Open Journal of Asthma
Immunosenescence and ACE2 protein expression: Association with SARS-CoV-2 in older adults
Gustavo Acosta Altamirano1, Carlos E Miguel Rodríguez1, María del Rocío Reyes-Montes3, Esperanza Duarte-Escalante3, Rocío Acosta-Reyes2, Carlos U Torres-Estrella1 and Omar E Valencia-Ledezma1*
2Medical Towers, La Joya Hospital, San Miguel de Allende Guanajuato Mexico
3Mycology Unit, Faculty of Medicine, Department of Microbiology and Parasitology, National Autonomous University of Mexico, Mexico City 04510, Mexico
Cite this as
Altamirano GA, Miguel Rodríguez CE, Reyes-Montes MDR, Duarte-Escalante E, Valencia-Ledezma OE, et al. (2022) Immunosenescence and ACE2 protein expression: Association with SARS-CoV-2 in older adults. Open J Asthma 6(1): 008-017. DOI: 10.17352/oja.000018Copyright License
© 2022 Altamirano GA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.At the end of 2019, in Wuhan, China, an outbreak of cases of respiratory tract infection emerged and its progressive infection mainly affects adults, generating many cases of pneumonia. A type of coronavirus named SARS-CoV-2, with genomic similarity to SARS-CoV and MERS-CoV, was identified as the etiological agent. The evolution of this pandemic has made it possible to verify the similarity in the pathophysiological mechanisms between these three viruses, identifying the Angiotensin-Converting protein-Enzyme 2 (ACE2) as the primary receptor for SARS-CoV-2. This age group is more prone to developing extrapulmonary complications from SARS-CoV-2 since the clinical and pathological findings suggest a particular relationship between greater expression of ACE2 and the comorbidities of chronic degenerative diseases and the greater expression of ACE2 at the level of the respiratory tract. It has also revealed the mechanisms by which the virus evades the innate immune response and the Th1-type adaptive response. The objective of this work was to analyze immunosenescence and its relationship with SARS-CoV-2 infection, through the review of the most recent articles (2021-2022), which describes the senescent state of the elderly. In addition, it intends to highlight the probable causes for which the most vulnerable population group (adults over 60 years of age) is more prone to presenting complications during the infection.
Introduction
On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic due to the rapid spread of a new coronavirus whose origin dates back to 2019 in Wuhan, China and causes COVID-19 disease [1]. This pathogen shares 79% genomic similarity with SARS-CoV and 51% with MERS-CoV, for which it was subsequently officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the WHO [2]. SARS-CoV-2 has shown a greater transmission capacity than SARS-CoV, causing the COVID-19 disease, characterized by the presence of fever, cough and fatigue as the main signs and symptoms. The evolution of this pandemic has made it possible to understand the behavior and pathophysiological mechanisms of the virus and identify the people most vulnerable to infection and risk factors that can trigger the development of severe illness from COVID-19. Risk factors include from the genetic level to age-related factors, a study carried out through a meta-analysis of the human genome, showed a correlation between a group of genes on chromosome 3 and susceptibility to SARS-CoV-2 infection. These genes, in turn, were shown to correlate with genes encoding the ABO blood group system, suggesting that certain blood groups, particularly type A, are more susceptible to SARS-CoV-2 infection [3]. Further study showed that these risk genes are probably inherited by Neanderthals and are found in greater abundance in South Asian and European populations [4]. Comorbidities are an essential factor in the development of COVID-19 disease because, in some way, it compromises the patient’s health status, due to all inflammatory processes, the risk of clinical complications increases. At the beginning of the pandemic, the population aged 60 years or older, with the presence of some comorbidities such as type 2 diabetes, Systemic Arterial Hypertension (SAH), Chronic Obstructive Pulmonary Disease (COPD) and asthma, with incomplete medical treatment, had a higher risk of infection and complications [5]. According to reports, mild to moderate disease occurs in 30 to 80% of populations, in 70,000 cases of COVID-19, the mild disease was presented in 81% with mild pneumonia or without it, moderate in 14% presented pneumonia with hypoxemia and severe disease was presented in 5% with respiratory failure requiring mechanical ventilation, shock or multi-organ failure [6,7]. However, the immunological ability of each individual is also affected by age. This phenomenon known as immunosenescence impairs the body’s ability to deal with infectious agents, thus compromising the immune response [8]. The objective of this work was to analyze immunosenescence and its relationship with SARS-CoV-2 infection, through the review of the most recent articles (2021-2022), which describes the senescent state of the elderly. In addition, it intends to highlight the probable causes for which the most vulnerable population group (adults over 60 years of age) is more prone to presenting complications during the infection.
Pathophysiology of COVID-19
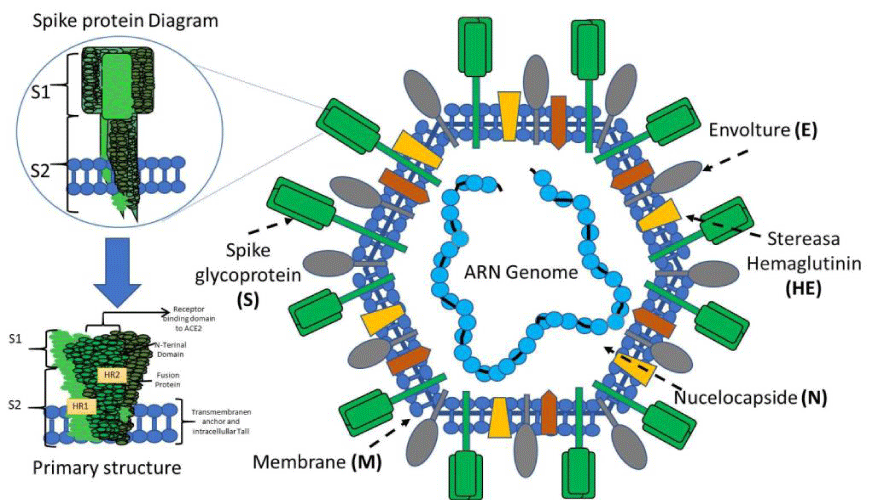
The general structure of the virus: To understand the pathogenic mechanisms of the virus, we must address its general structure. The virus capsid is made up of four main structural proteins: Spike (S), Membrane (M), Envelope (E) and proteins of the Nucleocapsid (N) (Figure 1). Inside this capsid, is the virus genome. The CoV genome is conformed of a positive-sense, single-stranded RNA, 29.9 kb in size [9], Within the genome are the sequences that encode the structural proteins, 16 non-structural proteins (NSP1-16) involved in the replication of the viral genome and, finally, 11 accessory proteins encoded by different open reading frames (ORFs): ORF3a, ORF3b, ORF3c, ORF3d, ORF6, ORF7a, ORF7b, ORF8, ORF9b, ORF9c and ORF10 [6]. One of the functions of accessory proteins is to antagonize the host’s interferon type 1 (IFN-1) response, regulating the cellular apoptosis response [10].
