (Lemur catta), China. Parasitol Res 111: 935-937. Link: http://bit.ly/32iBrlW
- Waghorn TS, Leathwick DM, Rhodes AP, Lawrence KE, Jackson R, et al. (2006) Prevalence of anthelmintic resistance on sheep farms in New Zealand. N Z Vet J 54:271-278. Link: http://bit.ly/34nDKFY
- Dowling G, Cantwell H, O’Keeffe M, Smyth MR (2005) Multi-residue method for the determination of benzimidazoles in bovine liver. Anal Chim Acta 529: 285-292. Link: http://bit.ly/36Bl33g
- Commission Decision No 37/2010, Official Journal of the European Communities, L 15/1; 20.1.2010.
- Danaher M, de Ruyck H, Crooks SRH, Dowling G, O’Keeffe M (2007) Review of methodology for the determination of benzimidazole residues in biological matrices. J Chromatogr B 845: 1-37. Link: http://bit.ly/2Njhebz
- Leticia Norma Carpentieri Rodrigues (2016) Adriana Karla Cardoso AmorimReis and Luciana Morita Katiki. Int Res J Pharm 7: 2230-8407. Link: http://bit.ly/2rdQAby
- Whelan M, Kinsella B, Furey A, Moloney M, Cantwell H, et al. (2010) Determination of anthelmintic drug residues in milk using ultra high performance liquid chromatography–tandem mass spectrometry with rapid polarity switching. J Chromatogr A 2012: 4612-4622. Link: http://bit.ly/33hh8a7
- Takeba K, Fujinuma K, Sakamoto M, Miyazaki T, Oka H, et al. (2000) Simultaneous determination of triclabendazole and its sulphoxide and sulphone metabolites in bovine milk by high-performance liquid chromatography. J Chromatogr A 882: 99-107. Link: http://bit.ly/2JOnw0w
- Liu H, Jiang C, Hui Y, Shen X, Zhang X, et al. (2010) SPE-LC Multi-Residue Analysis of Mebendazole and its Methabolites in Grass Carp and Shrimp. Chromatographia 72: 1083-1088. Link: http://bit.ly/32jN6ku
- Chen D, Tao Y, Zhang H, Pan Y, Liu Z, et al. (2011) Development of a liquid chromatography-tandem spectrometry with pressurized liquid extraction method for the determination of benzimidazole residues in edible tissues. J Chromatogr B 879: 1659-1667. Link: http://bit.ly/2CbY5lt
- De Ruyck H, Van Rentergherm R, De Ridder H, De Barbanader D (2000) Determination of anthelmintic residues in milk by high performance liquid chromatography. Food control 11: 165-173. Link: http://bit.ly/2NeOW1U
- Frederique L, van Holthoon, Tina Zuidema Multiresidue method for the simultaneous determination of benzimidazoles in bovine milk by on-line SPE-LC-MS/MS. Rikilt Institute of food safety Wageningen. Link: http://bit.ly/2Nj5kOI
- Constantinou S, Hadjigeorgiou M, Papachrysostomou C, Nicolaidou P (2000) Proceedings of the EuroResidue IV Conference on Residues of Veterinary Drugs in Food. Veldhoven Netherlands 297.
- Fletouris D, Botsoglou N, Psomas I, Mantis AI (1996) Trace analysis of albendazole and its sulphoxide and sulphone metabolites in milk by liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications 687: 427-435. Link: http://bit.ly/32hz3Mq
- Arenas RV, Johnston NA (1994) J AOAC Int 77: 710.
- European Committee for Standartization, European standard ref. No EN 14333-1, October 2004, ICS 67.080.01.
- Roudaut B, Garnier M (2000) in: van Ginkel LA, Ruiter A (Eds), Proceedings of EuroResidue IV Conference on Residues of Veterinary Drugs in Food, Veldhoven, Netherlands. National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands 950.
