Open Journal of Analytical and Bioanalytical Chemistry
Potentiometric sensing platform for selective determination of pregabalin in pharmaceutical formulations
Eman H El-Naby*
Cite this as
El-Naby EH (2019) Potentiometric sensing platform for selective determination of pregabalin in pharmaceutical formulations. Open J Anal Bioanal Chem 3(1): 049-056. DOI: 10.17352/ojabc.000011Pregabalin is a structural analogue of, but functionally unrelated to, the naturally occurring neurotransmitter γ-aminobutyric acid (GABA) with potent analgesic, anticonvulsant and anxiolytic activity. The abuse potential of pregabalin is a well-documented with high risk of addiction that may be fatal with overdoses particularly, among opiate addicts and polydrug use dependency syndrome, consequently, it is very pertinent to determine pregabalin in a sensitive and selective manner. Herein, for the first time the use of β-cyclodextrin:phosphomolybdic acid organic-inorganic hybrid material as a neutral ionophore in polymeric membrane based selective sensors for the determination of pregabalin in its pharmaceutical formulations has been reported. The fabricated sensors yielded an excellent performance, with a linear range from 1.0×10-6 to 1.0×10-1M pregabalin, a response slope closer to the ideal Nernstian value; 59.6 mV per decade as well as a detection limit was 6.0×10-7M and very short response time of about 5 s for high concentrations. The most interesting feature of the sensors is their remarkably selective recognition for pregabalin compared with opioids and this is an important issue that is suited to practical applications. The sensors were effectively used for the determination of pregabalin in pharmaceutical formulations with a recovery of 100.97%±0.17. On comparison with β-cyclodextrin, the proposed sensors exhibited the best performance characteristics.
Introduction
Globally, non-medical use of prescription drugs is a widespread and alarmingly prevalent phenomenon. It is well known that the abuse of prescription drugs together with its related significant health consequences and socioeconomic effects is a complex problematic and multi-faceted issue that has climbed steadily year on year. Nowadays, there are numerous concerns regarding their recreational use that is associated with the risk of intoxication and unexpected death among younger people who are the main target for these types of drugs. Pregabalin (PRG); Figure 1 belongs to gabapentinoids (pregabalin and gabapentin) prescription medications, is an alkylated analogue of gamma-aminobutyric acid (GABA); the main inhibitory neurotransmitter in the central nervous system. Nonetheless, pregabalin has no demonstrated effects on GABAergic mechanisms.
Pregabalin binds to the α-2-δ subunit site of voltage-gated calcium channels in the central nervous system, resulting in a reduction in calcium-dependent release of excitatory neurotransmitters; glutamate, noradrenaline, serotonin, dopamine, and substance P. through modulation of calcium channel function leading to analgesic, anticonvulsant, and anxiolytic effects. Pregabalin is recommended as a first-line approved by Food and Drug Administration (FDA) for the management of neuropathic pain and partial-onset seizures “epilepsy” [1], as well as for the treatment of fibromyalgia in USA in 2007 [2]. Pregabalin is approved as a significantly efficacious treatment for generalized anxiety disorder (GAD) that licensed by European Union in 2006 as one of the newer anxiolytic medications [3]. Interestingly, pregabalin with a significant effect is one of opioid sparing analgesics (ketamine, gabapentin, pregabalin, and clonidine) [4]. In addition, PRG might be a promising efficacious and safe novel candidate in the treatment of both alcohol and benzodiazepines dependence [5].
On the other side, special concerns about the potential risk of the gabapentinoids misuse have recently been raised concurrent with growing black market and illegal websites, with drawn attention to pregabalin as more desirable than gabapentin in the recreational use [6]. As described, pregabalin is an “ideal psychotropic drug” to achieve specific mindsets such as alcohol/γ-hydroxybutyric acid (GHB)/benzodiazepine-like effects mixed with euphoria, entactogenic feelings and dextromethorphan (DXM)-like dissociative as well as to cope with opiate/opioid withdrawal [7]. Most importantly, it is demonstrated that pregabalin frequently taken as part of a poly-drug cocktail scenario with high risks of serious adverse effects that could be fatal particularly in the opioids misuse population [8,9].
According to that mentioned earlier, the development of an appropriate and reliable method for the determination of PRG is an urgently required. Several reports in literature for PRG determination in its pharmaceutical formulations and biological matrices were based mainly on chromatographic methods including high performance liquid chromatography “HPLC” combined with fluorescence or UV detection of derivatized pregabalin [10-12], liquid chromatography-tandem mass spectrometry “LC-MS-MS” [13,14], however, the low molecular mass and high polarity of GABA derivatives present a challenge for this methodology. Only a few gas chromatography-mass spectrometry “GC-MS” [15,16], has also been performed with pay attention that derivatization is a must so as to apply GC–MS method as it is difficult to analyze pregabalin; an amino acid with amphoteric character using the conventional GC-MS. Spectrophotometry [17,18] and spectrofluorimetry [19] have also been developed. Even though used to a lesser extent, capillary electrophoresis; capillary electrophoresis and nuclear magnetic resonance technique was reported for PRG determination involving complexation with cyclodextrins [20]. Indeed, the determination of PRG is hard to achieve that challenging tightly related to its amphoteric nature, as well its structure as an aliphatic substituted amino acid with lacking of chromophore and fluorophore functionalities. This is resulted in a poor absorbtivity in the ultraviolet region as well as no visible absorption which reveals the need for derivatization protocol with the consumption of high cost potentially toxic organic solvents, which are hazardous to health and environment in addition to laborious sample pre-treatment process.