Homotrimers of the S proteins form the spikes on the viral surface and are responsible for binding to host receptors [11]. The Mpro or 3CLpro protein is the viral protease. It is responsible for cleaving the polyproteins of the virus, resulting in non-structural proteins [12]. Protein E has transmembrane domains, so it can function as a viroporin, embedding itself in host cell membranes and promoting virus assembly and release [13]. The N protein is vital for the packaging of the viral genome. It has two domains, both of which can bind to the RNA genome of the virus through different mechanisms [9]. The Membrane protein (M) crosses the host cell membrane and can attach to the other structural proteins. For example, binding to the N protein stabilizes the N-RNA complex within the virion, promoting viral assembly [14].
Mechanism of infection
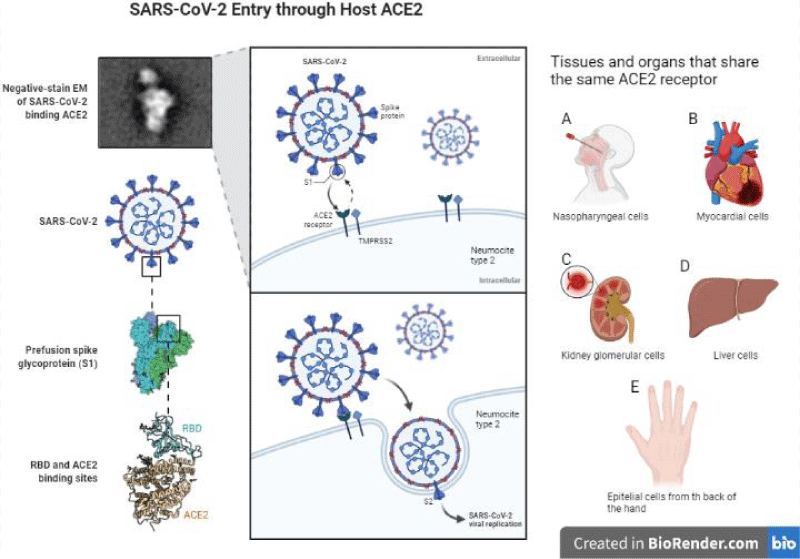
The infection mechanism of SARS-CoV-2 is very similar to that of SARS-CoV since they have angiotensin-converting enzyme 2 (ACE2) as a receptor ligand [15,16]. SARS-CoV-2 infection is triggered by the binding of the RBD domain of the S1 subunit to the host receptor ACE2 (Figure 2). These receptors have an expression in the nose, mouth, heart, lungs, liver and kidney [17,18]. It is pertinent to mention that our work team has observed the presence of these receptors on the back of the hand (data not shown). ACE2 is expressed primarily in a small subset of lung cells called type II pneumocytes [18]. Other studies have shown the presence of ACE2 in epithelial cells of the upper respiratory tract, macrophages and dendritic cells, with this we infer that contagious infections are favored in the elderly population [19]. These findings could explain some mechanisms by which this virus alters the patient’s innate immune response.
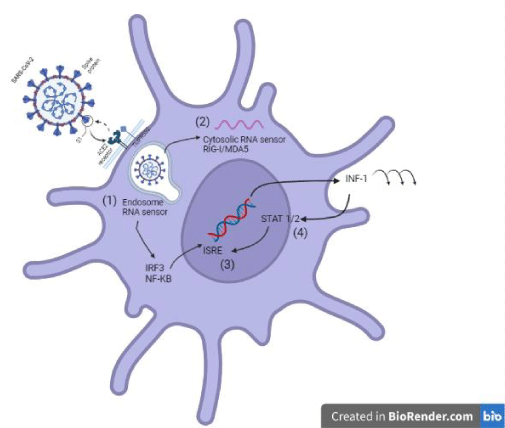
Once SARS-CoV-2 binds to its receptor, the host’s transmembrane protease serine 2 (TMPRSS2) binds the S protein, favoring virus entry [20]. Thus, the virus enters the cell, and viral replication takes place. To initiate an antiviral response, cells of the innate immune response recognize viral invasion through pathogen-associated molecular patterns (PAMPs). For an RNA virus like the coronavirus, genomic RNA is recognized by endosomal RNA receptors, TLR3 and TLR7 and cytosolic RNA sensor, RIG-/MDA5. The previous leads to the activation of an intracellular signaling cascade of the NF-κB and IRF3 types. In the nucleus, these transcription factors induce the expression of interferon type 1 (IFN-I) [19], which acts as the first line of defense against viral infection at the site of entry; thus, this IFN-I-like response should be able to suppress viral replication and spread at an early stage.
On the other hand, SARS-CoV can interfere with IFN-I production, and this mechanism is closely related to the severity of the disease [19]. It has been shown that different SARS-CoV-2 proteins can antagonize the host IFN-I response in different ways; for example, SARS-CoV-2 ORF3b is a potent IFN-I inhibitor [20,21]. The prior is associated with the ORF3b C-terminal domain length and its ability to inhibit IRF3 translocation to the nucleus [21]. Also, the ORF6 protein inhibits it by blocking STAT translocation to the nucleus [20,22] and the ORF7a inhibits IFN-I signaling by polyubiquitinating, thus enhancing its ability to antagonize IFN-I [20,23]. Other proteins capable of antagonizing the IFN-I response are ORF8 and the structural proteins M and N (Figure 3) [20].
The patient’s innate immune response is crucial for recovery. However, the adaptive immune response is more relevant in reducing vulnerability to reinfection and the body’s ability to combat intracellular microorganisms [24-26].
Evidence indicates that the Th1-type response is key to successfully controlling SARS-CoV, MERS-CoV and probably SARS-CoV-2 [12]. This response is also efficient against infections by intracellular pathogens (viruses and bacteria). Targeting a Th1 response is favored by dendritic cells and interleukin-12 (IL-12)-producing phagocytic cells [27]. It has been reported that patients admitted to intensive care units (ICU) showed a hyperactive immune response characterized by elevated levels of granulocyte colony-stimulating factor (GCSF), inducible protein 10 (IP10), monocyte chemotactic protein 1 (MCP1), macrophage inflammatory protein 1-alpha (MIP1α) and tumor necrosis factor-alpha (TNFα), collectively referred to as a cytokine storm [28]. This cytokine storm is proportional to the severity of the disease. However, SARS-CoV-2 infection also elicits a Th2-type immune response that induces the secretion of IL-4 and IL-10, cytokines that suppress inflammation [1] but favor antibody production.
