Open Journal of Analytical and Bioanalytical Chemistry
HPLC-DAD method for simultaneous determination of natural polyphenols
Vanya Dimcheva1*, Nikolay Kaloyanov1, Maria Karsheva1, Milena Funeva-Peycheva2 and Nadezhda Stoilova2
2Central Laboratory of Veterinary Control & Ecology (CLVCE), Bulgarian Food Safety Agency (BFSA), 5 “Iskarsko house”str., 1528 Sofia, Bulgaria
Cite this as
Dimcheva V, Kaloyanov N, Karsheva M, Peycheva MF, Stoilova N (2019) HPLC-DAD method for simultaneous determination of natural polyphenols. Open J Anal Bioanal Chem 3(1): 039-043. DOI: 10.17352/ojabc.000009A simple and reliable high-performance liquid chromatography with diode-array detection (HPLC-DAD) method was developed for simultaneous determination of 9 natural substances common in plants: three major catechins ((-) - epicatechin gallate, (-) - catechin, (-) - epigallocatechin), four major flavonoids (rutin, quercetin, myricetin, kaempferol), gallic and vanillic acid. The optimized method was carried out for 40 minutes with detection wavelengths of 278 and 368 nm, gradient elution system on a C18 reversed-phase column. The developed system was evaluated for several validation characteristics, as system suitability, specificity, linearity, limit of detections (LODs) and limit of quantifications (LOQs). The newly established HPLC method was proved to be specific, sensitive, linear and precise. The received results showed good chromatographic separation and assumed that the described method was validated. The HPLC-DAD method can be a useful tool for the quantitative and qualitative evaluation of the selected polyphenol compounds and can be improved in the future for the examination of the polyphenols of interests in individual herbal infusions.
Introduction
The interest in the phenolic compounds, in particular flavonoids, have increased last years. This is due to their antioxidant and bioactive properties associated with human health benefits. Hydroxybenzoic acids are phenolic compounds with a general structure C6-C1. The related acids such as gallic acid, protocatechuic acid, syringic acid, and vanillic acid are major compounds in the plants and foods [1]. Due to their structural diversity, phenolic acids have antipyretic, analgesic and anti-microbial activities [1,2].
Flavonoids have a C6-C3-C6 structure [2]. Based on their chemical structure, they are classified as flavonols, flavones, isoflavones, anthocyanidins, and flavanols [3]. The typical flavonoid spectrum consists of two maxima in the range 240–285 nm, and 300–550 nm [4]. Flavonoids exhibit anti-fungal, antibacterial, anti-viral, anti-inflammatory and anti-ulcer activities [5].
Catechins, or flavan-3-ols, consist of the most important flavonoid group. They are the main polyphenol compounds found in the tea. The main catechins are (-) - epicatechin, (-) - epigallocatechin, (-) - epicatechin-3-gallate, and (-) - epigallocatechin-3-gallate. They all possess antioxidant, antibacterial, and anti-inflammatory effects and alleviate cardiovascular diseases [6]. The UV spectra of catechins show maximum absorption at non-specific wavelengths (270–290 nm), at which many phenolics also absorb. Thus, does not permit their selective detection and identification [4].
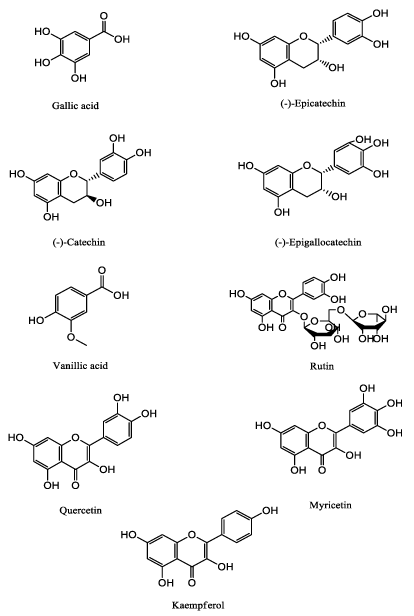
The major objective of the current study was to develop a simple, sensitive and accurate reversed-phase HPLC-DAD method for simultaneous separation of nine natural substances common in plants - (-) - epicatechin gallate, (-) - catechin, (-) - epigallocatechin, rutin, quercetin, myricetin, kaempferol, gallic and vanillic acid. The used reference standards were chosen because they are reported to be widely represented in the plant world and have a strong antioxidant capacity (Figure 1).