In comparison, ion selective sensors are electrochemical transducers that respond directly to the concentration “activity” of the target analyte through a potentiometric signal based on the measurement of the phase boundary potential at the sample/membrane interfaces [21]. Ion selective sensors have attained tremendous interest for the sensitivity, wide analyte concentration range, fast response, cost effectiveness of analysis, minimal matrix effect and reliability of the analytical information as well as what is most important, they are eco-friendly and use in situ applications. Therefore, ion selective sensors are attractive tools offering great opportunities for monitoring the analytes in many fields. The development of ion selective sensors is a well-established technique that has been implemented for the determination of pharmaceuticals for many years [22,23]. A through literature search has revealed that only one ion selective sensor has been developed for the determination of PRG in bulk drug and pharmaceutical formulations based on p-ClTPB as an ion exchanger [24]. However, the main drawback of ion exchanger based selective sensors is the selectivity mainly correlated with the lipophilcity of the analyte with respect to the interferents that commonly related to not optimal selectivity.
On the other hand, ion-ionophore mechanism has attracted considerable attention in the ion selective sensors, as they are based mainly on specifically non covalent interactions that evidently of a considerable importance in the selectivity of the potentiometric response [25,26]. In this respect, intensive research has been focused on the design and synthesis of ionophores with specific properties and functions providing the affinity and selectivity towards relevant molecules that resulted in ionophore-based sensors with superb selectivity [27-29]. So there is a lot of scope for the development of ion selective sensors for pregabalin, however, it is important to mention that molecular recognition and determination of amino acids by artificial receptors is still challenging because of their highly hydrophilic character as well as their zwitterionic nature in a wide pH range of aqueous solutions which makes impossible their ionometric determination. For that reasons, a great deal of attention has been devoted to the sensing element “ionophore” that plays the decisive role in the performance of ion selective sensors as well as the ionophore must be synthesized according to the nature of target analyte that providing its strongly entrapped within the hydrophobic membrane.
In this respect, β-cyclodextrin:phosphomolybdic acid “β-CD:PMA” organic-inorganic hybrid material was synthesized, however, to the best of our knowledge, there have been no attempts at using this hybrid material as a receptor for amino acid or a neutral ionophore in electrochemical sensors. For the sake of simplicity, our approach is the development of polymeric membrane based sensors incorporating “β-CD:PMA” hybrid material and conceived it as a neutral ionophore for the selective determination of pregabalin with the emphasis on the role of plasticizers. Furthermore, it is of interest to compare with β-cyclodextrin polymeric membrane based sensor “β-CD”.
Experimental
Reagents and materials
All chemicals used were of analytical reagent grade, unless otherwise specified and doubly distilled water was used throughout. phosphomolybdic acid (PMA, H3PMo12O40), ο-nitrophenyl octyl ether (o-NPOE), potassium tetrakis (p-chlorophenyl) borate (KTpClPB) and lanthanum chloride LaCl3.7H2O were obtained from Sigma Chemical Co., St. Louis, MO, USA. Tetrahydrofuran (THF) and dioctylphthalate (DOP) were purchased from British Drug Houses, Poole, England. High molecular weight poly (vinyl chloride) (PVC) and β-cyclodextrin (β-CD) were obtained from Aldrich Chemical Co. Milwaukee, WI. Pregabalin (PRG) was kindly supplied by International Advanced Pharmaceutical Ind. “INAD” Pharma, Giza, Egypt and Lyrica® capsules (150 mg/capsule) from Pfizer Ltd was obtained from a local community pharmacy. A stock solution of PRG (1.0×10-1M) was prepared by dissolving the calculated weight of PRG in 5 ml of 1.0×10-1M HCl and diluted to the mark with distilled water and series of working standard solutions at concentrations of (1.0×10-7 to 1.0×10-2M) were prepared by successive dilutions of the stock solution. Solutions of interferents (1.0×10-2M) were prepared for the study of potentiometric selectivity by dissolving an accurate weight of the interferent in a minimum volume of doubly distilled water, followed by dilution to 100 ml and pH was adjusted with 1×10-2M citrate buffer at pH 2.5±0.05.
Apparatus
Potentiometric measurements were performed at 25±1°C using an ORION model Star 4 digital pH/mV meter and pregabalin membrane sensors in conjunction with an Orion double junction Ag/AgCl reference electrode (90-02) containing 10% (w/v) potassium nitrate in the outer compartment. An Orion Ross pH electrode (Model 81-02) was used for pH adjustment. The cell assembly was: Ag/AgCl/10-2M KCl–10-2M PRG//sensor membrane//sample test solution/Orion double junction reference electrode.