Currently, other factors that alter the innate immune response and facilitate the infection and replication of the virus are being investigated. Some of these factors are environmental temperature and vitamin D3 deficiency, as they affect high-affinity nuclear receptors such as the Vitamin D Receptor (VDR) [29]. These receptors, when stimulated, favor cellular stress in response to an infection by microorganisms. In this way, the high infection rate in countries with a greater latitude could be related to individuals’ physical proximity in cold climates. However, more studies are still needed to validate these hypotheses [30]. In addition, experimental and meta-analysis studies have shown an association between serum vitamin D levels and the severity of COVID-19. It is also stated that the amount of CD4+ and CD8+ T cells is reduced when there is a lack of vitamin D. In addition, it has been proven that T lymphocytes increase with vitamin D supplementation. In turn, the increase in the PD-L1 expression is inhibited, thus eliminating the suppressive effect of PD-L1 on CD4+ and CD8+ T lymphocytes, and lymphopenia is prevented, reducing severity and mortality [31,32].
Immunosenescence: A decreased progression of the immune system
It has been shown that more than half of the deaths related to COVID-19 were individuals over 60 years of age, with an average age of 72.5 years. A study of 100 cases of COVID-19 deaths in China showed that elderly patients with chronic diseases, cardiovascular diseases and diabetes accounted for most COVID-19-related deaths [33]. Although an increase in life expectancy is anticipated worldwide in the coming years (WHO), it is not necessarily associated with a better quality of life for older adults. The aging process triggers pathophysiological dysfunctions in different tissues, organs and systems, including the immune system. Changes in the immune response associated with aging result in decreased effectiveness of the humoral and cellular immune responses. These changes in the immune system are collectively termed “immunosenescence” and are characterized by decreased innate and adaptive immune responses and latent production of pro-inflammatory cytokines [34]. It has been described that when the death of aged T cells occurs, the thymus replenishes its reserve with virgin T cells, which does not occur in individuals aged 60 to 70 years, where thymic production is reduced by 90% compared to newly borns.
Inflammation
Chronic inflammation is closely related to immunosenescence and the development of some comorbidities; although the mechanisms have not been fully elucidated, some factors favor this pro-inflammatory state. It is known that the decrease in cell repair mechanisms is affected with age, which causes damage at the genome and proteome level; for example, accelerated shortening of telomeres in the leukocyte population has been related to the development of coronary heart disease [35].
Likewise, the latent production of pro-inflammatory cytokines during aging is called inflammatory aging. This concept refers to a low-grade pro-inflammatory phenotype associated with aging progression, along with an increase in serum proinflammatory cytokines such as IL-6, IL-1RA, TNF-α, IL-1 and C-reactive protein [36-38]. This pro-inflammatory state that causes damage to healthy tissue due to the latent production of pro-inflammatory cytokines results from the inability of the immune system to eliminate pathogens, as well as infected, neoplastic, and senescent cells [35,39]. In addition, the increase in pro-inflammatory cytokines such as TNF and/or IL-6 has been related to the development of Alzheimer’s and cardiovascular diseases [40-42]. On the other hand, the increase in pro-inflammatory cytokines due to the deregulation of monocyte populations and functions is also influenced by age [43].
On the other hand, it has been shown that external agents, such as some pathogens, can affect the inflammatory state of the host; for example, the specific response of T cells, generated pre-infections by cytomegalovirus (CMV) in older people, cause deregulation of the process inflammatory, promoting greater severity in patients with SARS-CoV-2 infection [44].
Cellular senescence
Cellular senescence consists of multiple phenotypic changes in long-lived (old) cells, including short telomeres, cessation of proliferation, size increase and acquisition of increased activity of lysosomal acid beta-galactosidase called Senescence-Associated (SA)-βGal that serves as a biomarker of senescent cells regardless of their tissue or organ of origin. The most important characteristic of senescent cells is the acquisition of the Senescence-Associated Secretory Phenotype (SASP), which allows them to secrete significant amounts of pro-inflammatory cytokines (including IL-6, TNF-α, IL-1β, IL-8 and others) [45,46]. Immunosenescence is further characterized by decreased immune responses such as antigen presentation and naive T-cell priming, increased myeloid lineage differentiation, altered type I interferon (IFN) responses, decreased cytotoxic function of CD8+ T cells and decreased phagocytic function for many innate response cells [46]. In addition, senescent cells change their intracellular homeostasis, including telomere shortening and oxidative stress, which induce the activation of signaling pathways such as NF-κB. The latter favors the production of cytokines, chemokines, growth factors and bioactive lipids [47,48], assuming that senescent cells change their profile to a cytokine-secreting phenotype (senescence-associated secretory phenotype, SASP) [49,50].
The cell death process that occurs daily due to physical and chemical stress, as well as the accumulation of metabolic and catabolic products that are not efficiently eliminated through phagocytosis by cells of the innate immune system, accumulate and induce the activation of cell recognition receptors patterns (pattern recognition receptors, PRRs) [51]. Additionally, infectious processes during aging can exacerbate the pro-inflammatory condition due to the accumulation of pathogen-associated molecular patterns (PAMPs) and the release of damage-associated molecular patterns (DAMPs), which trigger the inflammatory process through their interaction with PRRs. Signaling through PRRs results in the activation of the transcription factor NF-kB, which is the primary activator and inducer of the SASP phenotype [52]. DAMPs can signal through the Nod-3 receptor (Nod-like receptor 3, NLRP3), inducing inflammasome activation and secretion of pro-inflammatory cytokines such as IL-1β and IL-18 [53].
Inflammatory aging also induces the degeneration of autophagy processes through which cell content is recycled, generating nutrients and energy to maintain cell homeostasis, thus contributing to the elimination of cell debris (senescent cells) and metabolic products, preventing their recognition by PRRs, as well as the development and progression of the inflammatory process [54,55]. In this context, deficiencies in autophagy induce protein aggregation and the accumulation of dysfunctional or damaged mitochondria that cause alterations in oxidative respiration, triggering the production of reactive oxygen species (ROS). Oxidative stress induces the inflammasome pathway through activation of the NLRP3 receptor [56-58], which activates the pro-inflammatory caspase pathway, caspase-1, to cleavage inactive cytokine precursors such as IL-1β and IL-18 [54,59,60].