The identification of phenolic acids and flavonoids in plant extracts was of major interest in determining their properties and their antioxidant or antibacterial properties [7]. Increasing knowledge of the health effects of polyphenols in food has led to the need to develop new techniques for separating bioactive molecules. There is an increasing demand for a highly sensitive and selective analytical method not only for extraction but for the analysis of polyphenols [8]. A very common method used for the qualitative and quantitative determination of polyphenols is HPLC coupled with DAD. The HPLC is a powerful technique for analysis of natural substances such as polyphenols, their identification, and evaluation [2].
Literature survey reveals that a number of analytical methods have been developed for the determination of natural polyphenols. Typically, the chromatographic conditions of these HPLC methods include the use of a C18 reverse phase column, a diode matrix detector [6].
Generally, in the reversed-phase HPLC polyphenol analyses for the separation of desired bioactive components are used two mobile phases. However, the mobile phase’s selection depends on the type and nature of the polyphenol compounds to be separated by HPLC [9].
Usually, the mobile phase A content water with small concentrations of acetic acid, formic acid and trifluoroacetic acid (TFA) and the mobile phase B content 100 vol. % organic solvents. The organic solvents such as methanol or acetonitrile are necessary to reduce the peak tailing and giving sharper peaks [10].
Regardless, the large number of studies the simultaneous determination of polyphenolics of different groups raiment difficult [4,11]. In the literature, various HPLC procedures in the separation of different classes of polyphenolic compounds are investigated for quantification and identification of flavonoids and phenolic acids [12-14], catechins [15], phenolic acids [16], polyphenols, flavonoids, and phenolic acids [14].
No analytical method based on reversed-phase HPLC have been reported in the literature for the simultaneous estimation of (-) - epicatechin gallate, (-) - catechin, (-) - epigallocatechin, rutin, quercetin, myricetin, kaempferol, gallic and vanillic acid.
Experimental
Reagents and standards: Ethanol (96 %) was supplied by Valerus, (Sofia, Bulgaria). Trichloroacetic acid (TCA), (> 99 %), acetonitrile (HPLC grade), gallic acid anhydride (> 99 %), vanillic acid (> 99 %), rutin hydrate (> 98 %), quercetin hydrate (≥ 95 %) were supplied by Merck (Sofia, Bulgaria). (-) - Epicatechin gallate (100 %), (-) - catechin (100 %), (-) - epigallocatechin (100 %), myricetin (> 98 %) and kaempferol (> 98 %) were supplied by Alfa Aezar (Sofia, Bulgaria). Deionized water (18.6 MΩ cm resistivity) generated by ELGA water purification system (Sofia, Bulgaria).
Standard and working solutions: All Stock standard solutions of reference standards (100 μg mL−1) were prepared by dissolving the proper amounts of them in 30 % water solution of ethanol. The working standards with a proper concentration were prepared by appropriate dilutions of the stock solution with 30 % water solution of ethanol. All working standards were filtered through 0.45 μm nylon-membrane syringe filter (Acrodisc, Sigma-Aldrich, Bulgaria) and put into the 2 mL vials. All filtered standards were kept at -18 °C. All standards were thawed before analysis.
Equipment: The analyses were performed with an Agilent 1100 HPLC system (Agilent 1100 HPLC, Agilent Technologies, California, USA) equipped with DAD detector (G1315B, Agilent Technologies, California, USA), and managed by HP Chemstation Software. The chromatography column used was Purospher star, Hiber RT 125-4; RP18 (Purospher star, Merck, Bulgaria), equipped with the same pre-column.
Chromatographic conditions: The separation was carried out using a linear gradient elution program with 0.1 % TCA (A) and 100 % acetonitrile (B) for 40 minutes. The gradient elution program started with 5 % B, 15 % B at 16.5 min, 33 % B at 22.5 min, 100 % B at 30.5 min, 5 % B at 35 min until 40th for equilibration. The flow rate was 1.6 μg mL−1 and the column temperature - 25 °C. The injection volume was set to 30 μL. Diode-array detection was set to collect data in the range of 200÷400 nm. Calibration curves of the HPLC-DAD method were obtained by plotting the peak area (counts) versus the concentration of the standards (μg mL−1). All calibrations points were chosen to be five. The concentration range was varied from 20 to 100 μg mL−1 for all analytes.