Synthesis and characterization of sensing element; β-cyclodextrin:phosphomolybdic acid organic-inorganic hybrid material [La(H2O)9]{[PMo12O40]⊂[β-CD]2} ″β-CD: PMA″
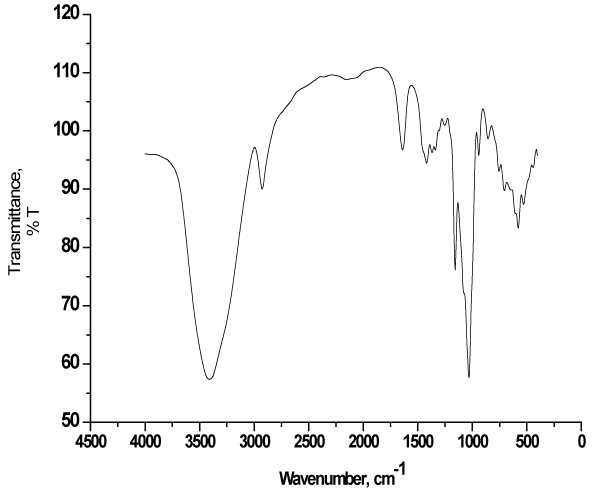
The hybrid material “ionophore” was synthesized as described in the literature, a mixture of β-CD (1.13 g, 1 mmol) and H3PMo12O40 (0.91 g, 0.5 mmol) in deionized H2O (10 mL) was stirred at room temperature for 30 min. A solution of lanthanum chloride; LaCl3·7H2O (0.56 g, 1.5 mmol) in H2O (5 mL) was added to the aqueous solution, and the resulting mixture was cooled at 4 °C in a refrigerator overnight. The yellow microcrystals that formed were collected by filtration, washed with ethanol, and in air to afford the final product “β-CD:PMA”; [La(H2O)9]{[PMo12O40]⊂[β-CD]2} which have a sandwich-like structure, wherein one [PMo12O40]3- trianion is encapsulated by the primary faces of two CD tori through intermolecular hydrogen bonding interactions and [La(H2O)9]3+ as counter cations [30]. The synthesized hybrid material was characterized with Fourier-transform infrared “FTIR”; Figure 2 and energy-dispersive X ray spectroscopy (EDX).
As shown from Figure 2, FTIR spectral signature of “β-CD:PMA” exhibited four characteristic bands for PMA around 1030, 941, 856 and 755 cm−1 with a shift to lower frequencies instead of 1064, 962, 869 and 787 [31], probably due to the formation of hydrogen bond C−H···O=Mo] [30]. Meanwhile, the strong peaks present at 2926, 1640, 1157 cm−1 are characteristics of β-CD and the broad peak with high intensity around 3413 cm−1 is corresponding to–OH of β-CD [32]. Additionally, the EDX analysis spectrum of “β-CD:PMA” includes five elements C, O, P, Mo and La as expected.
Membrane and sensor preparation
The general procedure for casting the membranes is as follows: PVC (67 mg), a plasticizer (124 mg), a sensing element (4 mg), and a lipophilic additive (50 wt% of β-CD:PMA or β-CD as an ionophore) were dissolved in THF (ca. 3 ml). The membrane cocktail was poured into a flat Petri dish (28 mm i.d.). Gradual evaporation of the THF at ambient temperature afforded an elastic, transparent membrane with a thickness of about 0.1 mm. A ‘blank’ membrane refers to the same membrane cocktail described above, however omitting the presence of a sensing element. Disks (~4 mm o.d.) were punched from the master membrane and glued with a PVC/THF slurry to plasticized PVC tubing fixed onto a 1000 ml pipette tip. The tube was then filled with equal volumes of 1.0×10-2M PRG and 1.0×10-2M KCl (internal solution) and an Ag/AgCl wire (~1 mm diameter) was immersed in this solution as an internal reference electrode. Before calibration, all sensors were conditioned by soaking in 1.0×10-3M PRG solution for ~1h before use. During the intervals between the individual measurements the sensors can be stored in the dry state after rinsing with distilled water.
EMF measurement and sensor calibration
The sensors were calibrated by spiking with successive aliquots of PRG standard solutions (1.0×10-6–1.0×10-1M) into a 9.0 ml citrate buffer at pH 2.5±0.05. Alternatively, the calibration was carried out by immersing the PRG membrane sensors in conjunction with a double-junction Ag/AgCl reference electrode into a 50-ml beaker containing 20 ml aliquots of 1.0×10-7–1.0×10-1M PRG solutions starting from low to high concentrations at constant pH value. The potential response of the stirred solutions was measured after stabilization to ±0.2 mV, and a calibration plot was constructed by plotting the emf readings against the logarithm of PRG+ concentrations. The plot was used for subsequent determination of PRG in pharmaceutical formulation.