Additionally, oxidative stress induces the release of CpG-rich mitochondrial DNA with the ability to activate the inflammatory response through its interaction with Toll-Like Receptors (TLRs) and Nod-Like Receptors (NLRs) and the subsequent production of pro-inflammatory cytokines [61,62]. Furthermore, impaired proteasome function also occurs during aging, contributing to the accumulation of misfolded protein aggregates that also induce pro-inflammatory pathways’ activation [63]. The intestinal microbiota during aging plays a fundamental role in the activation of the inflammatory response, due to the large number of molecules that they secrete and that are capable of activating the inflammatory response of the host, among these is the LPS, which constitutes a constant stimulation for the immune system in the cells of the intestine, due to changes in composition and diversity and develops a predominantly Th-1 type inflammatory response characterized by a reduced number of associated intestinal bacteria to an anti-inflammatory response. In addition, intestinal permeability increases during aging, which favors bacterial translocation and inflammation [64-66].
Evidence associated with vaccination shows an imbalance in pro- and anti-inflammatory mechanisms, lower production and diversification of T lymphocytes, altered immunosurveillance and synthesis of antibodies before immunization with SARS-CoV-2 and a decrease in immunological memory. Nutrition in geriatric individuals is essential to combat sarcopenia and bone fragility. Some food components that contribute to immunocompetence are proteins, vitamin D, n-3 fatty acids, antioxidant vitamins (vitamins C and E), zinc, selenium and iron, which are relevant for the senior population [67].
Immunosenescence and SARS-CoV-2
Immunosenescence is a process associated with the deterioration of the immune system caused by aging. This process has become relevant in recent years because it has been reported that COVID-19 disproportionately affects older adults. The CDC has also reported that people ages 65 to 74 are 90 times more likely to die from COVID-19 than people ages 18 to 29 [68].
Recent research mentions that aging is related to the immune system’s ability to maintain the organism in homeostasis with the individual’s intestinal microbiota. In addition, it is inferred that there is no single pattern that can define the immunological profile of the population over 60 years of age since there are multiple factors that alter the immune response, such as environmental exposure, nutritional status, and psychological status as humans age [66]. The phenomenon of senescence implies apoptosis when cells reach the end of their replicative potential or are exposed to various stressors, such as infection. Senescent cells accumulate in the tissues of older individuals and contribute to the development of age-related disorders. However, it was only in 2011 that it was determined that eliminating senescent cells could delay the appearance of diseases associated with aging. This discovery confirmed senescence as a hallmark of aging [69]. Immunosenescence originates as the inability of the innate and adaptive immune responses to act efficiently [70].
Older adults present deterioration of multiple functions such as the neutrophils’ phagocytic capacity, the synthesis of reactive oxygen intermediates, the efficiency of intracellular destruction, secretion of cytokines and chemokines, antibacterial defenses, wound infiltration and repair, and antigen presentation [71,72]. On the other hand, a mortality study in patients with a molecular diagnosis of COVID-19 at the Regional Hospital of High Specialty of Ixtapaluca showed that of 646 hospitalized adults, 426 were under 60 years of age, and 220 were 60 years of age or older. The authors pointed out that diabetes and hypertension were two of the most frequent comorbidities in Mexican patients. More febrile symptoms were also found in older adults, emphasizing that cellular senescence may be a condition for presenting more complex symptoms of hypertension during SARS-Cov-2 infection. It was also shown that mortality was significantly higher in the older adult group [73]. Similarly, a statistical analysis performed in the USA showed a higher prevalence for adolescents and young people than for older adults [74]. However, another study on the prevalence of COVID-19 in children, adolescents, and adults conducted through a database of the COVID-19 Monitoring Program in school children in Brazil, reported 3.5%, 3.6%, and 6.0% in children, adolescents and adults, respectively [75]. The previous confirmed that population diversity has a direct correlation with the Figures reported cases of COVID-19 [76].
Lymphopenia
In the process of immunosenescence, there is lymphopenia marked by decreased function of regulatory T cells, which increases susceptibility to autoimmune and inflammatory responses [77]. On the other hand, the condition and reduced ability of senescent macrophages to phagocytize apoptotic cells promote a proinflammatory state. During COVID-19, there is a significant reduction in peripheral lymphocytes, mainly CD4 and CD8 T cells and is associated with an increased risk of developing a secondary bacterial infection and viral sepsis. The exact mechanism leading to lymphopenia is unknown; however, it has been proposed that SARS-CoV-2 could directly infect T cells [78]. Older adults have a less efficient response to eliminate the viral load, which generates a cytokine storm with inadequate immune response and immunological memory, as well as a direct attack by SARS-CoV-2 on other organs. Systemic immune pathogenesis caused by cytokine storm and microcirculatory dysfunctions leads to viral sepsis due to lymphocyte depletion and dysfunction, therefore an adaptive immune response cannot be initiated early and effectively. This uncontrolled infection leads to increased macrophage infiltration and further damage to the lung [79]. The increased susceptibility to viral lower respiratory tract infections is primarily related to defective innate immunity [80].
Currently, only limited information is available on the innate response of the host infected with SARS-CoV-2, some studies show the involvement of innate immunity in the defense against COVID-19. Among these is a study of 99 cases in Wuhan, where an increase in total neutrophils (38%), a decrease in total lymphocytes (35%), an increase in serum IL-6 (52%) and an increase in C-reactive protein (84%) [81]. Another study conducted in the same city confirmed the same results in 41 patients, showing an increase in neutrophils and a decrease in lymphocytes, which correlated with the severity of the disease and death of the patients [82,83]. On the other hand, among the main risk factors for acute respiratory distress syndrome (ARDS) are diabetes, obesity, poorly controlled systemic arterial hypertension [SAH], low levels of T lymphocytes (CD3 and CD4), levels elevated aspartate aminotransferase, - albumin, creatinine, glucose, low-density lipoproteins, serum ferritin, lactate dehydrogenase and D-dimer [84,85].