- Edder P, Ortelli D, Jan P, Corvi C (2004) in: van Ginkel LA, Bergwerff AA (Eds), Proceedings of EuroResidue V Conference on Residues of Veterinary Drugs in Food, Noordwijkerhout, The Netherlands, Faculty of Veterinary Medicine, University of Utrecht, Utrecht Netherlands 566.
- Hu XZ, Wang JX, Feng YQ (2010) Determination of Benzimidazole Residues in Edible Animal Food by Polymer Monolith Microextraction Combined with Liquid Chromatography−Mass Spectrometry. J Agric Food Chem 58: 112-119. Link: http://bit.ly/2POaFj4
- Smith S, Gieseker Ch, Reimschuessel R, Sue Decker Ch, Carson MC (2009) Simultaneous screening and confirmation of multiple classes of drug residues in fish by liquid chromatography-ion trap mass spectrometry. J Chromatogr A 1216: 8224-8232. Link: http://bit.ly/2NeRIEm
- Lugomer M, Pavlicek D, Bilandzic N, Majnaric D (2017) Determination of benzimidazole residues and their metabolites in raw milk using high performance liquid chromatography-diode array detection. Mljekarstvo 67: 231-238. Link: http://bit.ly/2NE9NuB
- Xia X, Dong Y, Luo P, Wang X, Li X, et al. (2010) Determination of benzimidazole residues in bovine milk by ultra-high performance liquid chromatography–tandem mass spectrometry. Journal of chromatography B 878: 3174-3180. Link: http://bit.ly/2Cc1PDo
- Danaher M, O’Keeffe M, Glennon JD (2003) Development and optimisation of a method for the extraction of benzimidazoles from animal liver using supercritical carbon dioxide. Anal Chim Acta 483: 313-324. Link: http://bit.ly/2WHDU8x
- Kinsella B, Lehotay SJ, Mastovska K, Lightfield AR, Furey A, et al. (2009) New method for the analysis of flukicide and other anthelmintic residues in bovine milk and liver using liquid chromatography–tandem mass spectrometry. Anal Chim Acta 637: 196-207. Link: http://bit.ly/2JPUCNw
- Ortelli D, Cognard E, Jan P, Edde P (2009) Chromatogr J B 877: 2363.
- Stolker AAM, Rutgers P, Oosterink E, Lasaroms JJP, Peters RJB, et al. (2008) Comprehensive screening and quantification of veterinary drugs in milk using UPLC–ToF-MS. Anal Bioanal Chem 391: 2309-2322. Link: http://bit.ly/33ffshi
- Kaufmann A, Butcher P, Maden K, Widmer M (2007) Ultra-performance liquid chromatography coupled to time of flight mass spectrometry (UPLC–TOF): A novel tool for multiresidue screening of veterinary drugs in urine. Anal Chim Acta 586: 13-21. Link: http://bit.ly/34wubo4
- Kaufmann A, Butcher P, Maden K, Widmer M (2008) Quantitative multiresidue method for about 100 veterinary drugs in different meat matrices by sub 2-μm particulate high-performance liquid chromatography coupled to time of flight mass spectrometry. J Chromatogr A 1194: 66-79. Link: http://bit.ly/2WGSxsy
- Stoyke M Method for determination of Anthelmintics in muskle and liver, EU Reference Laboratory for Residues of Veterinary Drugs in Berlin, www.bvl.bund.de
- Horne DS, Knox JH, McLaren L (1996) A Comparison of Mobile-Phase Peak Dispersion in Gas and Liquid Chromatography. Sep Sci 1: 531-554. Link: http://bit.ly/2oMqtYh
- Kirkland JJ (1992) Superficially porous silica microspheres for the fast high-performance liquid chromatography of macromolecules. Anal Chem 64: 1239-1245. Link: http://bit.ly/2WHtZzK
- www.phenomenex.com, KinetexTM columns with core-shell particles
- Agilent. Link: http://bit.ly/2qmG3dA
- HALO fused-core HPLC columns. Link: http://bit.ly/32hAhXO
- Commission Decision 2002/657/EC (12.8.2002), Official Journal of the European Communities L 221: 8.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