Determination of pregabalin in pharmaceutical formulation
Five capsules (Lyrica, 150 mg/capsule) powdered and the required weight equivalent to one capsule was dissolved in the 5 ml 1.0×10-1M HCl. The solution was filtered into a 50-ml calibrated flask and diluted to the mark with distilled water. A 1.0 ml aliquot of the drug solution was added to 9.0 ml citrate buffer solution and potentiometrically measured as described then the potential readings were compared with the calibration plot. Alternatively, the standard addition method was used by measuring the potentials displayed by the drug test solution before and after the addition of a 0.1 ml aliquot of 1.0×10–1M standard PRG solution. The change in the electrode potential (ΔE) was recorded and used to calculate the concentration of the drug applying equation 1.
Where, and are the concentration and the volume of the unknown, respectively, and and are the concentration and the volume of the standard solution, respectively and S is the slope of calibration plot.
Results and Discussion
Performance characteristics of pregabalin selective sensors
Given the fact that, pregabalin as an amino acid derivative with amino, carboxylic groups and hydrophobic moiety, must to be taken into consideration to be recognized, attention will be turned toward a multitopic receptor approach [33,34].
It is well known that cyclodextrins (CDs) are a class of natural host molecules obtained from the enzymatic degradation of starch, referred as “all-purpose molecular containers” due to their abilities for the formation of inclusion complexes with various organic and inorganic guest molecules including drugs that make them especially attractive materials. Importantly, considerable attention may be paid to the unique character of cyclodextrins that form inclusion complexes with diverse guest molecules by encapsulating the non-polar part of the guest into its hydrophobic cavity and stabilizing the polar part by the polar “hydrophilic” rims [35], which is the carboxylate group as the most dominant one in the complexation of PRG with β-cyclodextrin [36].
On the other hand, inorganic anions with oxygen-rich surfaces; polyoxometalates (POMs) are diverse class of anionic metal-oxo clusters continue to engage significant attraction due to their remarkable molecular and electronic structural diversity, unexpected functionalities, and their applications in diverse scientific fields. Interestingly, the ability of host’s network of Keggin anions; the most attractive type of polyoxometalates for the selective affinity of intermolecular interactions with the amino group in the amino acids through hydrogen bonding interactions; N+–H......O has been reported [37]. It is important to mention that hydrogen bonding interactions play a prominent role in the performance of ionophore based sensors [38]. More important, POMs open a wide scope for the design of hybrid materials that accumulate synergistic functionalities and the organic-inorganic hybrid materials based on POMs have received a special concern in potential applications for sensors in the recent years [39]. However, little is known about drug determination, the advantage of the hybrid PMA–crown ether moieties lies in the novel properties that can combine the key merits of both sources and might be relevant for the sensitivity as well as selectivity of electrochemical sensors [40].
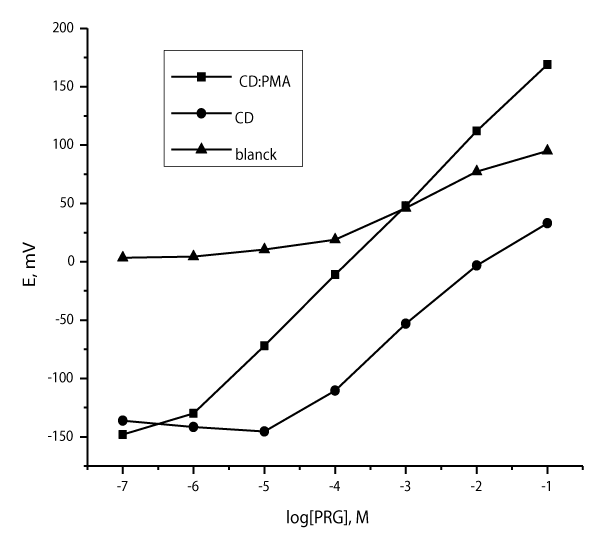
Accordingly, β-cyclodextrin:phosphomolobdic acid organic-inorganic hybrid material was synthesized with the specific purpose of studying this hybrid material as a neutral ionophore immobilized in a PVC plasticized membrane designed for PRG based selective sensors. To ensure permselectivity for neutral ionophore based sensors, the addition of a lipophilic ion exchanger (anionic additive); KTpClPB to the membrane bulk of PRG sensors is necessary for proper functioning. This additive lowers the electric membrane resistance and reduces interference by lipophilic anions [41]. Moreover, the anionic additives diminish the activation barrier for the cation-exchange at the membrane-solution interface that improving the interfacial ion-exchange kinetics resulting in a fast response time [42]. The performance characteristics of the developed sensors were electrochemically evaluated according to IUPAC recommendations at 25ºC [43], typical calibration plots of the sensors and their characteristic responses are presented in Figure 3 and Table 1, respectively. It is of important to note first that the blank membrane exhibited no significant response towards PRG. As can be seen, “β-CD:PMA” ‘not just molecule’ but ‘works’ and carries out tasks that it seemed to be effective enough as an ionophore for the response of PRG. It was shown that the DOP plasticized based sensors incorporating β-CD:PMA respond to PRG with a cationic Nernstian response slope closer to the ideal Nernstian value; 59.6±0.49 mV per decade and the detection limit as the intersection of the extrapolated linear regions of the calibration plot was 6.0×10-7M. As well it exhibited a good linear response within the range of 1.0×10-6 -1.0×10-1M (r2= 0.9998).