Vaccines in immunosenescence
The vulnerability of older adults to COVID-19 prioritizes vaccination in this age group. Immunization studies in older adults against influenza have shown that immunosenescence decreases its efficacy since an efficacy of 30% - 40% has been shown in older adults, who are the most vulnerable population. However, in the case of vaccination against SARS-CoV-2, in older adults, the results of clinical trials have been promising. In adults 70 years and older, a dose of the Pfizer/BioNTech vaccine was 57% to 61% effective in preventing COVID-19 and a dose of the AstraZeneca/Oxford University vaccine was 60% effective to 73%%. Administration of a second dose increased the efficacy of the Pfizer vaccine by 85% - 90% [86]. On the other hand, it is essential to consider epigenetics to predict the variability in vaccination efficacy in different regions. In addition, a second factor to consider is the individual’s nutritional status, since it is essential to generate a protective, effective and long-lasting immune response in the host [87]. On the other hand, a study shows that the expression of ACE2 receptors in the nasal epithelium depends on age, and is important since it is the first point of contact between SARS-CoV-2 and the host. In children, it has been shown that there is a lower expression of ACE2 than in older adults, which explains the high frequency of COVID-19 in older adults. These factors may affect the severity of illness and late recovery from pneumonia caused by SARS-CoV-2 infection in older adults [88,89]. Since March 2020, older adults around the world have shown disproportionate effects caused by SARS-CoV-2, reflected in high mortality rates, compared to young adults and children, so it is recommended to vaccinate the world population and prevent the generation of new viral variants [90].
Conclusion
The present work suggests that decreased cellular-type immune response presented by individuals over 65 years of age and the presence of comorbidities such as uncontrolled type 2 diabetes or SHA favor the development of complications such as ARDS, sepsis, multi-organ dysfunction and subsequent death of the patient. Aging and the state of immunosenescence, accompanied by the effect of inflammation, affect the immune response against viruses in older adults. The imbalance in the pro-and anti-inflammatory mechanisms, the reduced production and diversification of T lymphocytes and the lowered synthesis of antibodies against immunization are pointed out. Likewise, the overexpression of ACE2 receptors in older adults and decreased generation of antibodies to the vaccines are highlighted. The preserved and diminished Th2-type response in patients older than 65 years will allow their recovery, especially by producing IgM, IgG and IgA isotype antibodies, which can limit fatal outcomes. It is necessary to evaluate the immune response in elderly patients in saliva or in nasopharyngeal swabs at intervals to determine the immune status and thus have senescence data. Additionally, other factors can improve the response immunity of this age group, including warm climate, exposure to UV rays and adequate nutrition with vitamin D intake [91]. To reduce infections in immunized older adults with greater susceptibility to infections, the correct use of nasal and mouth masks [92], frequent hand washing and healthy social distancing will help protect the elderly.
I’ve Commons Attribution 4.0 International License (http://creat ivecommons .org/licen ses/by/4.0/), which permits unrestricted use, distribute-tion, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made
Statement of human and animal rights
This is a review article and none of the authors have performed studies with human or animal participants for it.
Disclaimer
The opinions expressed in this document are those of the authors.
The sponsors had no role in the design, methods, data collection, analysis and preparation of this paper.
- Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020 Mar 19;91(1):157-160. doi: 10.23750/abm.v91i1.9397. PMID: 32191675; PMCID: PMC7569573.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30;: PMID: 31986264; PMCID: PMC7159299.
- Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, Karlsen T H. The ABO blood group locus and a chromosome 3 gene cluster associate with SARS-CoV-2 respiratory failure in an Italian-Spanish genome-wide association analysis. medRxiv. 2020. https://doi.org/10.1101/2020.05.31.20114991
- Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020 Nov;587(7835):610-612. doi: 10.1038/s41586-020-2818-3. Epub 2020 Sep 30. PMID: 32998156.
- Witkowski JM, Fulop T, Bryl E. Immunosenescence and COVID-19. Mech Ageing Dev. 2022 Jun;204:111672. doi: 10.1016/j.mad.2022.111672. Epub 2022 Apr 1. PMID: 35378106; PMCID: PMC8975602.
- Gil R, Bitar P, Deza C, Dreyse J, Florenzano M, Ibarra C, Jorquera J, Melo J, Olivi H, Parada MT, Rodríguez JC, Undurraga Á. Cuadro clínico del COVID-19 clinical presentation of COVID-19. Revista Médica Clínica Las Condes. 2021;32(1):20–9. doi: 10.1016/j.rmclc.2020.11.004.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-1242. doi: 10.1001/jama.2020.2648. PMID: 32091533.
- Cunha LL, Perazzio SF, Azzi J, Cravedi P, Riella LV. Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front Immunol. 2020 Aug 7;11:1748. doi: 10.3389/fimmu.2020.01748. PMID: 32849623; PMCID: PMC7427491.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565-574. doi: 10.1016/S0140-6736(20)30251-8. Epub 2020 Jan 30. PMID: 32007145; PMCID: PMC7159086.
- Redondo N, Zaldívar-López S, Garrido JJ, Montoya M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front Immunol. 2021 Jul 7;12:708264. doi: 10.3389/fimmu.2021.708264. PMID: 34305949; PMCID: PMC8293742.
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020 Apr 16;181(2):281-292.e6. doi: 10.1016/j.cell.2020.02.058. Epub 2020 Mar 9. Erratum in: Cell. 2020 Dec 10;183(6):1735. PMID: 32155444; PMCID: PMC7102599.
- Ziebuhr J, Snijder EJ, Gorbalenya AE. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol. 2000 Apr;81(Pt 4):853-79. doi: 10.1099/0022-1317-81-4-853. PMID: 10725411.
- Ruch TR, Machamer CE. The coronavirus E protein: assembly and beyond. Viruses. 2012 Mar;4(3):363-82. doi: 10.3390/v4030363. Epub 2012 Mar 8. PMID: 22590676; PMCID: PMC3347032.
- Astuti I, Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr. 2020 Jul-Aug;14(4):407-412. doi: 10.1016/j.dsx.2020.04.020. Epub 2020 Apr 18. PMID: 32335367; PMCID: PMC7165108.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020 Mar;38(1):1-9. doi: 10.12932/AP-200220-0772. PMID: 32105090.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020 Mar 17;94(7):e00127-20. doi: 10.1128/JVI.00127-20. PMID: 31996437; PMCID: PMC7081895.
- Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD Jr, Thackray LB, Young MD, Mason RJ, Ambrosino DM, Wentworth DE, Demartini JC, Holmes KV. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15748-53. doi: 10.1073/pnas.0403812101. Epub 2004 Oct 20. PMID: 15496474; PMCID: PMC524836.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-260. doi: 10.1038/s41569-020-0360-5. PMID: 32139904; PMCID: PMC7095524.
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020; doi: doi.org/10.1101/2020.01.26.919985.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627.doi.org/10.1016/j.cell.2020.02.052.
- Schreiber G. The Role of Type I Interferons in the Pathogenesis and Treatment of COVID-19. Front Immunol. 2020 Sep 30;11:595739. doi: 10.3389/fimmu.2020.595739. PMID: 33117408; PMCID: PMC7561359.