Method validation
When performing the validation of the analytical method were evaluated fallowing validation parameters - system suitability, specificity, linearity, limit of detections and limit of quantifications in compliance with the International Conference on Harmonization (ICH) guidelines Q2 R1 [17].
System suitability: To be sure that the HPLC system and developed chromatographic conditions were suitable were evaluated the fallowing parameters: retention time, a number of theoretical plates, tailing factor, resolution, and precision. As acceptance criteria for achieving the system suitability of the HPLC system were considered the number of theoretical plates more than 3000, tailing factor less than 2.0, the resolution between peaks more than 2.0 and % RSD of analyte peaks areas of six replicate injections, less than 2 %. Relative standard deviation (RSD) of the area of six replicate injections of analytes were observed as a measure of precision. For this purpose, the working standard solution (50 μg mL-1) was injected into the HPLC system and analyzed with the described method and chromatogram was recorded. The resolution between peaks was accepted to be not more than 2.0.
Specificity: The specificity of the chromatographic method is to be avoided the appearance of other components other than those desired to appear on the chromatogram and to lead to an ambiguous assessment of the analytes [17]. To check the specificity was performed the peak purity tests.
Linearity: The linearity was assessed by the relationship between the concentration of the analytes and their area obtained during the HPLC analysis. The linearity of the proposed method was evaluated using five concentration levels in the concentration range 20 ÷ 100 μg mL-1. The working standard solutions were analyzed in six replicates. All the chromatograms were recorded. Calibration curves were obtained for each of the studied compounds. The calibration graphs were constructed by plotting the peak area of the studied analytes against respective concentrations. The correlation coefficients (R2), slopes and the intercepts were calculated. The acceptance criterion for correlation coefficients was assumed to be more than 0.99.
Limit of detection and limit of quantification: LODs and LOQs were determined for each of the studied compounds. Their values were calculated using the expressions 3.3σ/s and 10σ/s, respectively, in which σ is intercepted standard deviation and s is the slope of calibration curve [18].
Results and Discussion
To be optimized the proposed method during the development process a various composition of mobile phases were tried until the necessary separation was achieved. Different concentrations of TCA in water were tried for mobile phase A and for mobile phase B acetonitrile and methanol were tested. After several runs, 0.1 % of TCA in water for mobile phase A and 100 % acetonitrile for mobile phase B were found to be optimal. Diode-array detection was set to collect data in the range of 200 ÷ 400 nm. The wavelength of 278 nm was found as optimal for determination of the three catechins and two polyphenol acids and wavelength of 368 nm was found as optimal for quantification of the flavonoids - rutin, myricetin, quercetin, and kaempferol. The optimum wavelengths were corresponding to the optimum area of the peaks studied.
The method optimization has an object a good separation between the peaks but also in the short run time. The analytical conditions were optimized mainly and based on the results from several experiments each reference standard was well-separated with the flow rate of 1.6 μg mL−1, stationary phase type Hiber RT 125-4; RP18 and gradient eluting program of 40 minutes. The injection volume and a column oven temperature were also optimized. The chromatographic conditions were optimized on the basis of system suitability parameters such as retention time, a number of theoretical plates, tailing factor, resolution, and precision. Good separation was achieved with peaks identified as the 9 individual polyphenol compounds separately. The resolution between individual peaks was more than 2.0. The tailing factors for each individual peak were less than 2.0. The column performance was demonstrated by the number of the theoretical plates for each individual analyte and was recorded to be more than 3000. The precise of the HPLC system was indicated with the RSD of the peak areas of six replicate injections of each reference substance which was less than 2.0.
The system suitability parameters were tested by injecting standard solutions at the beginning of the HPLC method optimization and were recorded from the chromatograms. They were monitored during all performed analyses and have indicated that the developed HPLC system was suitable.
After the analytical condition’s optimization, the HPLC method was evaluated for several validation characteristics such as specificity, linearity, LOD, and LOQ.