Quite on the contrary, β-CD based sensor plasticized with ο-NPOE displayed the worse one; narrow linear range; 1.0×10-4–1.0×10-2M with a limit of detection which was about two decades poorer than “β-CD:PMA” based sensor; 5.0×10-5M. This distinction is thought to be due to the synergistic hybrid material effect that resulting in an enhancement of the non-covalent interactions providing a strong complexation process between β-CD:PMA and PRG at membrane-solution interface that dominates “induce” a sensor potentiometric signal; phase boundary potential. Additionally, the comparison with β-CD evidently revealed the significant role of PMA in the potential determining process. Consequently, the results are consistent with the expected multifunctional molecular design of the proposed ionophore; the reason for the very good performance as it is necessary to introduce more than binding site in one molecule to interact effectively with all of the recognition sites of amino acid in the aqueous phase as stated earlier as well as to radically change the properties of a sensor usually requires structural modifications of the ionophore itself.
Plasticizer is of a core importance in the optimum performance of ion selective sensors, they improve the working stability, the detectable concentration range by decreasing the zero-current ion diffusion from the membrane into the solution as well as the lifetime [44]. For this fact, DOP and ο-NPOE are representatives of low and high dielectric constant plasticizers, respectively have been investigated. As can be seen from Table 1, Figure 4, both plasticizers gave close-Nernstian response to PRG with rather similar detection limits. This indicated that PRG sensors with high sensitivity can easily be designed irrespective of the nature and dielectric constant of the plasticizer. DOP was used for further studies because it showed relative better selectivity towards PRG as described later.
One of the most relevant characteristics of an ion selective sensor is its response time; the time required to achieve a steady-state potential of PRG sensors within ±1.0 mV of the final equilibrium value after successive immersion of the sensors in a series of PRG solutions, each has a 10-fold difference from low to high concentrations. It is obvious that β-CD:PMA based sensors achieved the equilibrium response in a very short time of about 5 s for high concentrations from 1.0×10-4 to 1.0×10-1M indicating reversible and fast exchange kinetics process. However, a relatively long time of 25 s for β-CD based sensors has been showed. Apart from the specific binding sites, it is obvious that the ionophore has been possessed sufficient lipophilicity as well as chemically stablility inside the membrane that preventing it from leaching out of the membrane within four weeks providing that both β-CD:PMA based sensors could be used within this period without significant divergence in the performance characteristics of the sensors. On the other hand β-CD based sensor exhibited a lifetime of two weeks.
Because of the charged species of the analyte have to be transported into a lipophilic polymeric membrane from the water phase to allow its potentiometric detection with ionophore based ion selective sensors, the requirement of strongly acidic media for the PRG to achieve their full protonation is necessary. The influence of the pH on the potential response of proposed sensors was tested using 1.0×10-4 and 1.0×10-3M PRG solutions over the pH range 0.5–4.5. Adjustment of pH was carried out using either HCl or NaOH. The pH-potential profiles show that PRG sensors display good stability and potential remains constant over the pH range 1.5–3.7. At lower pH values < 1.5, the proposed sensors respond to PRG and hydronium ions simultaneously, while at higher pH values > 3.7, the formation of carboxylate moiety excludes PRG from the membrane, hence decreasing the potential response of the electrode. Therefore, a pH value of 2.5 was chosen in which PRG remains mainly as cationic species and this pH value was maintained using a citrate buffer (0.01M) for further measurements.
Selectivity
The potentiometric selectivity coefficient; is of paramount importance of an electrochemical sensors particularly for practical applications as it measures the relative affinity of target ion (PRG) and interfering ion (j) toward the ion selective sensor. It is well established that the selectivity of ionophore based sensors is a concerning issue of ionophore design [27]. Likewise, plasticizer acts as a membrane solvent that may significantly affect the membrane selectivity through both extraction of ions into organic phase and influencing their complexation with the ionophore [41,45]. Consequently, the relative influence of the neutral ionophore as well as plasticizers on the selectivity of PRG based sensors was performed using the separate solution method [46], and the obtained data was illustrated in Table 2. The concentrations of PRG and the interferents are kept at a level of 1.0×10-3M solutions and the potentiometric selectivity coefficients were evaluated according to equation 2.
Where and are the electrode potential for interfering ions and PRG, respectively, is the charge of interfering ions and S is the slope of calibration plot.