- Castaño-Rodriguez C, Honrubia JM, Gutiérrez-Álvarez J, DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, Regla-Nava JA, Fernandez-Delgado R, Verdia-Báguena C, Queralt-Martín M, Kochan G, Perlman S, Aguilella VM, Sola I, Enjuanes L. Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. mBio. 2018 May 22;9(3):e02325-17. doi: 10.1128/mBio.02325-17. PMID: 29789363; PMCID: PMC5964350.
- Konno Y, Kimura I, Uriu K, Fukushi M, Irie T, Koyanagi Y, Sauter D, Gifford RJ; USFQ-COVID19 Consortium, Nakagawa S, Sato K. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep. 2020 Sep 22;32(12):108185. doi: 10.1016/j.celrep.2020.108185. Epub 2020 Sep 4. PMID: 32941788; PMCID: PMC7473339.
- Miorin L, Kehrer T, Sanchez-Aparicio MT, Zhang K, Cohen P, Patel RS, Cupic A, Makio T, Mei M, Moreno E, Danziger O, White KM, Rathnasinghe R, Uccellini M, Gao S, Aydillo T, Mena I, Yin X, Martin-Sancho L, Krogan NJ, Chanda SK, Schotsaert M, Wozniak RW, Ren Y, Rosenberg BR, Fontoura BMA, García-Sastre A. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):28344-28354. doi: 10.1073/pnas.2016650117. Epub 2020 Oct 23. PMID: 33097660; PMCID: PMC7668094.
- Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H, Menachery VD, Rajsbaum R, Shi PY. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020 Oct 6;33(1):108234. doi: 10.1016/j.celrep.2020.108234. Epub 2020 Sep 19. PMID: 32979938; PMCID: PMC7501843.
- Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008 Mar;123(3):326-38. doi: 10.1111/j.1365-2567.2007.02719.x. Epub 2007 Nov 5. PMID: 17983439; PMCID: PMC2433332.
- Navarro VG. Inmunopatogenia del envejecimiento: el deterioro de la inmunidad innata y su repercusión sobre la inmunidad específica. Restauración por AM3. Rev. Esp. Geriatr. Gerontol. 2000; 35(1):30-42.
- Perez GTL, Sandoval MDLPR, Altamirano MST. Fisiopatología del daño multiorgánico en la infección por SARS-Cov2. Acta Pediatr. de Mex. 2020; 41(4S1), 27-41.
- Hanel A, Carlberg C. Vitamin D and evolution: Pharmacologic implications. Biochem Pharmacol. 2020 Mar;173:113595. doi: 10.1016/j.bcp.2019.07.024. Epub 2019 Aug 1. PMID: 31377232.
- Carlberg C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front Immunol. 2019 Sep 13;10:2211. doi: 10.3389/fimmu.2019.02211. PMID: 31572402; PMCID: PMC6753645.
- Shah Alam M, Czajkowsky DM, Aminul Islam M, Ataur Rahman M. The role of vitamin D in reducing SARS-CoV-2 infection: An update. Int Immunopharmacol. 2021 Aug;97:107686. doi: 10.1016/j.intimp.2021.107686. Epub 2021 Apr 17. PMID: 33930705; PMCID: PMC8052476.
- Aygun H. Vitamin D can reduce severity in COVID-19 through regulation of PD-L1. Naunyn Schmiedebergs Arch Pharmacol. 2022 Apr;395(4):487-494. doi: 10.1007/s00210-022-02210-w. Epub 2022 Jan 31. PMID: 35099571; PMCID: PMC8802291.
- Wang Y, Pang SC. Yang Y. Una asociación potencial entre la inmunosenescencia y la alta mortalidad relacionada con COVID-19 entre pacientes ancianos con enfermedades cardiovasculares. Envejecimiento Inmune. 2021; 18, 25 doi.org/10.1186/s12979-021-00234-z
- Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, Accardi G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol. 2019 Sep 25;10:2247. doi: 10.3389/fimmu.2019.02247. PMID: 31608061; PMCID: PMC6773825.
- Spyridopoulos I, Hoffmann J, Aicher A, Brümmendorf TH, Doerr HW, Zeiher AM, Dimmeler S. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009 Oct 6;120(14):1364-72. doi: 10.1161/CIRCULATIONAHA.109.854299. Epub 2009 Sep 21. PMID: 19770396.
- Kadambari S, Klenerman P, Pollard AJ. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev Med Virol. 2020 Sep;30(5):e2144. doi: 10.1002/rmv.2144. Epub 2020 Jul 15. PMID: 32671966; PMCID: PMC7404358.
- Domingues R, Lippi A, Setz C, Outeiro TF, Krisko A. SARS-CoV-2, immunosenescence and inflammaging: partners in the COVID-19 crime. Aging (Albany NY). 2020 Sep 29;12(18):18778-18789. doi: 10.18632/aging.103989. Epub ahead of print. PMID: 32991323; PMCID: PMC7585069.
- Cortes WAG. Inmunosenescencia, multimorbilidad, fragilidad y COVID-19. Revista Endocrino, 2021; 8(1). doi.org/10.53853/encr.8.1.665
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003 Apr;132(1):24-31. doi: 10.1046/j.1365-2249.2003.02137.x. PMID: 12653832; PMCID: PMC1808682.
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000 Apr 18;101(15):1767-72. doi: 10.1161/01.cir.101.15.1767. PMID: 10769275.
- Licastro F, Grimaldi LM, Bonafè M, Martina C, Olivieri F, Cavallone L, Giovanietti S, Masliah E, Franceschi C. Interleukin-6 gene alleles affect the risk of Alzheimer's disease and levels of the cytokine in blood and brain. Neurobiol Aging. 2003 Nov;24(7):921-6. doi: 10.1016/s0197-4580(03)00013-7. PMID: 12928051.
- Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012 Oct;11(5):867-75. doi: 10.1111/j.1474-9726.2012.00851.x. Epub 2012 Jul 20. PMID: 22708967.
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 Jun;908:244-54. doi: 10.1111/j.1749-6632.2000.tb06651.x. PMID: 10911963.
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol. 2018 Jan 10;8:1960. doi: 10.3389/fimmu.2017.01960. PMID: 29375577; PMCID: PMC5767595.
- Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012 Oct;11(5):867-75. doi: 10.1111/j.1474-9726.2012.00851.x. Epub 2012 Jul 20. PMID: 22708967.