The specificity was demonstrated with the peak’s purity of studied analytes. No interferences were observed close to retention times of reference standards of interest, so it was confirmed that each analyte’ peak was attributable to a single compound. So, it can be concluded that the in-house reported HPLC method showed good specificity.
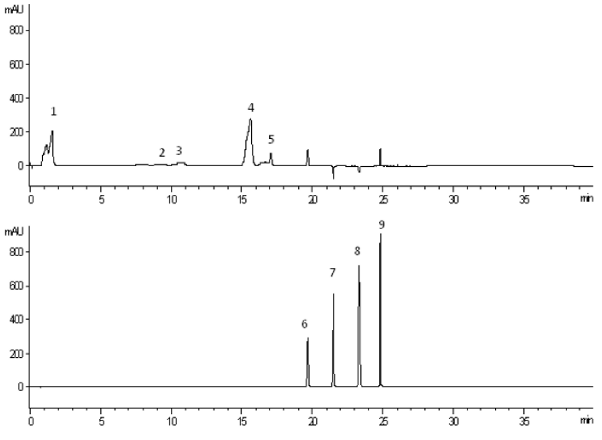
The HPLC chromatograms of the separated polyphenol substances are given in Figure 2.
The linearity of the established HPLC method was determined with the standard solutions with the concentration range of 20-100 μg mL-1 at five concentration levels. The linear calibration graphics were obtained through the observed peak areas of the studied analytes against respective concentration. The regression equations, correlation coefficients (R2), LOD and LOQ values, retention times (Rt) and maximum wavelengths (λmax) for the tested reference standards are shown in Table 1.
The regression analyses showed good linearity for all analytes. For individual analytes, R2 was achieved to be > 0.99. This suggests that the proposed method is linear.
The sensitivity of the developed HPLC method could be proved by the LOD and LOQ values calculated by the regression equations. It becomes clear that the method is more sensitive for the flavonoids, polyphenol acids and (-) - epicatechin considered by the low values of the LOD values ranged from 0.015 - 1.023 μg mL-1 and the LOQ values ranged from 0.028 - 1.023, respectively. The (-) - catechin and (-) – epigallocatechin shows highest LOD (2.82; 7.34) and LOQ concentrations (8.54; 22.26) than other polyphenols. This means no universal method can be used with all phenolic compounds: different approaches must be used depending on the different plant extract and polyphenols of interest.
There are different methods reported in the literature for the simultaneous estimation of different classes of polyphenols like the method presented by A. Alonso Garcia et al., [19], for determination of quercetin; gallic acid, (+) - catechin, (-) - epicatechin, caffeic acid, p-coumaric acid, salicylic acid and gentisic acid and the method presented by E. Altiok et al., [20], for assessment of hydroxytyrosol, tyrosol, rutin, luteolin-7-glucoside, verbascoside, apigenin-7-glucoside, oleuropein, luteolin, caffeic acid, vanillic acid and catechin. The reported in the literature methods could not be directly compared with our method because they are conducted at different chromatographic conditions.
The validation results showed that the newly established HPLC-DAD method for simultaneous separation of three different classes of polyphenol compounds can be used for identification and determination of the specific natural compounds in plant infusions. There is no data in the literature concerning such method optimized with similar chromatographic conditions. However, the method could be evaluated with exact plant extracts to give a better representation of its antioxidant profile.
Conclusions
The phenolic compounds especially phenolic acids, catechins and flavanoids having antioxidant properties which are well known with their pharmacological properties good for human health. However, for their identification and quantitate from foods and plants are required selective and comprehensive highly sensitive analytical technique as it is an HPLC-DAD technique. A simple and specific reversed-phase HPLC-DAD method for simultaneous determination of 9 natural substances common in plants - three major catechins, four major flavonoids, gallic and vanillic acid has been successfully developed and validated. The method was proved to be specific, sensitive, linear and precise for detection and assessment of the selected natural polyphenols. The described HPLC-DAD method could provide an efficient and comprehensive tool for quality and quantitative evaluation of polyphenols of interest in the different herbal infusions.