The results providing that sensors plasticized with less polar plasticizer DOP displayed better selectivity to a little extent with respect to the selected ions of interest than that of high dielectric one; ο-NPOE and both are superior than β-CD based sensors. This reflects the efficiency of the binding affinity of the proposed neutral ionophore towards pregabalin. Additionally, the most interesting feature of β-CD:PMA is that it enables remarkably selective recognition of PRG compared with opioids, the values showed that the response of β-CD:PMA/p-ClTPB based sensors toward pregabalin is about hundred times more selective in the presence of opioids, and this is an important issue that is suited to practical applications.
Analytical applications
The acceptable performance characteristics as well as high selectivity of proposed sensors make them more favorable for determining PRG in its pharmaceutical preparation without pretreatment. Direct potentiometric determination of PRG by DOP plasticized β-CD:PMA/p-ClTPB based selective sensors using the calibration plot method gave mean recovery of 99.16%±0.25. At the same time, mean recovery of 100.97%±0.17 was obtained applying the standard addition method.
Method validation
The validation of the proposed potentiometric sensors for determining pregabalin was assessed by measuring the range, lower limit of detection (LOD), precision or repeatability (CVw), between-day variability (CVb) “determined as relative standard deviation (RSD)”, linearity (correlation coefficient) and the sensitivity (slope). Data obtained with eight batches (eight determinations each) of PRG solutions are shown in Table 1. As well as accuracy (trueness or recovery), was expressed as the percentage of the average measured concentration of three replicates of lyrica samples compared to the nominal (theoretical) concentration and this gave satisfactory results as shown earlier. The sensors based on β-CD:PMA/p-ClTPB exhibit response towards PRG closer to the theoretical Nernstian value with a slope of 59.6±0.49 mV (r2= 0.9998) and 58.7±0.54 mV (r2= 0.9999) per decade over a linear working range of concentrations 1.0×10-6 to 1.0×10-1M and low detection limits of 6.0×10-7 and 8.0×10-7M for membranes plasticized with DOP and o-NPOE, respectively. On the other hand, β-CD/p-ClTPB based sensors plasticized with o-NPOE showed the worse performance; slope of 49.42±2.21 mV (r2= 0.9999) per decade over a linear range 1.0×10-4–1.0×10-2M. The results asserted that the sensors can easily be designed irrespective of the nature and dielectric constant of the plasticizer, however, multifunctional ionophore is a crucial requirement for the selective determination of PRG as an amino acid derivative.
Conclusion
Taking advantage of the hydrogen-bonding interaction together with the synergetic effect of the β-CD:PMA organic-inorganic hybrid material, potentiometric sensors for the first time have been designed for the determination of pregabalin in pharmaceutical formulations. The synthesized ionophore yielded sensors with analytically attractive properties and significant advantages over other techniques already described in the literature for determination of PRG. The detection limit is much more enough than the toxicological concentration range as well as this platform is a simple, fast and cost effective promising perspective for the determination of pregabalin even in biological matrices of opioid addicts.
- Blommel ML, Blommel AL (2007) Pregabalin: An antiepileptic agent useful for neuropathic pain. Am J Health Syst Pharm 64: 1475-1482. Link: http://bit.ly/2lv6XOb
- Kim L, Lipton S, Deodhar A (2009) Pregabalin for fibromyalgia: some relief but no cure. Cleve Clin J Med 76: 255-261. Link: http://bit.ly/2jWySWE
- Baldwin DS, Den Boer JA, Lyndon G, Emir B, Schweizer E, et al. (2015) Efficacy and safety of pregabalin in generalised anxiety disorder: A critical review of the literature. J Psychopharmacol 29: 1047-1060. Link: http://bit.ly/2ksB74m
- Perinpanayagam J, Abu-Asi MJ, Bustamante S, Kunnumpurath S (2015) Opioid-Sparing Drugs, Ketamine, Gabapentin, Pregabalin, and Clonidine. in Alan David Kaye, Nalini Vadivelu, Richard D. Urman (Eds), Substance Abuse Inpatient and Outpatient Management for Every Clinician 319-330. Link: https://tinyurl.com/yykwypj3
- Oulis P, Konstantakopoulos G (2010) Pregabalin in the Treatment of Alcohol and Benzodiazepines Dependence. CNS Neurosci Ther 16: 45-50. Link: http://bit.ly/2lTcmhX
- Evoy KE, Morrison MD, Saklad SR (2017) Abuse and Misuse of Pregabalin and Gabapentin. Drugs 77: 403-426. Link: http://bit.ly/2lCYF6B