- Witkowski JM, Fulop T, Bryl E. Immunosenescence and COVID-19. Mech Ageing Dev. 2022 Jun;204:111672. doi: 10.1016/j.mad.2022.111672. Epub 2022 Apr 1. PMID: 35378106; PMCID: PMC8975602.
- Barrera-Salas M, Morales-Hernández AE, Hernández-Osorio JJ, Hernández-Salcedo DR, Valencia-López R, Ramírez-Crescencio MA. Inmunosenescencia. Medicina interna de Méxic. 2017; 33(5):696-704. doi.org/10.24245/mim.v33i5.1204
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009 Aug;11(8):973-9. doi: 10.1038/ncb1909. Epub 2009 Jul 13. Erratum in: Nat Cell Biol. 2009 Oct;11(10):1272. Dosage error in article text. PMID: 19597488; PMCID: PMC2743561.
- Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011 Oct 15;25(20):2125-36. doi: 10.1101/gad.17276711. Epub 2011 Oct 6. PMID: 21979375; PMCID: PMC3205583.
- Cinat D, Coppes RP, Barazzuol L. DNA Damage-Induced Inflammatory Microenvironment and Adult Stem Cell Response. Front Cell Dev Biol. 2021 Oct 8;9:729136. doi: 10.3389/fcell.2021.729136. PMID: 34692684; PMCID: PMC8531638.
- Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019 May;117:15-22. doi: 10.1016/j.cyto.2019.01.013. Epub 2019 Feb 16. PMID: 30776684.
- Green DR, Oguin TH, Martinez J. The clearance of dying cells: table for two. Cell Death Differ. 2016 Jun;23(6):915-26. doi: 10.1038/cdd.2015.172. Epub 2016 Mar 18. PMID: 26990661; PMCID: PMC4987729.
- Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012 Apr;24(4):835-45. doi: 10.1016/j.cellsig.2011.12.006. Epub 2011 Dec 11. PMID: 22182507.
- Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018 Dec;40:61-73. doi: 10.1016/j.smim.2018.09.001. Epub 2018 Sep 26. PMID: 30268598.
- Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018 May;20(5):521-527. doi: 10.1038/s41556-018-0092-5. Epub 2018 Apr 23. PMID: 29686264.
- Wong SQ, Kumar AV, Mills J, Lapierre LR. Autophagy in aging and longevity. Hum Genet. 2020 Mar;139(3):277-290. doi: 10.1007/s00439-019-02031-7. Epub 2019 May 30. PMID: 31144030; PMCID: PMC6884674.
- Moreira OC, Estébanez B, Martínez-Florez S, de Paz JA, Cuevas MJ, González-Gallego J. Mitochondrial Function and Mitophagy in the Elderly: Effects of Exercise. Oxid Med Cell Longev. 2017;2017:2012798. doi: 10.1155/2017/2012798. Epub 2017 Aug 16. PMID: 28900532; PMCID: PMC5576425.
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20388-93. doi: 10.1073/pnas.0908698106. Epub 2009 Nov 16. PMID: 19918053; PMCID: PMC2787135.
- Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010 Mar;40(3):616-9. doi: 10.1002/eji.200940168. PMID: 20201014.
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011 Aug 26;333(6046):1109-12. doi: 10.1126/science.1201940. PMID: 21868666; PMCID: PMC3405151.
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194-217. doi: 10.1016/j.cell.2013.05.039. PMID: 23746838; PMCID: PMC3836174.
- Chowdhury A, Witte S, Aich A. Role of Mitochondrial Nucleic Acid Sensing Pathways in Health and Patho-Physiology. Front Cell Dev Biol. 2022 Feb 11;10:796066. doi: 10.3389/fcell.2022.796066. PMID: 35223833; PMCID: PMC8873532.
- Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004 Jun;75(6):995-1000. doi: 10.1189/jlb.0703328. Epub 2004 Feb 24. PMID: 14982943.
- Cuanalo-Contreras K, Mukherjee A, Soto C. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol. 2013;2013:638083. doi: 10.1155/2013/638083. Epub 2013 Nov 17. PMID: 24348562; PMCID: PMC3855986.
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larché MJ, Davidson DJ, Verdú EF, Surette MG, Bowdish DME. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe. 2017 Apr 12;21(4):455-466.e4. doi: 10.1016/j.chom.2017.03.002. Erratum in: Cell Host Microbe. 2018 Apr 11;23 (4):570. PMID: 28407483; PMCID: PMC5392495.
- DeJong EN, Surette MG, Bowdish DME. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe. 2020 Aug 12;28(2):180-189. doi: 10.1016/j.chom.2020.07.013. PMID: 32791111.
- Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. SARS-CoV-2, COVID-19 and the Ageing Immune System. Nat Aging. 2021 Sep;1(9):769-782. doi: 10.1038/s43587-021-00114-7. Epub 2021 Sep 14. PMID: 34746804; PMCID: PMC8570568.
- Lutz M, Arancibia M, Papuzinski C, Stojanova J. Inmunosenescencia, infecciones virales y nutrición: revisión narrativa de la evidencia científica disponible [Immunosenescence, viral infections and nutrition: A narrative review of scientific available evidence]. Rev Esp Geriatr Gerontol. 2022 Jan-Feb;57(1):33-38. Spanish. doi: 10.1016/j.regg.2021.08.003. Epub 2021 Nov 26. PMID: 34844781.
- Kumar A, Narayan RK, Prasoon P, Kumari C, Kaur G, Kumar S, Kulandhasamy M, Sesham K, Pareek V, Faiq MA, Pandey SN, Singh HN, Kant K, Shekhawat S, Raza K, Kumar S: Mecanismos del COVID-19 en el cuerpo humano: Lo que sabemos hasta ahora. Kompass. Neumol. 2022; 4:3-20. doi: 10.1159/000521507.
- Khan F, Anto Johnson P, Christy Johnson J, Singh J, Mardon A. Immunosenescence, COVID-19, and Vaccine Efficacy in the Elderly. Canadian Journal of Medicine. 2022; 4(1), 22-25. doi: 10.33844/cjm.2022.6017
- Rico-Rosillo MG, Oliva-Rico D, Vega-Robledo GB. Envejecimiento: algunas teorías y consideraciones genéticas, epigenéticas y ambientales [Aging: Some theories, genetic, epigenetic and environmental considerations]. Rev Med Inst Mex Seguro Soc. 2018 Oct 25;56(3):287-294. Spanish. PMID: 30394717.
- Muñoz Carlos-Sebastian. El daño genético y los macrófagos. Implicaciones en envejecimiento y en la inflamación. Tesis Doctoral. Universitat de Barcelona. 2008.