- Robbins RJ (2003) Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem 51: 2866-2887. Link: http://bit.ly/31jSVyi
- Stalikas CD (2010) Phenolic acids and flavonoids: occurrence and analytical methods. Method Mol Biol 610: 65-90. Link: http://bit.ly/2GTODGc
- Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821-1835. Link: http://bit.ly/33iKGEz
- Merken H, Gray B (2003) A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. J Agric Food Chem 48: 577-599.
- Cuyckens F, Claeys M (2004) Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom 39: 1-15. Link: http://bit.ly/2MLHn2Y
- Lu Z, Liu Y, Zhao L, Jiang X, Li M, et al. (2014) Effect of low-intensity white light mediated de-etiolation on the biosynthesis of polyphenols in tea seedlings. Plant Physiol Biochem 80: 328-336. Link: http://bit.ly/2M5w2LF
- Mihai CM, Mărghitaş LA, Dezmirean DS, Chirilă F, Moritz RF, et al. (2012) Interactions among flavonoids of propolis affect antibacterial activity against the honeybee pathogen Paenibacillus larvae. J Invertebr Pathol 110: 68-72. Link: http://bit.ly/2Ko0cHW
- Qian Liu, Wensheng Cai, Xueguang Shao (2008) Determination of seven polyphenols in water by high performance liquid chromatography combined with preconcentration Talanta, 77: 679-683. Link: http://bit.ly/33eAJYR
- Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K (2003) Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem 51: 571-581. Link: http://bit.ly/2yL026F
- Andersen ØM, Fossen T (2003) Characterization of anthocyanins by NMR, in Current Protocols in Food Analytical Chemistry, Wrolstad, R.E., Ed., New York: John Wiley 1.
- Tsao R, Yang R (2003) Optimization of a New Mobile Phase to Know the Complex and Real Polyphenolic Composition: Towards a Total Phenolic Index Using High-Performance Liquid Chromatography. J Chromatogr A 1018: 29-40. Link: http://bit.ly/33iLkBZ
- Zhongxiang Fang, Yuhuan Zhang, Yuan Lü, Guangpeng Ma, Jianchu Chen, et al. (2009) Phenolic compounds and antioxidant capacities of bayberry juices. Food Chemistry 113: 884-888. Link: http://bit.ly/33kklG4
- Zhi-hong Wang,Cheng-chin Hsu, Mei-chinYin (2009) Antioxidative characteristics of aqueous and ethanol extracts of glossy privet fruit. Food Chemistry 112: 914-918. Link: http://bit.ly/2yJt6LT
- Ulla Svedström, Heikki Vuorela, Risto Kostiainen, Into Laakso, Raimo Hiltunen (2006) Fractionation of polyphenols in hawthorn into polymeric procyanidins, phenolic acids and flavonoids prior to high-performance liquid chromatographic analysis. Journal of Chromatography A 1112: 103-111. Link: http://bit.ly/2KkQVAm
- Quansheng Chen, Jiewen Zhao, Sumpun Chaitep, Zhiming Guo (2009) Simultaneous analysis of main catechins contents in green tea ( Camellia sinensis (L.) by Fourier transform near infrared reflectance (FT-NIR) spectroscopy. Food Chemistry 113: 1272-1277. Link: http://bit.ly/2MIuIOh
- Ross KA, Beta T, Arntfield SD (2009) A Comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chemistry 113: 336-344. Link: http://bit.ly/2Tf1hEP
- International Conference on Harmonization (2005) Validation of Analytical Procedures: Text and Methodology, Q2 (R1). Link: http://bit.ly/2ThbWyt
- Shekarchi M, Hajimehdipoor H, Khanavi M, Adib N, Bozorgi M, et al. (2010) A validated method for analysis of Swerchirin in Swertia longifolia Boiss. by high performance liquid chromatography. Pharmacogn Mag 21: 13-18. Link: http://bit.ly/2TikaGH
- García AA, Grande BC, Gándara JS (2004) Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet absorbance detection for the determination of polyphenols in alcohol-free beers. J Chromatogr A 1054: 175-180. Link: http://bit.ly/33eD0TT
- Evren Altıok, Deniz Bayçın, Oguz Bayraktar, Semra Ulku (2008) Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Separation and Purification Technology 62: 342-348. Link: http://bit.ly/2MIwe2V

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