- Schifano F, D'Offizi S, Piccione M, Corazza O, Deluca P, et al. (2011) Is There a Recreational Misuse Potential for Pregabalin? Analysis of Anecdotal Online Reports in Comparison with Related Gabapentin and Clonazepam Data. Psychother Psychosom 80: 118-122. Link: http://bit.ly/2kudGHV
- Häkkinen M, Vuori E, Kalso E, Gergov M, Ojanperä I (2014) Profiles of pregabalin and gabapentin abuse by postmortem toxicology, Forensic Sci Int 241: 1-6. Link: http://bit.ly/2lZbqc4
- Elliott SP, Burke T, Smith C (2017) Determining the Toxicological Significance of Pregabalin in Fatalities. J Forensic Sci 62: 169-173. Link: http://bit.ly/2lRhSBI
- Dousa M, Gibala P, Lemr K (2010) Liquid chromatographic separation of pregabalin and its possible impurities with fluorescence detection after postcolumn derivatization with o-phtaldialdehyde. J Pharm Biomed Anal 53: 717-722. Link: http://bit.ly/2ktU3j1
- Yoshikawa N, Naito T, Yagi T, Kawakami J (2016) A Validated Fluorometric Method for the Rapid Determination of Pregabalin in Human Plasma Applied to Patients With Pain. Ther Drug Monit 38: 628-633. Link: http://bit.ly/2lCZJaB
- Arayne SM, Shahnaz H, Ali A, Sultana N (2014) Monitoring of Pregabalin in Pharmaceutical Formulations and Human Serum Using UV and RP-HPLC Techniques: Application to Dissolution Test Method. Pharm Anal Act 5: 1-7. Link: http://bit.ly/2kf3bIt
- Kostić N, Dotsikas Y, Malenović A, Stojanović BJ, Rakić T, et al. (2013) Stepwise optimization approach for improving LC-MS/MS analysis of zwitterionic antiepileptic drugs with implementation of experimental design. J Mass Spectrom 48: 875-884. Link: http://bit.ly/2lZcBZ2
- Jang KH, Seo JH, Yim SV, Lee KT (2011) Rapid and Simple Method for the Determination of Pregabalin in Human Plasma using Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS): Application to a Bioequivalence Study of Daewoong Pregabalin Capsule To Lyrica® Capsule (Pregabalin 150 mg). J Pharm Invest 41: 255-262. Link: http://bit.ly/2khGkfq
- Hložek T, Bursová M, Coufal P, Čabala R (2016) Gabapentin, Pregabalin and Vigabatrin Quantification in Human Serum by GC–MS After Hexyl Chloroformate Derivatization. J Anal Toxicol 40: 749 -753. Link: http://bit.ly/2lWriMl
- Tafesse TB, Mazdeh FZ, Chalipour A, Tavakoli M, Hajimahmoodi M, et al. (2018) Gas Chromatography–Mass Spectrometry Determination of Pregabalin in Human Plasma Using Derivatization Method. Chromatographia 81: 501-508. Link: http://bit.ly/2lZdqkA
- Gouda AA, Malah ZA (2013) Development and validation of sensitive spectrophotometric method for determination of two antiepileptics in pharmaceutical formulations. Spectrochim Acta A Mol Biomol Spectrosc 105: 488-496. Link: http://bit.ly/2kug9lF
- Bali A, Gaur P (2011) A novel method for spectrophotometric determination of pregabalin in pure form and in capsules. Chem Cent J 5: 59. Link: http://bit.ly/2khCva2
- Derayea SM, Attia TZ, Elnady M (2018) Development of spectrofluorimetric method for determination of certain antiepileptic drugs through condensation with ninhydrin and phenyl acetaldehyde. Spectrochim Acta A Mol Biomol Spectrosc 204: 48-54. Link: http://bit.ly/2k2pTDu
- Béni S, Sohajda T, Neumajer G, Iványi R, Szente L (2010) Separation and characterization of modified pregabalins in terms of cyclodextrin complexation, using capillary electrophoresis and nuclear magnetic resonance. J Pharm Biomed Anal 51: 842-852. Link: http://bit.ly/2lD2loV
- Lindner E, Pendley BD (2013) A tutorial on the application of ion-selective electrode potentiometry: An analytical method with unique qualities, unexplored opportunities and potential pitfalls; tutorial. Anal Chim Acta 762: 1-13. Link: http://bit.ly/2lY6DYq
- Gupta VK, Arunima N, Singhal B, Agarwal S (2011) Recent Advances on Potentiometric Membrane Sensors for Pharmaceutical Analysis. Comb Chem High Throughput Screen 14: 284-302. Link: http://bit.ly/2lThHWx
- Jansod S, Afshar MG, Crespo GA, Bakker E (2016) Phenytoin speciation with potentiometric and chronopotentiometric ion-selective membrane electrodes. Biosens Bioelectron 79: 114-120. Link: http://bit.ly/2kr3a49
- Lotfy HM, Awad AM, Shehata MA (2012) Novel Ion Selective Electrode for Determination of Pregabalin in Pharmaceutical Dosage Form and Plasma. Anal Bioanal Electrochem 4: 507-517. Link: http://bit.ly/2ksHNzs
- Bühlmann P, Chen LD (2012) Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes. In: Jonathan W. Steed, Philip A. Gale (eds), Supramolecular Chemistry: From Molecules to Nanomaterials, John Wiley Sons, Ltd, New York 5: 2539-2579. Link: https://tinyurl.com/y4gav67r
- Mikhelson KN (2013) Ion-selective Electrodes. Springer, Heidelberg –New York–Dordrecht–London.