- Moskalev A, Stambler I, Caruso C. Inmunidad innata y adaptativa en el envejecimiento y la longevidad: la base de la resiliencia. Envejecimiento y enfermedad, 2020; 11(6), 1363–1373. doi.org/10.14336/AD.2020.0603
- Luis R Macias Kauffer, Cayetano MR, Bryan Medina Leyte, Alma R Sánchez Conejo, Gustavo Acosta Altamirano, Increased Serum Glucose is not a Covid-19 Mortality Predictor in Elderly Patients. 2020; 11(2). AJBSR.MS.ID.001613. doi:10.34297/AJBSR.2020.11.001613.
- Rumain B, Schneiderman M, Geliebter A. Prevalence of COVID-19 in adolescents and youth compared with older adults in states experiencing surges. PLoS One. 2021 Mar 10;16(3):e0242587. doi: 10.1371/journal.pone.0242587. PMID: 33690600; PMCID: PMC7946189.
- Cavalcante Pinto Júnior V, Moura LFWG, Cavalcante RC, Lima JRC, Bezerra AS, de Sousa Dantas DR, Amaral CML, Lima DF, Júnior ABV, Florindo Guedes MI. Prevalence of COVID-19 in children, adolescents and adults in remote education situations in the city of Fortaleza, Brazil. Int J Infect Dis. 2021 Jul;108:20-26. doi: 10.1016/j.ijid.2021.04.086. Epub 2021 May 1. PMID: 33945867; PMCID: PMC8088039.
- Malaguarnera L, Cristaldi E, Lipari H, Malaguarnera M. Acquired immunity: immunosenescence and physical activity. Eur. Rev. Aging. Phys. Act. 2008; 5(61) doi.org/10.1007/s11556-008-0039-0
- Gao L, Zhou J, Yang S, Wang L, Chen X, Yang Y, Li R, Pan Z, Zhao J, Li Z, Huang Q, Tang J, Hu L, Liu P, Zhang G, Chen Y, Ye L. The dichotomous and incomplete adaptive immunity in COVID-19 patients with different disease severity. Signal Transduct Target Ther. 2021 Mar 8;6(1):113. doi: 10.1038/s41392-021-00525-3. PMID: 33686064; PMCID: PMC7938043.
- Thomas R, Wang W, Su DM. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun Ageing. 2020 Jan 20;17:2. doi: 10.1186/s12979-020-0173-8. PMID: 31988649; PMCID: PMC6971920.
- Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020 May 9;395(10235):1517-1520. doi: 10.1016/S0140-6736(20)30920-X. Epub 2020 Apr 17. PMID: 32311318; PMCID: PMC7164875.
- Napoli C, Tritto I, Mansueto G, Coscioni E, Ambrosio G. Immunosenescence exacerbates the COVID-19. Arch Gerontol Geriatr. 2020 Sep-Oct;90:104174. doi: 10.1016/j.archger.2020.104174. Epub 2020 Jul 3. PMID: 32653765; PMCID: PMC7333612.
- Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel). 2016 Mar 15;8(3):36. doi: 10.3390/cancers8030036. PMID: 26999211; PMCID: PMC4810120.
- Tu W, Rao S. Mechanisms Underlying T Cell Immunosenescence: Aging and Cytomegalovirus Infection. Front Microbiol. 2016 Dec 27;7:2111. doi: 10.3389/fmicb.2016.02111. PMID: 28082969; PMCID: PMC5186782.
- Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015 May 1;194(9):4073-80. doi: 10.4049/jimmunol.1500046. PMID: 25888703; PMCID: PMC4452284.
- Caruso C, Candore G, Cigna D, DiLorenzo G, Sireci G, Dieli F, Salerno A. Cytokine production pathway in the elderly. Immunol Res. 1996;15(1):84-90. doi: 10.1007/BF02918286. PMID: 8739567.
- Cox LS, Bellantuono I, Lord JM, Sapey E, Mannick JB, Partridge L, Gordon AL, Steves CJ, Witham MD. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev. 2020 Nov;1(2):e55-e57. doi: 10.1016/S2666-7568(20)30011-8. Epub 2020 Nov 9. PMID: 33521768; PMCID: PMC7834195.
- Torres-Estrella CU, Reyes-Montes M del R, Duarte-Escalante E, Sierra Martínez M, Frías-De-León MG, Acosta-Altamirano G. Vaccines Against COVID-19: A Review. Vaccines. 2022; 10(3):414. doi.org/10.3390/vaccines10030414
- Zhang Z, Guo L, Huang L, Zhang C, Luo R, Zeng L, Liang H, Li Q, Lu X, Wang X, Ma CY, Shao J, Luo W, Li L, Liu L, Li Z, Zhou X, Zhang X, Liu J, Yang J, Kwan KY, Liu W, Xu Y, Jiang H, Liu H, Du H, Wu Y, Yu G, Chen J, Wu J, Zhang J, Liao C, Chen HJ, Chen Z, Tse HF, Xia H, Lian Q. Distinct Disease Severity Between Children and Older Adults With Coronavirus Disease 2019 (COVID-19): Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin Infect Dis. 2021 Dec 6;73(11):e4154-e4165. doi: 10.1093/cid/ciaa1911. PMID: 33388749; PMCID: PMC7799282.
- Bunyavanich S, Do A, Vicencio A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020 Jun 16;323(23):2427-2429. doi: 10.1001/jama.2020.8707. PMID: 32432657; PMCID: PMC7240631.
- Center for Infectious Disease Research and Policy. Pfizer, AstraZeneca COVID-19 vaccines may offer high efficacy in elderly, https://www.cidrap.umn.edu/news-perspective/2021/03/pfizer-astrazeneca-covid-19-vaccines-may-offer-highefficacy-elderly; 2021 accessed 1 mayo 2022.
- Sanz JM, Gómez Lahoz AM, Martín RO. Papel del sistema inmune en la infección por el SARS-CoV-2: inmunopatología de la COVID-19 [Role of the immune system in SARS-CoV-2 infection: immunopathology of COVID-19]. Medicine (Madr). 2021 May;13(33):1917-1931. Spanish. doi: 10.1016/j.med.2021.05.005. Epub 2021 May 27. PMID: 34075268; PMCID: PMC8158328.
- Frias AMG, Martínez MS, Acosta-Altamirano G. Nasal Mask-An Alternative to Prevent Contagion during Essential Activities. Biomed J Sci Tech Res Title. 2021; 34(4):27018-27022. doi: 10.26717/BJSTR.2021.34.005595

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