- Bühlmann P, Pretsch E, Bakker E (1998) Carrier-based ion-selective electrodes and bulk optodes. 2. ionophores for potentiometric and optical sensors. Chem Rev 98: 1593-1688. Link: http://bit.ly/2ktYqKX
- Ganjali MR, Norouzi P, Rezapour M, Faridbod F, Pourjavid MR (2006) Supramolecular Based Membrane Sensors. Sensors 6: 1018-1086. Link: http://bit.ly/2lUvfRG
- Guinovart T, Hernández-Alonso D, Adriaenssens L, Blondeau P, Rius FX, et al. (2017) Characterization of a new ionophore-based ion-selective electrode for the potentiometric determination of creatinine in urine. Biosens Bioelectron 87: 587-592. Link: http://bit.ly/2kujQYz
- Wu Y, Shi R, Wu YL, Holcroft JM, Liu Z, et al. (2015) Complexation of Polyoxometalates with Cyclodextrins. J Am Chem Soc 137: 4111-4118. Link: http://bit.ly/2lUwnVq
- Murugan AV, Kwon CW, Campet G, Kale BB (2003) Synthesis and characterization of novel organic-inorganic hybrid material of poly(3,4-ethylene dioxythiophene and phosphomolybdate anion. Active and Passive Elec Comp 26: 81-86. Link: http://bit.ly/2lBIVAQ
- Bulani VD, Kothavade PS, Kundaikar HS, Gawali NB, Chowdhury AA, et al. (2015) Inclusion complex of ellagic acid with β-cyclodextrin: Characterization and in vitro anti-inflammatory evaluation. J Molecul Structure 1105: 308-315. Link: http://bit.ly/2lvQfhE
- Blikova YN, Otkidach KN, Shvedene NV, Pletnev IV, Sheina NM, et al. (2004) Metal Phthalocyanine and Crown Ether–Based Membranes for the Potentiometric Determination of Phenylalanine Methyl Ester Using an Ion-Selective Electrode. J Analytic Chemistry 59: 584-589. Link: http://bit.ly/2lDGDRJ
- Yi YR, Kim KS, Helal A, Kim HS (2013) Molecular recognition of ώ-amino acids by thiazolobenzocrown receptors: a GABA-selective ionophore. J Supramolecular Chemistry 25: 16-23.
- Saha S, Roy A, Roy K, Roy MN (2016) Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci Rep 6: 35764. Link: http://bit.ly/2lC011s
- Popa G, Dragostin O, Buzia OD, Tartau LM, Profire L, et al. (2017) Studies on Obtaining and Characterization a Pregabalin Cyclodextrin Complex for Taste Masking Purpose. Rev Chim (Bucharest) 68: 337-380. Link: http://bit.ly/2lz3ga3
- Juan Li, Yanfei Qi, Jing Li, Wang H, Wu X, et al. (2004) Heteropolymolybdate–amino acid complexes: synthesis, characterization and biological activity, Journal of Coordination Chemistry. J Coord Chem 57: 1309-1319. Link: http://bit.ly/2k442vu
- Qin L, Zeng G, Lai C, Huang D, Zhang C, et al. (2017) A visual application of gold nanoparticles: Simple, reliable and sensitive detection of kanamycin based on hydrogen-bonding recognition. Sensors and Actuators B Chemical 243: 946-954. Link: http://bit.ly/2ktJX1D
- Ammam M (2013) Polyoxomealates: formation, structures, principal properties, main deposition methods and application in sensing. J Mater Chem A 1: 6291- 6312. Link: https://rsc.li/2lz4imr
- El-Naby EH (2014) Selective ketamine recognition based on membrane potential changes induced by a hybrid organic/inorganic supramolecular assembly. Anal Methods 6: 900-906. Link: https://rsc.li/2kumuxt
- Armstrong RD, Horvai G (1990) Properties of PVC based membranes used in ion selective electrodes. Electrochimica Acta 35: 1-7. Link: http://bit.ly/2lxaZp6
- Gehrig P, Morf WE, Welti M, Pretsch E, Simon W (1990) Catalysis of Ion Transfer by tetraphenylborons in Neutral Ionophore-Based Ion-Selective Electrodes. Helvetica Chemica Acta 73: 203-212. Link: https://tinyurl.com/y5nxslh7
- Lindner E, Umezawa Y (2008) Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes. Pure Appl Chem 80: 85-104. Link: http://bit.ly/2lC1EMC
- Stoica AI, Kleber C, Viñas C, Teixidor F (2013) Ion selective electrodes for protonable nitrogen containing analytes: Metallacarboranes as active membrane components. Electrochimica Acta 113: 94-98. Link: http://bit.ly/2kroUNa
- Peper S, Gonczy C (2011) Potentiometric Response Characteristics of Membrane-Based Cs+-Selective Electrodes Containing Ionophore-Functionalized Polymeric Microspheres. 8. Link: http://bit.ly/2jWO6Li
- Bakker E, Pretsch E, Bűhlmann P (2000) Selectivity of Potentiometric Ion Sensors. Anal Chem 72: 1127-1133. Link: http://bit.ly/2k3MPSS

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
